Abstract
The physicochemical and rheological properties of yoghurt made from unstandardised unhomogenised buffalo milk were investigated during fermentation and 28 days of storage and compared to the properties of yoghurt made from homogenised fortified bovine milk. A number of differences observed in the gel network can be linked to differences in milk composition. The microstructure of buffalo yoghurt, as assessed by confocal laser scanning microscopy (CLSM) and cryo scanning electron microscopy (cryo-SEM), was interrupted by large fat globules and featured more serum pores. These fat globules have a lower surface area and bind less protein than the homogenised fat globules in bovine milk. These microstructural differences likely lead to the higher syneresis observed for buffalo yoghurt with an increase from 17.4 % (w/w) to 19.7 % (w/w) in the weight of whey generated at days 1 and 28 of the storage. The higher concentration of total calcium in buffalo milk resulted in the release of more ionic calcium during fermentation. Gelation was also slower but the strength of the two gels was similar due to similar protein and total solids concentrations. Buffalo yoghurt was more viscous, less able to recover from deformation and less Newtonian than bovine yoghurt with a thixotropy of 3,035 Pa.s−1 measured for buffalo yoghurt at the end of the storage, at least four times higher than the thixotropy of bovine yoghurt. While the titratable acidity, lactose consumption and changes in organic acid concentrations were similar, differences were recorded in the viability of probiotic bacteria with a lower viability of Lactobacillus acidophilus of 5.17 log (CFU/g) recorded for buffalo yoghurt at day 28 of the storage. Our results show that factors other than the total solids content and protein concentration of milk affect the structural properties of yoghurt. They also illustrate the physicochemical reasons why buffalo and bovine yoghurt are reported to have different sensory properties and provide insight into how compositional changes can be used to alter the microstructure and properties of dairy products.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Yoghurt consumption has increased considerably in the last 35 years, with Australian consumption rising from 1.0 to 7.1 kg per capita per year (Dairy Australia 2010; Tamime and Robinson 2007). Yoghurt can be produced from a range of milk types, with bovine milk being the most popular raw ingredient and a small volume of buffalo or other milk used for yoghurt production (Tamime and Robinson 2007). The chemical composition of milk varies considerably between species and this difference impacts on both the processing steps in yoghurt production, as well as the properties of the final product. Buffalo milk, for example, has a higher concentration of fat (8.0 % w/w vs. 4.1 % w/w), protein (4.6 % w/w vs. 3.4 % w/w), total solids (17.0 % w/w vs. 12.1 % w/w), calcium (47 mM vs. 30.5 mM) and phosphorous (27.7 mM vs. 19.2 mM; Ahmad et al. 2008; Braun and Preuss 2008; Menard et al. 2010; Varricchio et al. 2007; Jensen 1995) than bovine milk.
Yoghurt is traditionally produced using a mixture of thermophilic starter cultures consisting of Lactobacillus delbrueckii ssp. bulgaricus and Streptococcus thermophilus. Recently, probiotic bacteria such as Lactobacillus acidophilus and Bifidobacterium spp. have also been included for their potential health benefits (Donkor et al. 2007). L. bulgaricus can, however, produce excess acid during yoghurt storage leading to significant reduction in the viability of probiotic bacteria, particularly L. acidophilus and also a strong flavour in the final product (Saccaro et al. 2009).
The set-type buffalo yoghurt found on the Australian market is typically produced with unhomogenised milk. This yoghurt is also made without milk solids fortification or the addition of artificial thickeners, as the solids content of the milk is high (Addeo et al. 2007). Moreover, buffalo milk powder is not readily available worldwide, making milk fortification more difficult (Tamine and Robinson 2007). In contrast, bovine milk often requires fortification with skimmed milk powder to increase the solids content and achieve the desired yoghurt firmness and texture (Gun and Isikli 2006; Tamime and Robinson 2007). Bovine milk is also typically homogenised, producing a homogenous product that prevents the formation of a cream layer.
While many studies have examined the structure and properties of bovine yoghurt, few have been conducted on buffalo yoghurt. The effect of fat (Cunha-Neto et al. 2005; Pandya et al. 2004; Raju and Pal 2009), solids content (Chawla and Balachandran 1994; Shiby and Mishra 2008) and storage time (Yadav et al. 2007) on buffalo yoghurt properties has been examined. Fortified buffalo yoghurt containing fruit pulp, honey and soymilk has also been investigated (Ghadge 2008; Kumar and Mishra 2003). These studies have primarily focused on the chemical composition of the final product and have often employed sensory evaluation to evaluate textural properties, which provides only limited insight into the underlying chemical and physical properties of the product.
The viscosity and flow properties of buffalo yoghurt are also not well understood. Many studies have measured the viscosity of buffalo yoghurt at a single shear rate (Raju and Pal 2009; Cunha-Neto et al. 2005) but as yoghurt is a non-Newtonian fluid, it is expected that the viscosity will be a function of the shear rate applied (Lee and Lucey 2010). Recently, Bezerra and co-authors (2012) investigated different formulations made from buffalo and goats milk and determined rheological properties including the consistency coefficient (K) and flow behaviour index (n) using a range of shear rates from 10 to 1,100 s−1, however, other important properties such as gelation and thixotropy of buffalo yoghurt have not yet been investigated. Several past studies have also ceased milk fermentation at a pH of 4.8–5.0 (Nahar et al. 2007; Yadav et al. 2007; Bezerra et al. 2012), which is significantly higher than the end pH of 4.5–4.6 commonly reported in studies for bovine yoghurt (Lee and Lucey 2004b; Robinson and Haddadin 2010; Tamime and Robinson 2007) and the legal requirement for yoghurt production in Australia (a pH of 4.5 or less; Australia and New Zealand Food Standards 2006).
Comparative analysis of buffalo and bovine yoghurt is likely to be insightful given the differences in bovine and buffalo milk composition and physical properties, yet there have been limited comparative studies. Khanna and Singh (1979) found a higher proteolytic activity, a higher titratable acidity and a better growth of S. thermophilus and L. bulgaricus when unstandardised unhomogenised buffalo milk was used in place of unstandardised unhomogenised bovine milk. Yoghurt made from buffalo milk was also found to be more nutritious than bovine or goat yoghurts due to the higher concentration of protein and fat, although bovine yoghurt was found to have a more acceptable smell and taste (Nahar et al. 2007). Interestingly, buffalo yoghurt made from unstandardised unhomogenised milk contains a higher concentration of acetaldehyde, the major volatile compound in yoghurt, compared to bovine, ewe and goat yoghurts made from unstandardised and unhomogenised milk (Erkaya and Sengul 2011), which may be responsible for the distinct aroma. The organic acid profile, which is known to contribute significantly to yoghurt flavour, has also been studied for bovine (Adhikari et al. 2002; Donkor et al. 2007; Fernandezgarcia and McGregor 1994; Thi et al. 2012; Tormo and Izco 2004) but not buffalo yoghurt.
While existing studies illustrate some of the possible similarities and differences between buffalo and bovine yoghurt, there has been no comprehensive study on buffalo yoghurt using the range of physicochemical and microscopic techniques now available, particularly for products made using processes relevant to a commercial scale. This study aimed to characterize the microstructure and properties of buffalo yoghurt made from unhomogenised unstandardised milk including the fermentation kinetics, texture, rheological properties, organic acid profile and the growth and viability of probiotic bacteria during fermentation and 28 days of cold storage. A second aim was to compare the properties of buffalo yoghurt with the properties of the well studied bovine yoghurt made from fortified and homogenised milk, in order to provide a better understanding of the nature of buffalo yoghurt and how changes to milk composition alter the properties of dairy products, such as yoghurt.
Materials and Methods
Yoghurt Preparation
Yoghurt was produced from either buffalo milk or bovine milk. Raw buffalo milk was obtained from a local dairy farm (VIC, Australia), while pasteurised homogenised bovine milk was purchased from a local supermarket (Pura Brand, VIC, Australia). Each litre of bovine milk was fortified with 37 g skim milk and 37 g full cream milk powder (Coles, VIC, Australia) to obtain a milk composition (Table 1) that enabled the production of yoghurt with a composition similar to a typical of commercial yoghurt (i.e., 3–4 % (w/w) fat, 5–6 % (w/w) protein, 6–8 % (w/w) carbohydrate and 150–200 mg of calcium per 100 g of milk). This experimental design, including the choice of non-homogenised and non-fortified buffalo milk and homogenised and fortified bovine milk was based on the milk preparations typically used commercially at an industrial scale. Moreover, in a preliminary screening experiment, bovine yoghurt produced from non-homogenised and non-fortified milk produced a soft gel with high synersis, which offered a poor comparison to buffalo yoghurt and was not representative of a commercial standard.
Four litre samples of buffalo milk or fortified bovine milk were heated at 85 °C for 30 min in a water bath for pasteurisation and the milk then cooled to 43 °C. Freeze-dried direct vat culture FDV ABT-5 (CHR Hansen, VIC, Australia) containing a mixture of S. thermophilus, L. acidophilus La-5 and Bifidobacterium lactis Bb-12, was then added to the milk. This culture had previously been inoculated in a 100 mL sample of ultra heat treated full cream milk (Devondale brand, VIC, Australia) and incubated at 43 °C for 30 min before addition to the milk for yoghurt production.
The milk was then distributed into plastic containers of two different volumes, 100 and 50 mL, for different analyses. Sample containers with a volume of 100 mL were used for chemical, microbiological, textural and rheological analysis. Sample containers with a volume of 50 mL were used for the analysis of syneresis and microstructure to allow centrifugation and easier access to the sample for microscopic analyses. The containers containing the milk samples were transferred and fermented at 43 °C in a thermostat-controlled water-bath (Qualtex, Watson Victor Ltd., Australia) containing water that had been tempered to 43°C. The fermentation was terminated at a pH of 4.5 and yoghurt samples were immediately stored in a cold room (4 °C) for 28 days. During fermentation, the pH, ionic calcium concentration, titratable acidity, lactose concentration, organic acid profile and bacterial growth were investigated (see below). Changes in the rheological properties, gel firmness and organic acid profiles of yoghurt were also evaluated at days 1, 7 and 28 of cold storage, while changes in the microstructure and viability of bacteria were assessed at the beginning (day 1) and at the end (day 28) of the storage period. Two yoghurt making trials were carried out on separate days and three independent containers of yoghurt were analysed in each trial for each method of analysis.
Chemical Analysis
pH Measurement
Changes in the pH during fermentation were measured using an electrode pH meter (Orion 720A plus, Orion Pacific Pty Ltd., VIC, Australia). Before measurement of the samples, calibrations were performed using three standard pH buffer solutions of pH 10.0, 7.0 and 4.0 (Ajax Fine Chem, VIC, Australia). The pH was measured at the start of the experiment and after the first hour of fermentation. Measurements were then made at an interval of 1.5 h until the pH reached 4.5. Three replicate containers of yoghurt were used for each pH measurement at each time point. These samples were then discarded and new containers used for the next time point.
Total Solids Content
The total solids content was determined according to the standard method of Association of Analytical Communities (2006). Milk samples were dried in a gravity and forced convection oven (S.E.M Pty. Ltd., SA, Australia) at 100 ± 3 °C. After drying, the samples were cooled in a desiccator before weighing. This procedure was repeated until the weight of the samples remained unchanged. The total solids content was expressed as the percentage relative to the initial weight of the sample.
Fat
The milk fat content of samples was determined using a modified version of the method described by Atwood and Hartmann (1992). Briefly, milk samples were diluted 10 times and warmed to 37 °C. A 50 μL aliquot of each sample was mixed with 600 μL ethanol, 100 μL of 2 M hydroxylamine hydrochloride and 100 μL of 3.5 N NaOH and this mixture incubated at room temperature for 30 min. After incubation, the mixture was acidified with 120 μL of 3.4 M HCl and this sample added to 100 μL of a ferric chloride-trichloroacetic acid solution, prepared by mixing 10 mL of the solution containing 0.37 M FeCl3 and 0.1 M HCl with 7.5 g tricloroacetic acid (TCA). The solution was then centrifuged at 1,500×g for 5 min and the supernatant was dispensed into a 96 well microplate (BD Falcon, NSW, Australia). The absorbance of the solution was measured at 540 nm using a spectrophotometer (Fluostar Optima, BMG labtech, Ortenberg, Germany). The fat globule surface area was determined using a Masterizer 2000 (Malvern Instruments, Malvern, UK) or confocal laser scanning microscopy (CLSM) and Imaris software (Bitplane, USA), as reported previously (Ong et al. 2010, 2012).
Protein
The milk protein content of samples was determined using a modified version of the method described by Pesce and Strande (1973). Briefly, a 100 μL sample was mixed with 1 mL of TCA-Ponceau S working reagent, which was prepared from Ponceau-S (Merck Pty Ltd., VIC, Australia) and TCA (Chem Supply, SA, Australia), as described by Pesce and Strande (1973). The solution was then centrifuged at 15,800×g for 5 min. The supernatant was discarded and the precipitate dissolved in 700 μL of 0.2 N NaOH. Each sample was then transferred to a 96 well microplate (BD Falcon, NSW, Australia). The absorbance was measured at 560 nm using the spectrophotometer described above.
Lactose
The lactose concentration of samples was determined using high performance liquid chromatography (HPLC) as described previously (Gosling et al. 2009). Briefly, the carbohydrate fraction was collected from the top layer of a mixture of methanol, chloroform and Mili Q water (a deionized and filtered water, purified to a resistivity of 18.2 mU, Millipore, Billerica, MA, USA). The extracted samples were then separated on an HPLC Shimadzdu Prominence system (NSW, Australia) equipped with a RID-10A refractive index detector and a 300 × 7.8 mm Rezex RCM-Monosaccharide Ca2+ column (Phenomenex, NSW, Australia). Milli Q water was used as mobile phase with a flow rate of 0.5 mL min−1. The column and detector were maintained at 80 °C and 40 °C, respectively. Five standard solutions of lactose were prepared from lactose monohydrate in powder form (Chem Supply, SA, Australia) using a 0.1 N sodium acetate/lactate buffer (Chem Supply, SA, Australia).
Ionic Calcium
The concentration of ionic calcium in samples was determined using an Orion 93-20 calcium half cell electrode in conjunction with an Orion 90-02 Ag/AgCl double junction reference electrode (Orion Pacific, VIC, Australia). The electrodes were connected to an Orion 720A + voltmeter. Samples were placed in a beaker on a magnetic stirrer and the electrodes immersed in the sample for 4–5 min to allow stabilisation before measurement. The concentration of ionic calcium was then determined by reference to a calibration curve set up from a series of CaCl2 standards with concentrations ranging from 0.2 to 10 mM. The CaCl2 standards were prepared from the dehydrate salt (Ajax Fine Chem, VIC, Australia) and potassium chloride (Chem Supply, SA, Australia) added to achieve constant ionic strength of 0.08 M across the range of standard solutions.
Total Calcium
The total calcium content in milk samples was determined using inductively coupled plasma optical emission spectrometry (Varian ICP-OES 720, Varian Inc, CA, USA). Briefly, 10 g of sample was weighed into a porcelain crucible and dried overnight in an oven at 100 ± 3 °C. Crucibles containing dry matter were then transferred to a furnace for ashing at 600 °C for at least 24 h. The ash was acidified with 69 % (w/w) nitric acid and 32 % (w/w) hydrochloric acid and diluted to 10 mL with Mili Q water. Finally, the diluted ash samples were analysed for total calcium by ICP-OES using a wavelength of 373 nm. The concentration of total calcium was determined by reference to a calibration curve of five standard CaCl2 solutions, prepared from the dihydrate salt (Ajax Fine Chem, VIC, Australia).
Titratable Acidity
The titratable acidity of yoghurt samples was assessed following the Association of Analytical Communities (AOAC) titration method using 0.1 N NaOH and phenolphthalein (2 % (w/v) in ethanol) as an indicator (AOAC 2006). A 10 g sample of yoghurt was diluted with 10 mL of Milli Q water before titration. Titratable acidity was expressed as a percentage of lactic acid, determined using the following equation:
Where V NaOH is the volume (mL) of 0.1 N NaOH solution required for titration, m sample is the weight (g) of yoghurt sample.
Organic Acid Profile
The organic acid content of yoghurt samples was determined using a modified version of the method described by Thi and co-authors (2012). Briefly, a 4 g sample was mixed with 70 μL of 15.6 M HNO3 and 4 mL of 0.009 N H2SO4, vortexed for 2 min and left to stand for 15 min before the addition of 17 mL of 0.009 N H2SO4. After mixing for 15 s, the mixture was centrifuged at 3,220×g for 30 min. The aqueous layer was filtered through a 0.22 μm filter membrane before analysis by HPLC using a Shimadzu Prominence system equipped with PDA UV detector and a Bio-Rad Aminex HPX 87H cation exchange column connected to a cation H+ guard column (Bio Rad Laboratories Pty Ltd, Hercules, CA, USA). The detector was set to record absorbance at both 220 and 290 nm. The mobile phase was 0.009 N H2SO4, delivered at a flow rate of 0.6 mL min−1 and the column maintained at 65 °C. Peaks were identified by their retention times and confirmed by spiking with organic acid standards including acetic, citric, formic, hippuric, lactic, propionic, pyruvic and orotic acids (all supplied by Sigma Aldrich, MO, USA). The standards were prepared as stock solutions in Milli Q water (except for uric acid and orotic acid which were dissolved in 0.1 N NaOH to ensure solubility). At least four concentrations of each organic acid were used to prepare the calibration curve. The coeluted acids (orotic and citric acid, uric and formic acid) were quantified based on their different absorbance at two different wavelengths (220 and 290 nm). Briefly, at 290 nm, the absorbance of citric and formic acid were negligible as compared to the absorbance of uric and orotic acid, which enabled the calculation of concentration of uric and orotic acid at this wavelength. The concentration of citric and formic acid were then obtained by subtraction of the total area of absorbance at 220 nm with the area of absorbance of uric and orotic acid.
Syneresis and Analysis of Whey Protein Composition
Yoghurt syneresis was quantified using centrifugation (Purwandari and Vasiljevic 2009). Milk fermented in 50 mL centrifuge tubes (BD Falcon, NSW, Australia) was centrifuged at 700×g at 8 °C for 10 min using an Eppendorf centrifuge (Eppendorf 5810R, VIC, Australia). The whey was then gently poured off and weighed. Syneresis was expressed as a weight percentage of the whey separated from the gel over the initial weight of the gel. Whey was also expelled from yoghurt samples after 1 day of storage by centrifugation at 700×g at 8 °C for 10 min and raw milk by ultra-centrifugation at 100,000×g at 20 °C for 30 min (Beckman Optima L-100XP Ultracentrifuge, Minnesota, USA) and the proteins in these whey samples were analysed using SDS-PAGE as previously described (Ong et al. 2010). Briefly, a similar mass of protein, as assessed by spectrometry, was mixed with the sample buffer solution containing 0.1 M dithiothreitol (Invitrogen, Australia) and boiled at 100 °C for 3 min. The samples and a molecular weight (MW) standard were then loaded onto the 4–15 % precast gel (both the MW standard and the gel were supplied by Bio Rad, CA, USA) and run for 1 h at 150 V with a Bio-Rad power supply unit. The protein bands were fixed and stained using a solution containing 0.025 % (v/v) Coomassie R-250 (ICN Biochemicals Inc., Aurora, Ohio, USA), 40 % (v/v) methanol and 7.5 % (v/v) acetic acid for 1 h and then de-stained in a solution containing 7.5 % (v/v) acetic acid until the background became clear. The gel images were recorded using a Fuji Film intelligent Dark Box II with Fuji Film LAS-1000 Lite V1.3 software (Brookvale, NSW, Australia).
Textural and Rheological Analysis
Firmness (Gel Strength)
The texture of yoghurt was accessed as described by Kumar and Mishra (2003), with some modifications, using a TA.XT-2 texture analyser (Stable Microsystems, Surrey, England) equipped with a 2 kg load cell and a 10 mm diameter cylindrical probe. Triplicate yoghurt samples in 120 mL plastic containers (filled with 100 mL of milk and fermented as described in section 2.1) were used to assess yoghurt texture. Samples were kept at 6–8 °C in an ice box until measurement at room temperature. The contact area was set at 1 mm2 and the contact force set at 5 g. The instrument speed was set at 1 mm s−1. The compression distance, the distance of penetration from the surface of sample, was set at 20 mm. Data were recorded at a rate of 200 points per second. The firmness of the yoghurt, or gel strength, was determined as the maximum force measured during sample compression.
Rheological Properties (Storage Modulus and Loss Tangent) During Fermentation
The rheological properties of yoghurt samples including the storage modulus (G’) and loss tangent (tan δ) during fermentation were determined using a controlled strain rheometer (Advanced Rheometrics Expansion System, TA Instruments, New Castle, USA) equipped with a cup 34 mm in diameter and a six-blade vane fixture 32 mm in diameter and 33 mm in height. A 38 mL milk sample was transferred into the cup immediately after inoculation with starter culture. A dynamic time sweep was performed on the samples with an oscillation frequency of 0.1 Hz and a constant strain of 1 % at the desired fermentation temperature. The measurements of storage modulus (G’) and loss tangent (tan δ) were recorded every 5 min until the fermented milk reached a pH of 4.5. The gelation time was defined as the time when the G’ measured exceeded 1 Pa (Lee and Lucey 2004a).
Rheological Properties (Thixotropy, Flow Behaviour Index and Consistency Coefficient) During Storage
The rheological properties of yoghurt during storage were determined using a method described in a previous study (Purwandari and Vasiljevic 2009), with some modifications. The measurements were carried out using the controlled stress rheometer (AR-G2, TA instruments Ltd., New Castle, USA) fitted with a cone plate (40 mm diameter/4o angle). All samples were stirred with a spatula 20 times clockwise prior to loading to achieve a homogenous mixture. After the sample was equilibrated to 20 °C, the yoghurt was pre-sheared at 500 s−1 for 60 s and then equilibrated for 300 s prior to rheological measurements. The flow curves measured the viscosity of the sample as a function of shear rate. The shear rate was increased logarithmically from 0.1 to 100 s−1 in 300 s (upward curve) followed by a logarithmic decrease from 100 to 0.1 s−1 in 300 s (downward curve). A holding time of 5 s at the shear rate of 100 s−1 was applied to the sample between the increasing and decreasing sweeps. The thixotropy (hysteresis loop area) between the upward and downward flow curves was determined using the Rheology Advantage data analysis software (Version V5.70, TA instruments Ltd., New Castle, USA). Data obtained from the upward curve were fitted to the modified Ostwald-de Waele model given by the equation:
where η is the apparent viscosity (Pa.s), γ the shear rate (s−1), K the consistency coefficient (Pa.sn) and n the flow behaviour index (Purwandari and Vasiljevic 2009).
Microstructural Analysis
Microstructure Observation of Yoghurt Using Confocal Laser Scanning Microscopy (CLSM)
CLSM was used to assess the microstructure of yoghurt samples. Milk was fermented in 50 mL centrifuge tubes (BD Falcon, NSW, Australia) and then left undisturbed in a cold room at 4 °C over the storage period. On the day of analysis, yoghurt was gently taken out of the container by inverting the tube. A thin slice, approximate 2 mm in thickness, was carefully obtained from the middle of the yoghurt sample using a surgical blade. Samples, approximately 3 × 3 × 2 mm in size, were placed on a microscope slide. Samples were stained with Nile Red and Fast Green FCF (both supplied by Sigma Aldrich, NSW, Australia). Stock solutions of Nile Red (1 mg mL−1) and Fast Green FCF (1 mg mL−1) were diluted 10 times with Milli Q water immediately prior to staining. The diluted staining solutions were added to the samples for 15 min at 4 °C to allow the stains to penetrate into the sample. Excess stain was removed and sample was covered with a glass coverslip (0.17 mm thick; ProSciTech, QLD, Australia). Samples were observed using an inverted confocal scanning laser microscope (Leica TCS SP2; Leica Microsystems, Heidelberg, Baden-Wurttemberg, Germany) powered by Ar/Kr and He/Ne lasers with a ×63 oil-immersion objective. During observation, the pinhole diameter was maintained at 1 Airy unit. The excitation/ emission wavelength were set at 488 nm/ 500–600 nm for Nile Red and 633 nm/ 650–710 nm for Fast Green FCF. Two samples were used for CLSM for each trial. Four images were collected for each sample and a typical image presented.
Microstructure Observation of Yoghurt Using Cryo Scanning Electron Microscopy (Cryo-SEM)
Cryo-SEM was used to assess the yoghurt microstructure using a previously described method (Ong et al. 2011). Samples 5 × 2 × 2 mm in size were mounted on a copper holder attached to a vacuum transfer device (VTD) and then rapidly immersed into a liquid nitrogen slush maintained at −210 °C. After freezing, the frozen specimens were immediately transferred into an attached cryo preparation chamber using the VTD. The sample was fractured using a chilled scalpel blade within the chamber, which was maintained at −140 °C under a high vacuum conditions (<10−4 Pa). The specimen was then etched to facilitate the removal of ice from the surface of the fractured sample by vacuum sublimation at −95 °C for 20 min and coated using a cold magnetron sputter coater with 300 V, 10 mA of sputtered gold/palladium alloy (60/40) for 120 s resulting in a gold layer (∼6 nm in thickness). Samples were then transferred under vacuum onto a nitrogen gas cooled module, maintained at −140 °C and observed using a field emission gun SEM (Quanta, Fei Company, Hillsboro, Oregon, USA) and a solid state backscattered electron detector (SSD). Two samples were used for cryo-SEM for each trial. Four images were collected for each sample and a typical image presented.
Microbiological Analysis
The enumeration of bacteria was carried out at the beginning and at the end of the fermentation when the sample reached a pH of 4.5 and at days 1 and 28 of the storage to assess bacterial growth and viability. A 10 g yoghurt sample was diluted in 90 mL of sterile peptone 0.1 % (w/w) solution (Oxoid, Basingstoke, UK), then serially diluted using 9 mL of sterile peptone solution and added to the media using a pour-plate technique. M17 agar (Oxoid, Basingstoke, UK) was used for the enumeration of S. thermophilus (aerobic conditions, 42 °C, 48 h). MRS agar (Oxoid, Basingstoke, UK) supplemented with clindamycine (0.1 mg L−1) was used for the selective enumeration of L. acidophilus La-5. MRS agar supplemented with cysteine hydrochloride (0.5 g L−1), lithium chloride (1 g L−1) and dicloxacillin (0.5 mg L−1) was used for the enumeration Bifidobacterium animalis BB-12. Clindamycine and dicloxacilin were supplied by Sigma Aldrich (NSW, Australia) while cysteine hydrochloride and lithium chloride were obtained from Merck (VIC, Australia). The inoculated plates were incubated under anaerobic conditions using an anaerobic gas jar (Oxoid, SA, Australia) at 37 °C for 72 h. Plates with 25–250 colonies were selected for manual counting.
Statistical Analysis
Experiments were carried out on two separate days and three separate samples taken from each batch for analysis so n = 6. Data were analysed using statistical Minitab software (V16, Minitab Inc., Stage College, PA, USA). One way analysis of variance (ANOVA) and Tukey’s paired comparison were used to assess the differences between means, with a significance level of P = 0.05.
Results and Discussion
A set style yoghurt was made from buffalo milk to examine the properties and structure of this dairy product. A similar yoghurt was also produced from bovine milk to allow comparisons to a well studied yoghurt system. The buffalo milk preparation was unhomogenised, unstandardised and unfortified, as occurs in commercial buffalo yoghurt production. In contrast, the bovine milk preparation was homogenised and fortified with skim milk and whole milk powder, as unhomogenised unfortified bovine milk is known to produce a weak gel (Tamine and Robinson 2007), which offers little comparative value. The homogenisation and fortification of bovine milk also reflects current manufacturing processes. Buffalo milk has a similar concentration of protein and total solids to fortified bovine milk, as shown in Table 1. The major differences between the two types of milk after protein standardisation are the concentrations of lactose, fat and calcium. The compositions in Table 1 are consistent with the raw materials reported for commercial production processes (Lee and Lucey 2003; Robinson and Haddadin 2010).
The Changes in the Concentration of H+ and Ionic Calcium During Fermentation
The changes in the concentration of hydrogen ions (H+) and ionic calcium during the fermentation of buffalo or fortified bovine milk are presented in Fig. 1. The fermentation time, defined here as the time required for the milk to reach an H+ concentration of 3.2 × 10−5 M (equivalent to a pH of 4.5), was 1 h longer for buffalo milk compared to fortified bovine milk (Fig. 1a). This longer fermentation time could be due to the lower initial concentration of dissociated H+ in the buffalo milk compared to bovine milk (0.02 × 10−5 M vs. 0.03 × 10−5 M, equivalent to pH 6.7 and 6.5, respectively; P < 0.05; Fig. 1a inset). When buffalo milk was pre-acidified to a pH of 6.5, similar to the initial pH of standardised bovine milk, there was no significant difference in fermentation time (Supplementary Fig. 1), confirming that difference in the initial milk pH was the major reason for the longer fermentation time for buffalo milk. This result suggests that the standardisation of milk pH could be used to reduce variability in the length of fermentation for buffalo milk products.
Changes in the concentration of dissociated H+ as a function of fermentation time (a) and ionic calcium as a function of H+ ion concentration (b) in buffalo (blue filled circle) or fortified bovine milk (red filled square). Each data point is the average of six replicates (n = 6) and the error bars are the standard deviation of the mean. The inset in (a) shows the first two data points and errors on an enlarged scale
The concentration of ionic calcium increased during fermentation of both yoghurts (Fig. 1b), as calcium dissociated from the casein micelles as a result of increased H+ ion concentration (i.e. decrease in pH). Considerable variation was observed between the replicate measurements, as is often observed for this technique (Lewis 2011; Lin et al. 2006; Mekmene et al. 2010). The ionic calcium concentration recorded at the start of fermentation for both buffalo and fortified bovine milk was similar. As fermentation progressed, however, the level of ionic calcium present in buffalo milk began to exceed that in fortified bovine milk at the same H + concentration. By the end of the fermentation, the concentration of ionic calcium in buffalo milk was significantly higher (P < 0.05) than that in fortified bovine milk. This difference is possibly due to the higher concentration of total calcium in this milk compared to fortified bovine milk (Table 1). The higher total calcium concentration and similar ionic calcium concentration at the beginning of the fermentation, suggests that buffalo milk contains more colloidal calcium phosphate (CCP), the insoluble form of calcium associated with casein micelles. The equilibrium between CCP and ionic calcium appears different in buffalo milk, particularly at lower pH, where more calcium was dissociated from the casein micelles. This is similar to the results of Ahmad and co-authors (2008), who found more than 82 % of total calcium is present as CCP in buffalo milk, while only 72 % of total calcium is present as CCP in bovine milk. Similarly, Abd El-Salam (1978) observed a higher concentration of calcium present in the casein micelles of buffalo milk (28.5 – 35.8 g per kg of casein) compared to bovine milk (25.2 – 29.8 g per kg of casein).
A linear relationship was also observed between the pH and log [Ca2+] (ionic calcium) during the acidification, with a gradient of −0.50 and −0.38 for buffalo milk and fortified bovine milk respectively (Supplementary Fig. 2). The higher absolute value of the gradient for buffalo milk confirms that CCP was more readily solubilised as a function of pH during the fermentation of buffalo milk.
Lactose Consumption, Acid Production and Changes in Organic Acid Profiles During Fermentation and Storage
During fermentation there was a decrease in lactose concentration and an increase in titratable acidity for both buffalo and bovine milk (Supplementary Fig. 3). The amount of lactose consumed by the LAB was not significantly different in buffalo or fortified bovine milk. The titratable acidity of buffalo and fortified bovine yoghurt at the end of the fermentation was also similar with 1.08 % and 1.05 % lactic acid equivalents, respectively. The titratable acidity of buffalo yoghurt was in the range previously reported, although this range varies significantly from 0.68 % to 1.59 % lactic acid equivalents (Raju and Pal 2009; Cunha-Neto et al. 2005; Yadav et al. 2007).
A linear relationship was found between the titratable acidity and pH (Supplementary Fig. 4), as could be expected, given the shape of the curve in Supplementary Fig. 3b. The similar correlations between titratable acidity and pH in buffalo yoghurt and fortified bovine yoghurt (Supplementary Fig. 4) may be due to the similar protein content of the two yoghurts.
The concentration of organic acids in buffalo and fortified bovine yoghurt during fermentation and storage are presented in Fig. 2. Buffalo yoghurt contained a significantly lower concentration of all organic acids, except for pyruvic acid and citric acid. Among the eight organic acids investigated, citric acid and lactic acid were the most abundant acids in both types of yoghurt. The changes in organic acid concentration during fermentation and storage followed similar trends for both buffalo and fortified bovine yoghurt. The concentration of lactic acid, acetic acid and pyruvic acid increased (Fig. 2c, d, g), while the concentration of other acids remained relatively constant. These results are consistent with previous studies where citric and lactic acids were found to be the dominant acids in bovine yoghurt, with the concentration of lactic acid also increasing on storage (Adhikari et al. 2002; Donkor et al. 2007; Fernandezgarcia and McGregor 1994).
Changes in the concentration of citric acid (a), orotic acid (b), pyruvic acid (c), lactic acid (d), formic acid (e), uric acid (f), acetic acid (g) and hippuric acid (h) during the fermentation and storage of buffalo (blue filled circle with a solid line across) or fortified bovine yoghurt (red filled square with a broken line across). Each data point is the average of six replicates (n = 6) and the error bars are the standard deviation of the mean. Storage commenced after 6 h of fermentation for buffalo yoghurt and 5 h for bovine yoghurt
The significant difference in the concentration of some organic acids within the two yoghurts is likely the consequence of the lower initial concentration of these acids in buffalo milk (Fig. 2). The significantly lower concentration of orotic and uric acid in buffalo milk were also reported in previous studies, with values ranging from 1.9 to 2.9 and 0.2 to 0.4 mg/100 g for buffalo milk and from 8.3 to 11.3 and 3.5 to 8.7 mg/100 g for bovine milk respectively (Fernandezgarcia and McGregor 1994; Larson and Hegarty 1979; Okonkwo and Kinsella 1969; Prakash and Sharma 1986; Sikka et al. 2001; Tormo and Izco 2004; Venkatappaiah and Basu 1952).
Storage Modulus G’ and Loss Tangent (tan δ) During Fermentation
The changes in the storage modulus G’ and loss tangent (tan δ) measured during yoghurt production for buffalo or fortified bovine milk are presented in Fig. 3. The storage modulus G’ and the loss tangent (tan δ) are two parameters used to study the small deformation and rheological properties of gels, such as yoghurt. The storage modulus G’ measures the stored energy, which reflects the gel stiffness (Lee and Lucey 2003), while the loss tangent (tan δ) is defined as the ratio of the loss modulus (viscous modulus) to the storage modulus (elastic modulus; Rao 2007).
Changes in the storage modulus G’ (a) and the loss tangent tan δ (b) as a function of fermentation time for buffalo (blue filled circle with a solid line across) or fortified bovine yoghurt (red filled square with a broken line across). Each data point is the average of two fermentations (n = 2) and the error bars are the standard deviation of the mean. The inset in a corresponds to data between 120 and 165 min of fermentation time
The point of gelation, defined as the point when the G’ reaches 1 Pa, occurred at ∼150 and ∼160 min for fortified bovine and buffalo milk respectively (Fig. 3a inset). The slower gelation for buffalo milk is likely a product of the delayed acidification observed for non standardised buffalo milk (Fig. 1a), behaviour also described as a lag phase (Tamine and Robinson 2007). Large fluctuations in tan δ were observed prior to this point of gelation, indicating instability in the gel. Following gelation the G’ increased and this increase was more rapid for the bovine sample (Fig. 3a). The G’ for the buffalo gel was smaller than for the fortified bovine gel for most time points during fermentation but the G’ at the end of fermentation was similar, indicating similar product stiffness (Fig. 3a). The tan δ was not significantly different for the two preparations. It remained almost stable in the range of 0.84 ± 0.18 and 1.09 ± 0.15 for the buffalo gel and decreased slightly for the fortified bovine gel between 1.10 ± 0.13 to 0.74 ± 0.12 (Fig. 3b).
The similarity of the tan δ observed for buffalo and bovine yoghurt is interesting, as it suggests the different fat content in these preparations does not significantly impact on gelation. Both yoghurts have a similar protein and total solids content and this appears to be main component determining the rate of change in the viscous and elastic modulus for these yoghurts, rather than the fat.
In this study, a cup and vane fixture was used for rheological characterisation, while most previous studies used a cup and bob fixture. In our preliminary trials with a cup and bob fixture, the G’ at the end of the fermentation for the bovine gel was in the range of 161 to 209 Pa, similar to previous studies (Lee and Lucey 2003, 2004b; Lucey et al. 1998). The G’ profile for the buffalo gel, however, contained an abnormal peak (Supplementary Fig. 5), possibly due to slippage as a result of syneresis. This phenomenon has previously been observed during the gelation of fortified bovine yoghurt (Haque et al. 2001) and a cup and vane fixture was therefore used in our study to prevent slippage.
Gel Firmness of Buffalo Yoghurt and Fortified Bovine Yoghurt During Storage Time
The firmness of buffalo and fortified bovine yoghurt as assessed by a TAXT-2 texture analyser is presented in Fig. 4a. In general, no significant differences in gel firmness were observed between the two yoghurts (P > 0.05) but the firmness of both yoghurts increased significantly over 28 days of storage (P < 0.05). It is notable that the buffalo yoghurt is firm without the need for fortification, unlike bovine yoghurt (data not shown) and this result highlights the importance of protein and total solids contents in determining gel firmness.
Changes in the gel firmness (a), syneresis (b) and rheological properties including thixotropy (c), consistency coefficient (d) and flow behaviour index (e) of buffalo (blue filled circle with a solid line across) or fortified bovine yoghurt (red filled square with a broken line across) during storage at 4 °C. Each data point is the average of six replicates (n = 6) and the error bars are the standard deviation of the mean. The R square values for fitting the rheological data to the power law model (η = K. γ n – 1) are 1.00 for buffalo yoghurt throughout the storage period and 0.93, 0.94 and 0.99 for bovine yoghurt at days 1, 7 and 28 of storage, respectively
An increase in yoghurt firmness during storage has previously been observed (Salvador and Fiszman 2004; Domagala 2009; Saccaro et al. 2009). In the study of Salvador and Fiszman (2004) the magnitude of the firmness and change in firmness on storage were found to be dependent on composition of the yoghurt, particularly the protein concentration. An increase of ∼3 g force (from ∼30.6 g force to ∼33.7 g force) was observed for yoghurt with 3.5 % (w/w) protein, while a larger increase of ∼9 g force (from ∼56 g force to ∼65 g force) was observed for yoghurt with 4.4 % (w/w) protein (Salvador and Fiszman 2004). Similarly, Domagala (2009) found a firmer gel after storage in yoghurt made from bovine milk with 3.2 % (w/w) protein or sheep milk with 5.2 % (w/w) but not in yoghurt made from sheep milk with only 2.5 % (w/w) protein. The presence of B. lactis in the starter culture has been shown to increase gel firmness in the study of Saccaro and co-authors (2009). Due to the relatively high protein concentration in both buffalo and fortified bovine milk (4.1–4.6 % w/w) and the use of starter culture consisting of B. lactis in our study, the gel firmness in the two yoghurts is also expected to increase during storage.
Syneresis of Buffalo Yoghurt and Fortified Bovine Yoghurt During Storage Time
Syneresis or whey separation is a major visible defect that develops during yoghurt storage and affects yoghurt acceptance by consumers (Gundogdu et al. 2009; Purwandari et al. 2007; Salvador and Fiszman 2004). The mass of whey that separates from the yoghurt can be determined by a number of methods including centrifugation (Purwandari et al. 2007), draining through a mesh (Yazici and Akbulut 2007) or by observation of spontaneous syneresis in a volumetric flask (Lee and Lucey 2003). Spontaneous syneresis is preferable for a set-style yogurt, as the gel structure is maintained (Lee and Lucey 2010). In our study, a thick layer of fat on the surface of buffalo yoghurt prevented the expulsion of whey, resulting in whey pooling along the sides of the volumetric flask. The centrifugation method was therefore used to assess the extent of syneresis as a function of storage time.
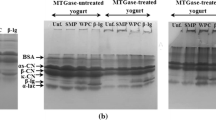
Significantly higher syneresis was observed for buffalo yoghurt throughout the storage period of 28 days at 4 °C (P < 0.05) compared to fortified bovine yoghurt (Fig. 4b), with almost ten times more whey expelled from buffalo yoghurt despite the similar total solids content of the two yoghurts. Syneresis also increased as a function of storage time for the buffalo yoghurt (P < 0.05) but syneresis did not increase for the fortified bovine yoghurt. A significant amount of syneresis was also observed by Bezerra and co-authors (2012), with 20.2 mL of whey expelled from 200 g of buffalo yoghurt, equivalent to 10.1 % (v/w), using the draining method. The higher mass of whey expelled from the buffalo yoghurt here and in other studies is likely a result of the low surface area of the fat globules in this preparation compared to the homogenised bovine milk. Protein can absorb to the surface of the homogenised bovine fat globules leading to a network that can hold water. The specific surface area of unhomogenised buffalo milk fat was significantly lower than in the homogenised fortified bovine milk preparation, with 1.61 m2 per g fat vs. 18.87 m2 per g fat as determined using light scattering. A similar trend was observed when the surface of the fat globules was determined by CLSM (Supplementary Table 1). The specific surface area of buffalo milk fat in this study is similar to that observed by Menard and co-authors (2010), who recorded a specific surface area of 1.78 m2 per g fat of buffalo milk. A decrease in yoghurt syneresis has been observed when the surface area of bovine milk fat globules was increased (Gun and Isikli 2006; Keogh and O’Kennedy 1998) consistent with this hypothesis. A preliminary analysis of the whey proteins expelled during syneresis using electrophoresis (Supplementary Fig. 6) showed that there was no difference in the composition of the whey expelled from the buffalo and fortified bovine yoghurt. The whey proteins present in buffalo or fortified bovine milk were also fractionated for comparison. The SDS PAGE results show the absence of higher molecular weight proteins in whey samples expelled from buffalo or bovine yoghurt, whereas serum albumim and lactoferrin are present in the fractionated milk preparations, consistent with these proteins being incorporated within the gel network after the heat treatment during yoghurt production.
Rheological Properties of Buffalo Yoghurt and Fortified Bovine Yoghurt During Storage Time
A shear rate sweep (0.1 to 100 s−1) was next used to investigate the flow properties of the two yoghurts. Typical flow curves for the yoghurts at days 1 and 28 of storage are presented in Supplementary Fig. 7. The rheological properties measured including thixotropy, the consistency coefficient (K) and flow behaviour index (n), are presented as a function of storage time in Fig. 4c, d, e.
Several similarities were found in the rheological properties of buffalo and fortified bovine yoghurts. Both buffalo and fortified bovine yoghurts were shear-thinning and thixotropic and the apparent viscosity of yoghurts was strongly dependent on shear rate (Supplementary Fig. 7). These results are consistent with previous observations for bovine (Purwandari et al. 2007) or buffalo yoghurt (Bezerra et al. 2012). The degree of thixotropy, flow behaviour index (n) and consistency coefficient (K), however, were considerably different for buffalo and fortified bovine samples (Fig. 4c, d, e).
Yoghurt produced from buffalo milk was significantly more thixotropic than the yoghurt made from fortified bovine milk throughout the storage period (P < 0.05; Fig. 4c). A prolonged storage of 28 days also led to a significant increase in thixotropy for both types of yoghurt (P < 0.05). The lower thixotropy of fortified bovine yoghurt indicates the structure is better able to recover from deformation, especially when the yoghurt is fresh.
The consistency coefficient (K) was highly influenced by yoghurt type and storage time (Fig. 4d). A significantly higher consistency coefficient (K) was recorded for buffalo yoghurt (P < 0.05), indicating buffalo yoghurt is more viscous. The yoghurt viscosity also increased with storage time, as shown by a significant increase in the consistency coefficient (K) for both buffalo and fortified bovine yoghurt between days 7 and 28 of storage (P < 0.05).
Contrary to the consistency coefficient (K), the flow behaviour index (n) was found to be significantly higher in fortified bovine yoghurt (P < 0.05; Fig. 4e). Storage was found to have a significant effect on the flow behaviour index of both yoghurts (P < 0.05). These data indicate that the yoghurts are more non-Newtonian, more sensitive to shear and more susceptible to a break-down in the network structure on deformation as the yoghurt gel network ages during storage. The lower flow behaviour index for buffalo yoghurt observed indicate this yoghurt is more sensitive than bovine yoghurt.
Our results are similar to the findings of Abu-Jdayil and Mohameed (2002), who also observed an increase in the consistency coefficient (K) from 96 Pa.sn to 165 Pa.sn and decrease in the flow behaviour index (n) from 0.20 to 0.12 when bovine yoghurt was stored for 14 days. Similarly, Purwandari and co-authors (2007) found a significant increase in the consistency coefficient (K) and thixotropy with storage time for bovine yoghurt. If buffalo yoghurt is sheared at a much higher shear rate (10 to 1,100 s−1), however, a lower consistency index of 4.9 Pa.sn has been observed (Bezerra et al. 2012). The quantitative differences between buffalo and bovine yoghurt observed in our study explain potential differences in the sensory properties of bovine and buffalo yoghurt. Our data also suggest that more care is required in handling buffalo yoghurt and that there are potential underlying differences in the network structure of the two yoghurts.
Microstructure of Buffalo Yoghurt and Bovine Yoghurt During Storage Time
The microstructure of the bulk network within buffalo and fortified bovine yoghurt was assessed by CLSM for samples stored for 1 and 28 days as shown in Fig. 5. The structure of the same samples was also observed by cryo-SEM, as shown in Figs. 6 and 7.
Microstructure of buffalo (a, b) or fortified bovine yoghurt (c, d) after 1 day of storage (a, c) or 28 days of storage (b, d) as observed by CLSM. Nile Red stained fat appears red and FCF stained protein appears green. The black areas are serum pores. Images were captured using a ×63 objective using a ×1 digital zoom (upper images) or ×5 digital zoom (lower images). The scale bars are 10 μm in length in the upper images and 2 μm in length in the lower images
Microstructure of buffalo (a1–a3) or fortified bovine yoghurt (b1–b3) after 1 day of storage as observed by cryo-SEM. Images were captured using a solid state detector at ×1,000 magnification (left), ×4,000 magnification (middle) and ×16,000 magnification (right). The scale bars are 100 μm in length (left), 20 μm in length (middle), or 5 μm in length (right). Arrows indicate the presence of serum pores within the network. Individual fat globules and the protein network are also highlighted in Fig. 6a3 and b3
Microstructure of buffalo (a1–a3) or fortified bovine yoghurt (b1–b3) after 28 days of storage observed by cryo-SEM. Images were captured using a solid state detector at ×1,000 magnification (left), ×4,000 magnification (middle) and ×16,000 magnification (right). The scale bars are 100 μm in length (left), 20 μm in length (middle), or 5 μm in length (right). Arrows indicate the presence of serum pores within the network
The microstructure of both buffalo yoghurt and fortified bovine yoghurt observed by CLSM appears similar (Fig. 5). Both yoghurts consist of a protein network with integrated fat globules and serum pores (which appear as unstained black regions; Fig. 5a1, c1). The only significant difference observed between the microstructure of the two yoghurts is the appearance of the bigger fat globules within buffalo samples (Fig. 5a2, b2), which was expected as the buffalo milk was not homogenised. The microstructure of both yoghurts was affected by storage with larger serum pores appearing after 28 days of storage particularly in the buffalo samples (Fig. 5b1).
The cryo-SEM images (Fig. 6a1–a3) confirm the presence of bigger fat globules within the microstructure of the buffalo yoghurt. The homogenised fat globules in bovine yoghurt are embedded within the protein network (Fig. 6b1–b3), whereas the unhomogenised fat globules in buffalo yoghurt appear within the pores of the protein network or are sufficiently large enough to disrupt the network (Fig. 6a1–a3). In general, the protein network within buffalo yoghurt appeared more irregular (Fig. 6a2 and b2) and contained a greater number of serum pores (Fig. 6a1) consistent with CLSM images (Fig. 5a1).
The major change in yoghurt microstructure during storage was the appearance of more serum pores in the network of both buffalo and fortified bovine samples (Fig. 7a1, b1). These pores were more numerous in buffalo yoghurt, consistent with observations made by CLSM (Fig. 5b1, d1) and may be linked to the increased whey separation (Fig. 4b) and changes in thixotropy and flow behaviour index observed on storage (Fig. 4c, e). These images therefore suggest a link between the product microstructure and functional properties.
Bacterial Growth During Fermentation and Viability of Bacteria During Storage Time
Both buffalo and fortified bovine yoghurts were inoculated with the same number of starter culture bacteria. The growth of these three strains during fermentation is presented in Table 2, while the viability during storage is summarized in Table 3. During fermentation, the number of bacteria increased significantly for each strain in each type of yoghurt (P < 0.05; Table 2). The most considerable change was found in the population of S. thermophilus, with an increase of ∼2 log CFU/g (from 7.2 log CFU/g to more than 9.2 log CFU/g) and this strain dominated the microbial population in both yoghurts. L. acidophilus La-5 and B. lactis Bb-12 showed a slower growth, with an increase of less than 1 log CFU/g observed for these populations of bacteria. In general, the bacteria grew better in fortified bovine milk than buffalo milk, except for L. acidophilus La-5, which showed no significant difference (P > 0.05).
The number of L. acidophilus La-5 bacteria decreased significantly in both buffalo and fortified bovine yoghurts on storage (P < 0.05; Table 3). In contrast, the number of S. thermophilus appeared constant in both samples and the number of B. lactis Bb-12 slightly reduced (P < 0.05) in buffalo yoghurt but unchanged in fortified bovine yoghurt (P > 0.05). The relative numbers of L. acidophilus La-5 and B. lactis Bb-12 also differed between the two yoghurt types, with higher numbers remaining viable in fortified bovine yoghurt, while the number of S. thermophilus was similar.
Previous studies have reported the dominant role of S. thermophilus in yoghurt fermentation, with average viable cell populations ranging from 8 log CFU/g to 9 log CFU/g (Damin et al. 2008; Oliveira et al. 2001; Saccaro et al. 2009). S. thermophilus typically exhibits superior growth and greater viability when mixed with a variety of cultures including L. bulgaricus, L. acidophilus, L. casei, L. rhamnosus or Bifidobacterium spp. (Damin et al. 2008; Dave and Shah 1997; Saccaro et al. 2009). This preferential growth has been observed in a range of milk types including bovine milk, goat milk and soymilk (Bozanic 2002; Bozanic et al. 2011). The better growth and viability of S. thermophilus is likely due to the higher proteolytic activity and lactose utilisation reported for this strain compared to the two other probiotic strains (Marafon et al. 2011; Ozer and Kirmaci 2010; Saccaro et al. 2009).
The significant reduction in viability of L. acidophilus observed here is consistent with the noted loss of proteolytic activity that is thought to occur for this strain during storage at 4 °C (Gilliland and Lara 1988). L. acidophilus is also sensitive to lactic acid and peroxide produced by other bacterial strains (Dave and Shah 1997; Saccaro et al. 2009). To solve this issue, several studies have attempted to increase and maintain the number viable of probiotic bacteria in bovine yoghurt by adding more probiotic bacteria at the beginning of the fermentation, encapsulating probiotic bacteria before inoculation, utilising heat-shocked starter cultures or combining L. acidophilus with the slow acid producing starter culture strains (Bozanic et al. 2011; El-Dieb et al. 2012; Priya et al. 2011).
The higher concentration of milk fat and calcium in buffalo milk are unlikely to affect the viability of Lactobacillus and Bifidobacterium in the buffalo yoghurt although these components have been reported to stimulate the growth of lactic acid bacteria in previous studies (Tan et al. 2012; Wishon et al. 2010). Differences observed in the microstructure are also unlikely to alter bacterial survival. The viability of the probiotic bacteria could be enhanced by the higher concentration of lactose in bovine yoghurt, as lactose may act as a protective coating. A further factor may be bioactive compounds that may differ in the two milk preparations. The presence of these bioactives and possible mechanism of action is worthy of further study.
Conclusion
Buffalo and bovine yoghurts differ in their microstructure and physicochemical properties following fermentation and during cold storage. Buffalo yoghurt exhibited a weaker network structure that was more porous, more irregular and more disrupted by large unhomogenised fat globules. This difference in microstructure leads to a significantly higher degree of syneresis, a greater degree of thixotropy, a greater consistency coefficient (K) and a smaller value of flow behaviour index (n). While the gel firmness of both yoghurts was similar, the number of probiotic bacteria was reduced during the cold storage of buffalo yoghurt. Our results suggest that the total solids content of the milk is not the only key factor that may affect yoghurt quality and that other factors such as the concentration of fat globules, surface area of fat, concentration of lactose and concentration of calcium can alter the structure and properties of yoghurt. The different microstructure and physiochemical properties observed here for buffalo and bovine yoghurt are also likely responsible for the different sensory properties of these two yoghurts. The different properties observed here as a function of milk composition suggest a possible route for the production of new dairy products with altered properties.
References
Abd El-Salam, M. H. (1978). Comparative study of the composition of casein micelles from buffalo and cow milk. Egypt Journal of Dairy Science, 6, 1–8.
Abu-Jdayil, B., & Mohameed, H. (2002). Experimental and modelling studies of the flow properties of concentrated yogurt as affected by the storage time. Journal of Food Engineering, 52(4), 359–365.
Addeo, F., Alloisio, V., & Chianese, L. (2007). Tradition and innovation in the water buffalo dairy products. Italian Journal of Animal Science, 6, 51–57.
Adhikari, K., Grun, I. U., Mustapha, A., & Fernando, L. N. (2002). Changes in the profile of organic acids in plain set and stirred yogurts during manufacture and refrigerated storage. Journal of Food Quality, 25(5), 435–451.
Ahmad, S., Gaucher, I., Rousseau, F., Beaucher, E., Piot, M., Grongnet, J. F., et al. (2008). Effects of acidification on physicochemical characteristics of buffalo milk: A comparison with cow's milk. Food Chemistry, 106(1), 11–17.
AOAC. (2006). Official methods of analysis. Washington: Association of Official Analytical Chemists.
Atwood, C. S., & Hartmann, P. E. (1992). Collection of fore and hind milk from the sow and the changes in milk-composition during suckling. The Journal of Dairy Research, 59, 287–298.
Australia New Zealand Food Standards (2006) Fermented milk products F2011C00622, Standard 2.5.3.
Bezerra, M. F., Souza, D. F. S., & Correia, R. T. P. (2012). Acidification kinetics, physiochemical properties, and sensory attributes of yoghurts prepared from mixtures of goat and buffalo milks. International Journal of Dairy Technology, 65(3), 437–443.
Bozanic, R. (2002). Fermentation and storage of probiotic yoghurt from goat's milk. Mljekarstvo, 52, 93.
Bozanic, R., Lovkovic, S., & Jelicic, I. (2011). Optimising fermentation of soymilk with probiotic bacteria. Czech Journal of Food Sciences, 29(1), 51–56.
Braun, P. G., & Preuss, S. E. (2008). Nutritional composition and chemico-physicai parameters of water buffalo milk and milk products in Germany. Milchwissenschaft Milk Science International, 63(1), 70–72.
Chawla, A. K., & Balachandran, R. (1994). Studies on yoghurt buffalo milk: Effect of different solid nonfat content on chemical, rheological and sensory characteristics. Indian Journal of Dairy Science, 47(9), 762–765.
Cunha-Neto, O. C., Olivera, A. A. F., Hotta, R. M., & Sorbal, P. J. A. (2005). Physico-chemical and sensory evaluation of plain yoghurt manufactured from buffalo milk with different fat content. Science and Technology of Agriculture, 25(3), 448–453.
Dairy Australia (2010) Consumption summary for year 2009–2010. Production and sale. Available at: www.dairyaustralia.com.au. Accessed 30 November 2011.
Damin, M. R., Minowa, E., Alcantara, M. R., & Oliveira, M. N. (2008). Effect of cold storage on culture viability and some rheological properties of fermented milk prepared with yogurt and probiotic bacteria. Journal of Texture Studies, 39(1), 40–55.
Dave, R. I., & Shah, N. P. (1997). Effect of level of starter culture on viability of yoghurt and probiotic bacteria in yoghurts. Food Australia, 49(4), 164–168.
Domagala, J. (2009). Instrumental texture, syneresis and microstructure of yoghurts prepared from goat, cow and sheep Milk. International Journal of Food Properties, 12(3), 605–615.
Donkor, O. N., Tsangalis, D., & Shah, N. P. (2007). Viability of probiotic bacteria and concentrations of organic acids in commercial yoghurts during refrigerated storage. Food Australia, 59(4), 121–126.
El-Dieb, S. M., Abd Rabo, F. H. R., Badran, S. M., Abd El-Fattah, A. M., & Elshaghabee, F. M. F. (2012). The growth behaviour and enhancement of probiotic viability in bioyoghurt. International Dairy Journal, 22(1), 44–47.
Erkaya, T., & Sengul, M. (2011). Comparison of volatile compounds in yoghurts made from cows', buffaloes', ewes' and goats' milks. International Journal of Dairy Technology, 64(2), 240–246.
Fernandezgarcia, E., & McGregor, J. U. (1994). Determination of organic-acids during the fermentation and cold-storage of yogurt. Journal of Dairy Science, 77(10), 2934–2939.
Ghadge, P. N. (2008). Effect of fortification on the physico-chemical and sensory properties of Buffalo milk yoghurt. Electronic Journal of Environmental Agricultural and Food Chemistry, Electronic journal of environmental, agricultural and food chemistry, 7(5), 2890.
Gilliland, S. E., & Lara, R. C. (1988). Influence of storage at freezing and subsequent refrigeration temperatures on beta-galactosidase activity of Lactobacillus-acidophilus. Applied and Environmental Microbiology, 54(4), 898–902.
Gosling, A., Alftren, J., Stevens, G. W., Barber, A. R., Kentish, S. E., & Gras, S. L. (2009). Facile pretreatment of Bacilius circulans beta-galactosidase increases the yield of galactosyl oligosaccharides in milk and lactose reaction systems. Journal of Agricultural and Food Chemistry, 57(24), 11570–11574.
Gun, O., & Isikli, N. (2006). The effects of fat and non fat dry matter concentration and storage time on the physical properties and acidity of yoghurts made with probiotic cultures. Food Science and Technology International, 12(6), 467–476.
Gundogdu, E., Cakmakci, S., & Dagdemir, E. (2009). The effect of garlic (Allium sativum L.) on some quality properties and shelf-life of set and stirred yoghurt. Turkish Journal of Veterinary and Animal Sciences, 33(1), 27–35.
Haque, A., Richardson, R. K., & Morris, E. R. (2001). Effect of fermenation temperature on the rheology of set and stirred yogurt. Food Hydrocolloids, 15, 593–602.
Jensen, R. G. (1995). Handbook of milk composition. San Diego: Academic Press.
Keogh, K. M., & O’Kennedy, B. T. (1998). Rheology of stirred yoghurt as affected by added milk fat, protein and hydrocolloids. Journal of Food Science, 63, 108–112.
Khanna, A., & Singh, J. (1979). A comparison of yoghurt starter in cow's and buffalo milk. The Journal of Dairy Research, 46(04), 681.
Kumar, P., & Mishra, H. N. (2003). Effect of mango pulp and soymilk fortification on the texture profile of set yoghurt made from buffalo milk. Journal of Texture Studies, 34(3), 249–269.
Larson, B. L., & Hegarty, H. M. (1979). Orotic acid in milks of various species and commercial dairy-products. Journal of Dairy Science, 62(10), 1641–1644.
Lee, W. J., & Lucey, J. A. (2003). Rheological properties, whey separation, and microstructure in set-style yogurt: Effects of heating temperature and incubation temperature. Journal of Texture Studies, 34(5–6), 515–536.
Lee, W. J., & Lucey, J. A. (2004a). Effect of starter inoculation rates and incubation temperatures on physical properties of yogurt. Journal of Animal Science, 82, 93–93.
Lee, W. J., & Lucey, J. A. (2004b). Structure and physical properties of yogurt gels: Effect of inoculation rate and incubation temperature. Journal of Dairy Science, 87(10), 3153–3164.
Lee, W. J., & Lucey, J. A. (2010). Formation and physical properties of yoghurt. Asian-Australasian Journal of Animal Sciences, 23(9), 1127–1136.
Lewis, M. J. (2011). The measurement and significance of ionic calcium in milk - A review. International Journal of Dairy Technology, 64(1), 1–13.
Lin, M. J., Lewis, M. J., & Grandison, A. S. (2006). Measurement of ionic calcium in milk. International Journal of Dairy Technology, 59(3), 192–199.
Lucey, J., Tamehana, M., Singh, H., & Munro, P. (1998). A comparison of the formation, rheological properties and microstructure of acid skim milk gels made with a bacterial culture or glucono-delta-lactone. Food Research International, 31(2), 147–155.
Marafon, A. P., Sumi, A., Alcantara, M. R., Tamime, A. Y., & de Oliveira, M. N. (2011). Optimization of the rheological properties of probiotic yoghurts supplemented with milk proteins. LWT- Food Science and Technology, 44(2), 511–519.
Mekmene, O., Le Graet, Y., & Gaucheron, F. (2010). Theoretical model for calculating ionic equilibria in milk as a function of pH: comparison to experiment. Journal of Agricultural and Food Chemistry, 58(7), 4440–4447.
Menard, O., Ahmad, S., Rousseau, F., Briard-Bion, V., Gaucheron, F., & Lopez, C. (2010). Buffalo vs. cow milk fat globules: Size distribution, zeta-potential, compositions in total fatty acids and in polar lipids from the milk fat globule membrane. Food Chemistry, 120(2), 544–551.
Nahar, A., Amin, M. A., Alam, S. M. K., Wadud, A., & Islam, M. N. (2007). A comparative study on the quality of Dahi (yoghurt) prepapared from cow, goat and buffalo milk. International Journal of Dairy Science, 2(3), 260–267.
Okonkwo, P., & Kinsella, J. E. (1969). Orotic acid in yoghurt. Journal of Dairy Science, 52(11), 1861–1862.
Oliveira, M. N., Sodini, I., Remeuf, F., & Corrieu, G. (2001). Effect of milk supplementation and culture composition on acidification, textural properties and microbiological stability of fermented milks containing probiotic bacteria. International Dairy Journal, 11(11–12), 935–942.
Ong, L., Dagastine, R. R., Kentish, S. E., & Gras, S. L. (2010). The effect of milk processing on the microstructure of the milk fat globule and rennet induced gel observed using confocal laser scanning microscopy. Journal of Food Science, 75, 135–145.
Ong, L., Dagastine, R. R., Kentish, S. E., & Gras, S. L. (2011). Microstructure of milk gel and cheese curd observed using cryo scanning electron microscopy and confocal microscopy. LWT- Food Science and Technology, 44(5), 1291–1302.
Ong, L., Dagastine, R. R., Kentish, S. E., & Gras, S. L. (2012). The effect of pH at renneting on the microstructure, composition and texture of Cheddar cheese. Food Research International, 48, 119–130.
Ozer, B. H., & Kirmaci, H. A. (2010). Functional milks and dairy beverages. International Journal of Dairy Technology, 63(1), 1–15.
Pandya, N., Kanawjia, S., & Dave, R. (2004). Effects of fat content on physico-chemical and sensory properties of buffalo milk dahi (yoghurt). Journal of Animal Science, 82, 236–236.
Pesce, M. A., & Strande, C. S. (1973). New micromethod for determination of protein in cerebrospinal-fluid and urine. Clinical Chemistry, 19, 1265–1267.
Prakash, B. S., & Sharma, R. S. (1986). Orotic acid in milk and milk products. Journal of Food Science and Technology Mysore, 23(2), 85–87.
Priya, A. J., Vijayalakshmi, S. P., & Raichui, A. M. (2011). Enhanced survival of probiotic Lactobacillus acidophilus by encapsulation with nanostructured polyelectrolyte layers through layer-by-layer approach. Journal of Agricultural and Food Chemistry, 59(21), 11838–11845.
Purwandari, U., & Vasiljevic, T. (2009). Rheological properties of fermented milk produced by a single exopolysaccharide producing Streptococcus thermophilus strain in the presence of added calcium and sucrose. International Journal of Dairy Technology, 62(3), 411–421.
Purwandari, U., Shah, N. P., & Vasiljevic, T. (2007). Effects of exopolysaccharide-producing strains of Streptococcus thermophilus on technological and rheological properties of set-type yoghurt. International Dairy Journal, 17(11), 1344–1352.
Raju, P., & Pal, D. (2009). The physico-chemical, sensory, and textural properties of misti dahi prepared from reduced fat buffalo milk. Food and Bioprocess Technology, 2(1), 101–108.
Rao, M. A. (2007). Rheology of fluid and semisolid foods: principles and applications. Rheology of fluid and semisolid foods: principles and applications (2nd ed.). New York: Springer.
Robinson, R. K., & Haddadin, M. S. Y. (2010). Improving the safety and quality of milk, volume 2: improving quality in milk products. In M. W. Griffiths (Ed.), Improving the safety and quality of milk, volume 2: Improving quality in milk products. Cambridge: Woodhead Ltd.
Saccaro, D. M., Tamime, A. Y., Pilleggi, A., & Oliveira, M. N. (2009). The viability of three probiotic organisms grown with yoghurt starter cultures during storage for 21 days at 4 degrees C. International Journal of Dairy Technology, 62(3), 397–404.
Salvador, A., & Fiszman, S. M. (2004). Textural and sensory characteristics of whole and skimmed flavored set-type yogurt during long storage. Journal of Dairy Science, 87(12), 4033–4041.
Shiby, V. K., & Mishra, H. N. (2008). Modelling of acidification kinetics and textural properties in dahi (Indian yogurt) made from buffalo milk using response surface methodology. International Journal of Dairy Technology, 61(3), 284–289.
Sikka, P., Saxena, N. K., Gupta, R., Sethi, R. K., & Lall, D. (2001). Studies on milk allantoin and uric acid in relation to feeding regimens and production performance in buffaloes. Asian-Australasian Journal of Animal Sciences, 14(11), 1634–1637.
Tamime, A. Y., & Robinson, R. K. (2007). Yoghurt: Science and technology, Third Edition. In A. Y. Tamime & R. K. Robinson (Eds.), Tamime and Robinsons Yoghurt: Science and Technology (3rd ed.). Cambridge: Woodhead Publ Ltd.
Tan, W. S., Budinich, M. F., Ward, R., Broadbent, J. R., & Steele, J. L. (2012). Optimal growth of Lactobacillus casei in a Cheddar cheese ripening model system requires exogenous fatty acids. Journal of Dairy Science, 95(4), 1680–1689.
Thi, M. P. N., Lee, Y. K., & Zhou, W. B. (2012). Effect of high intensity ultrasound on carbohydrate metabolism of bifidobacteria in milk fermentation. Food Chemistry, 130(4), 866–874.
Tormo, M., & Izco, J. M. (2004). Alternative reversed-phase high-performance liquid chromatography method to analyse organic acids in dairy products. Journal of Chromatography. A, 1033(2), 305–310.
Varricchio, M. L., Di Francia, A., Masucci, F., Romano, R., & Proto, V. (2007). Fatty acid composition of Mediterranean buffalo milk fat. Italian Journal of Animal Science, 6, 509–511.
Venkatappaiah, D., & Basu, K. P. (1952). Non-protein nitrogenous constituents of milk. I. Variation due to species, breed, individuality, season and stage of lactation. Indian Journal of Dairy Science, 5(2), 95–116.
Wishon, L. M., Song, D. F., & Ibrahim, S. A. (2010). Effect of metals on growth and functionality of Lactobacillus and Bifidobacteria. Milchwissenschaft-Milk Science International, 65(4), 369–372.
Yadav, H., Jain, S., & Sinha, P. R. (2007). Evaluation of changes during storage of probiotic dahi at 7 degrees C. International Journal of Dairy Technology, 60(3), 205–210.
Yazici, F., & Akbulut, C. (2007). Impact of whey pH at drainage on the physicochemical, sensory, and functional properties of Mozzarella cheese made from buffalo milk. Journal of Agricultural and Food Chemistry, 55(24), 9993–10000.
Acknowledgments
The authors would like to acknowledge the Australian Government for providing the Australian Postgraduate Award (International) (APA-International) scholarship, Rural Industries Research and Development Cooperation (RIRDC) for financial support and Shaw River for kindly supplying the buffalo milk. The authors also thank the Particulate Fluids Processing Centre (PFPC) and the Bio21 Institute for access the equipment and Mr Roger Curtain for his help in operating the scanning electron microscope in cryo mode.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PPTX 524 kb)
ESM 2
(PPTX 492 kb)
ESM 3
(PPTX 67.0 kb)
ESM 4
(PPTX 528 kb)
ESM 5
(PPTX 58.8 kb)
ESM 6
(PPTX 398 kb)
ESM 7
(PPTX 91 kb)
Supplementary Table 1
Volume, surface area, mean diameter and number of fat globules (FGs) in buffalo milk and fortified bovine milk determined using CLSM or light scattering. (DOC 35 kb)
Rights and permissions
About this article
Cite this article
Nguyen, H.T.H., Ong, L., Lefèvre, C. et al. The Microstructure and Physicochemical Properties of Probiotic Buffalo Yoghurt During Fermentation and Storage: a Comparison with Bovine Yoghurt. Food Bioprocess Technol 7, 937–953 (2014). https://doi.org/10.1007/s11947-013-1082-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-013-1082-z