Abstract
The addition of maltodextrin DE 10 to red wine (Cabernet Sauvignon) followed by freeze-drying allowed to obtain a free-flowing (dealcoholized) wine powder (WP) having a phenolic concentration about 3.6 times higher than the original liquid red wine. The powder, having a water activity (a w) of 0.053 and 0.330 was stored at 28 °C and 38 °C and the content of ten different phenolic compounds was determined by HPLC. Caftaric acid, quercetin 3-glucoside, caffeic acid, gallic acid and resveratrol contents in the WP stored at 28 °C and 38 °C, remained almost constant during 70 days of storage, while epicatechin gallate, catechin, malvidin 3-G and epicatechin showed slight losses (about 15–25 %) during storage. On the contrary, epigallocatechin experienced a strong loss of concentration (around 61 % loss) during storage at the same conditions. A moderate decrease of antioxidant activity, determined by free radical scavenging capacity of the 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH*) and ferric reducing antioxidant power (FRAP) was observed along storage. This decrease was more important at higher temperature (38 °C) and higher a w (0.33). These results would allow the feasibility of using this WP as a healthy ingredient in alcohol-free powder drinks.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In the past years, evidence has accumulated suggesting that wine might be one of the most prominent elements contributing to the beneficial health effect of the so-called “Mediterranean diet” (Radovanovic and Radovanovic 2010). A regular and moderate wine consumption is a highly evoked fact for explaining the low incidence of cardiovascular events in France (known as “the French paradox”) compared with other industrialized countries (Gorelik et al. 2008; Nikfardjam et al. 2006; Soulat et al. 2006). It has been shown that small daily intakes of wine can reduce the risk of coronary heart disease and atherosclerosis, with this benefit ascribed to the antioxidant properties of the phenolic compounds (Díaz et al. 2012; Mazza et al. 1999; Radovanovic and Radovanovic 2010; Renaud and de Lorgeril 1992) which differ from those found in grapes or must. Although the mechanisms of action are yet not fully understood, it is well known that phenolic compounds behave as radical scavengers and antioxidants. It has also been demonstrated that they can protect cholesterol in the low-density lipoprotein (LDL) from oxidation (Brouillard et al. 1997; Cioroi and Musat 2007). The relationship between in vitro antioxidant capacity and the polyphenol total content of red wines has been documented (Avalos Llanos et al. 2003; Cioroi and Musat 2007) and total polyphenol content was found to be responsible for the in vivo effect (Camussoni and Carnevali 2004).
However, there are some clear drawbacks in wine consumption associated with the ingestion of alcohol: (a) consumption must be moderate (i.e., 1–2 glasses/day) in order to avoid alcohol related diseases, and (b) many people — for ethnical, social or religious reasons — do not consume wine (Midgley 1971; Sanchez et al. 2011).
In a previous work, Sanchez et al. (2011) reported preliminary results on the freeze-drying encapsulation of red wine added with maltodextrin (MD) obtaining an alcohol-free powder. Water and almost all alcohol from wine were removed during freeze-drying and the use of MD as a drying aid led to a glassy microstructure in which the wine phenolics — as well as other components of wine dry extract such as glycerol, sugars, organic acids, salts, etc. — were entrapped. Sanchez et al. (2011) suggested their free flowing (dealcoholized) wine powder (WP) might be added to other powdered drinks for enrichment in red wine phenolics. Munin and Edwards-Lévy (2011) noted that although polyphenols are compounds possessing interesting properties for use in medicine, they lack of long term stability since are usually very sensitive to light and heat, and encapsulation appears to be a promising approach to gain stability. Thus, further studies including the stability of individual phenolic compounds on the WP during storage were required.
The objective of the present work was to study the stability of several red wine phenolics in a protective enclosing within an MD matrix (WP) of low moisture content during storage at selected conditions of temperature and water activity (a w). Moreover, the change in its antioxidant activity during storage was monitored.
Materials and Methods
Materials
The wine used was a commercial Cabernet Sauvignon, “Postales del Fin del Mundo” (Bodega Fin del Mundo, vintage 2010) from a cold climate wine growing region (Neuquén province, Patagonia, Argentina). Its alcohol content, pH and total polyphenols content were 13.7 %, 3.8 and 2,299 ± 89 mg of Gallic Acid Equivalent (GAE)/l, respectively (using the Folin–Ciocalteu method, subsequently described in this section). All bottles were purchased at the same time in a winery located in the city of Buenos Aires.
Maltodextrin Dextrose Equivalent 10 (MD10) (Productos de Maíz S.A., Buenos Aires, Argentina) was used for encapsulation of wine and was selected on the basis of the well-known ability of this MD to encapsulate labile biomaterials (Chranioti and Tzia 2012; Galmarini et al. 2011; Roos 1995).
HPLC-grade reagents malvidin-3-glucoside chloride (malvidin-3-G), catechin, epicatechin, epicatechin gallate, epigallocatechin, caffeic acid, gallic acid, caftaric acid, quercetin 3-glucoside (quercetin 3-G) and resveratrol (all of them >95 % purity) were purchased from Extrasynthese, France; the solvents acetonitrile (>99.9 % purity) from Pancreac, France, formic acid (98 %) from Sigma Aldrich, France and methanol (>99.5 %) Chromasolv, France.
The Folin–Ciocalteau reagent was obtained from Merck KgaA Darmstadt, Germany.
Potassium chloride buffer (pH 1) and sodium acetate buffer (pH 4.5) used for chemical anthocyanins quantification were prepared using potassium chloride, chlorhydric acid and sodium acetate purchased from Biopack, Buenos Aires, Argentina.
The 2,2-diphenyl-1-picrylhydrazyl free radical (DPPH*) was purchased from Merck, France. For the FRAP assay, the 2,4,6-Tripyridyl-s-triazine (TPTZ) used for the was from Sigma Aldrich (France), while FeSO4⋅7H2O, FeCl3⋅6H2O and the sodium acetate (99 %) were purchased from Fluka (France).
Encapsulation Procedure
MD10 was dissolved in wine to a 20 % concentration (total weight basis) and freeze-dried to encapsulate the dry extract of wine (including polyphenols) in an amorphous carbohydrate microstructure (Sanchez et al. 2011). The solution of wine and MD10 was poured into a stainless steel tray (round tray of 20 cm diameter; depth of sample, 1 cm) and freeze-dried at room temperature in a FIC Ll-I-E300-CRT freeze dryer (Buenos Aires, Argentina) operated with a freezing plate and condenser at −40 °C and a vacuum below 200 μmHg. Freeze-drying time was 40 h at room temperature (22 ± 3 °C) and resulting samples had a water activity of 0.053. Along with water, almost all of the ethanol originally present in wine was eliminated during freeze-drying, leading to a dealcoholized WP, since residual alcohol was below 1 % (Sanchez et al. 2011). The freeze-dried product — a porous cake of glassy aspect — was milled in a domestic grain coffee grinder resulting in a free-flowing powder used in the storage stability experiments.
Storage Conditions
After freeze-drying, samples of WP (6 g) were placed in small opaque glass flasks and stored at selected (and controlled) temperatures and water activity (a w). WP was stored in constant temperature ovens kept at 28 °C and 38 °C in hermetically sealed flasks in order to preserve the low initial moisture conditions (a w = 0.053) and at 28 °C and 38 °C in opened flasks over a saturated solution of MgCl which provided a constant relative humidity of 33 % (a value sufficiently low so as not to have adverse physical changes but also high enough to be easily maintained during storage). Samples of all systems were removed from storage and analyzed after 0, 30, 50 and 70 days.
It is well known that amorphous hygroscopic powders (such as present DWP) may suffer undesirable physical changes during storage, namely stickiness and caking. These changes are mainly controlled by the glass transition temperature (T g) of the amorphous powder in relation to storage temperatures and relative humidity as well as storage time (Galmarini 2010; Galmarini et al. 2011; Roos 1995; Roos and Karel 1991). Therefore, storage conditions at higher temperature/a w were not selected because they might induce those adverse physical changes. The chosen temperatures represent normal and drastic ambient storage conditions.
Water Activity and Moisture Content
The water activity (a w) of DWP samples was determined using an electronic dew point water activity meter Aqualab series 3 (Decagon Devices, Pullman, WA, USA). The device was calibrated and tested with saturated salt solutions in the water activity range of interest (Favetto et al. 1983). The error in aw measurement was determined to be about ±0.004 a w.
Moisture content was determined gravimetrically; a thin layer of DWP was placed in a forced circulation oven at 70 °C (±1 °C) until constant weight. A relatively low temperature was used to avoid the possibility of any evaporation of glycerol in the DWP, originated from the liquid red wine.
Total Polyphenols
Total phenolics were measured by Folin–Ciocalteu method (Camussoni and Carnevali 2004; Singleton and Rossi 1965). WP samples were first rehydrated with water to their original weight and they were afterwards diluted (1:10). The same dilution was used for the liquid wine. All samples were treated according to the protocol described by the International Organization of Vine and Wine (International Organization of Vine and Wine 2009). As stated in the protocol, absorbance at 765 nm was measured using a spectrophotometer Shimadzu PharmaSpec UV-1700 (Shimadzu Corporation, Japan). All measurements were done in duplicate, and polyphenol concentrations were expressed as GAE in milligrams per liter calculated by means of a standard curve of gallic acid.
Monomeric Anthocyanin Content
Monomeric anthocyanin content (Monomeric AC) of the initial wine and of WP was determined using the pH differential method (Giusti and Wrolstad 2001; Ribereau-Gayon and Stonestreet 1968). WP samples were rehydrated with water to their original weight before freeze-drying and diluted in buffer to achieve a proper concentration range for reading. A Shimadzu PharmaSpecUV-1700 spectrophotometer (Shimadzu Corporation) and 1-cm path length disposable cells were used for spectral measurements. Absorbencies were read at 520 and 700 nm. Pigment content was calculated as malvidin-3-glucoside (mg/100 g WP) with molecular weight of 463.3 g mol−1 and extinction coefficient of 25,000 l cm−1 mol−1. All samples were prepared in duplicate and measurements were taken four times for each one.
Analysis of Phenolic Compounds by HPLC
Samples were prepared by weighting 60.0 ± 0.5 mg of WP which was dissolved in 1.5 ml of solvent composed of a mixture of water/methanol/HCl (89/10/1) (Souquet et al. 2006). Prior to injection they were filtered through a Whatman PTFE syringe filter (diameter = 13 mm, porosity = 0.45 μm). The injection volume was 10 μl. The original red wine was also analyzed by direct injection.
HPLC analysis was carried out in a 1100 series HPLC instrument (Agilent Technologies, Waldbronn, Germany) equipped with a vacuum degasser, a quaternary pump, an autosampler and a thermostated column compartment. Separation was achieved on a reverse phase Nucleosyl LC-18 column. The column’s temperature was maintained at 30 °C. Detection was performed using a diode array detector attached to a computer (HP Chemstation).
Two solvents were used during the analysis. Solvent A composed of distilled water/formic acid (95/5) and solvent B consisting of acetonitrile/water/formic acid (80:15:5). A constant flow of 1 ml/min was applied with a linear gradient elution profile. The following proportions of solvent B were used (Salas et al. 2004): 0–7 min, 3 %; 7–20 min, 13 %; 20–23 min, 14 %; 23–34 min, 20 %; 34–38 min, 20 %; 38–45 min, 24 %; and 45–54 min, 35 %.
Malvidin 3-G, catechin, epicatechin, epicatechin gallate, epigallocatechin, caffeic acid, gallic acid, caftaric acid, quercetin 3-G and resveratrol were identified according to their retention time and spectral properties. Quantification was done by external standard curves of authentic standards of each compound. These chosen phenolics are representative of important polyphenol groups found in red wine, namely, flavanols, hydroxycinnamic acids, hydroxybenzoic acids, tartaric acid derivatives, flavonols, anthocyanins and stilbenes. Total anthocyanins were also analyzed by absorption at 520 nm and expressed as g malvidin-3-G equivalent/100 g WP. Caftaric acid was expressed as g of caffeic acid/100 g WP. All samples of WP were prepared in triplicate and then analyzed.
Antioxidant Capacity
Changes in the antioxidant capacity along storage of the WP was analyzed using two independent methods: free radical scavenging capacity of the DPPH* (2,2-diphenyl-1-picrylhydrazyl) (Klopotek et al. 2005; Hukkanen et al. 2006; Marc et al. 2004; Stratil et al. 2006) and ferric reducing antioxidant power (FRAP) (Benzie and Strain 1996).
For the DPPH* method, the WP was first dissolved in water (1 g/100 ml) and then three dilutions were done in methanol in order to obtain solutions of WP within the desired range of % inhibition. After some previous explorative dilutions, the final determinations were done using the concentrations 9.6, 6.4 and 3.2 g/l. Each storage condition and time (0, 30, 50 and 70 days) was analyzed. All methanol solutions were centrifuged during 7 min at 4 °C in order to precipitate the undisolved MD10 before analysis.
For the analysis of WP samples, 150 μl of each dilution together with 3 ml of DPPH* solution (2.5 mg DPPH*/100 ml methanol) were placed in disposable 1-cm path length plastic spectrophotometer cells. A control was prepared by adding 150 μl of methanol to 3 ml of DPPH* solutions and a blank was done using 3 ml of methanol and 150 μl of each WP methanol dilution. All WP samples, control and blank were prepared in duplicate and were stored in the dark during 60 min before measuring their absorbance (Abs) at 517 nm. Measurements were done by means of a spectrophotometer UV-MC2. SAFAS, Monaco. The inhibition percent (inhibition %) was obtained by using Eq. 1
Finally, for each storage time the inhibition % was plotted against concentration obtaining a linear regression (R 2 always >0.995), which was used to obtain the concentration of WP necessary to inhibit 50 % of DPPH* (EC50). This change along time for each storage condition was represented. Results are expressed as 50 % inhibition, representing grams of WP needed to reduce DPPH* concentration in 50 %; thus, lower values mean higher antioxidant capacity.
The FRAP technique was adapted from Benzie and Strain (1996). The Frap reagent was prepared by mixing acetic buffer (300 mM, pH = 3.6), TPTZ 10 mM solution in HCl (40 mM) and FeCl3⋅6H2O (20 mM water solution) at a ratio of 10:1:1. FRAP reagent was incubated at 37 °C, and then 1.5 ml were added to 50 μl of the WP solution (125 mg WP/25 ml of water). After 5 min of reaction the absorbance of blue coloration was measured at 595 nm against a blank sample by means of a spectrophotometer UV-MC2 (SAFAS, Monaco). A standard curve of FeSO4.7 H20 expressed as Fe2+ μM was done in the range of 100–1,200 μmol Fe2+/l. Results (mean from three replicates) were expressed as micromoles of Fe2+ reduced per Kg of WP, based on the mentioned FeSO4⋅7H2O standard.
Data Analysis
Multiple factor analyses of variance (ANOVA) with subsequent Student–Newman–Keuls (SNK) test were carried out to detect differences along storage times for chemical and HPLC determinations using Infostat software v.2009 (Universidad Nacional de Córdoba, Argentina).
HPLC quantification of malvidin-3-G and chemical determination of anthocyanins were correlated by means of a simple regression using Statgraphics Plus v 5.1.
For all measurements the standard deviation (SD) was calculated.
Results and Discussion
Analysis of Phenolic Compounds
Table 1 shows content of the ten individual phenolic compounds investigated in Cabernet Sauvignon red wine and in the WP. As a result of freeze-drying and addition of MD10, the concentration of phenolics in the WP (mg/100 g) resulted to be in general about 3.6 times higher than in the liquid wine (also expressed in mg/100 g wine) revealing that the product could be considered a concentrate of wine polyphenols. Moisture content of WP after freeze-drying was determined to be 1.51 ± 0.07 % and that of the WP equilibrated to aw 0.330 was 4.53 ± 0.09 %.
As previously noted in the Materials and Methods section the phenolic compounds studied in present work are representative of important polyphenol groups found in red wine. The concentration of these selected phenolics in the used red wine (Table 1) falls within the range reported by several authors either for Argentinean Cabernet Sauvignon wines (Kruma et al. 2010; Fanzone et al. 2010) or from other countries (Anli and Vural 2009; Burin et al. 2011; Gambelli and Santaroni 2004; Kruma et al. 2010). Total polyphenols content (Table 1) also fits in the range reported for several red wines either from Argentina or other countries (Anli and Vural 2009; Camussoni and Carnevali 2004; Kruma et al. 2010).
Phenolic compounds in red wine have proved important for delivering health benefits and therefore their stability has been widely studied giving place to numerous publications in a variety of wines from different geographical regions. However, they are of limited applicability to a powder system such as the WP. In a powder food system as the WP, moisture (or a w) is a key factor affecting chemical and physical stability since it is well known that water sorbed in an amorphous food powder acts as a plasticizer accelerating (or decreasing) chemical reactions by influencing molecular mobility (Buera et al. 2006; Li et al. 2011; Roos 1995).
van Galde et al. (2003) used the technique of freeze-drying not to obtain a wine polyphenols concentrate but to remove alcohol and water from red wine, in order to study the effects of wine polyphenols on health without the influence of alcohol. When describing the procedure used to reconstitute the freeze-dried product they stated that: “distilled water was added to the dry powder”. The meaning of “dry powder” is not clear since a powder cannot be obtained after freeze-drying of red wine alone. In present and previous works (Sanchez et al. 2011), we observed that after freeze-drying of Cabernet Sauvignon wine without the addition of MD10, an amorphous rubbery mass (very difficult to handle) was obtained. However, when MD10 was added before freeze-drying a free flowing powder (after milling) was obtained. This free flowing powder is readily dissolved in water, it is easy to handle and could be integrated as an ingredient in different food systems.
Freeze-drying has proven to be a suitable method for drying thermosensitive substances, minimizing thermal degradation reaction (Munin and Edwards-Lévy 2011). van Galde et al. (2003) also assessed the effect of freeze-drying on retention of four important red wine polyphenols (gallic acid, catechin, epicatechin and quercetin) by determining their concentration both in the original red wines and the freeze-dried products. In their case, recovery amounted to 68 % (average for all four phenolics in six different red wine brands). They mentioned that “apart from the intended loss of volatile substances like alcohol, about 30 % of the non volatile substances is also lost” in the freeze-drying process; although they acknowledged that further research was needed to analyze at which stage of their freeze-drying process the reported loss of polyphenols occurred. Volatility is the tendency of a substance to vaporize (or sublimate) and it is directly related to a substance's vapor pressure. Thus, one would expect that gallic acid, catechin, epicatechin and quercetin would be hardly “volatilized” during freeze-drying due to their very low vapor pressure. For gallic acid (25 °C) vapor pressure is 7.32 × 10−11 mmHg; for catechin (25 °C) 9.3 × 10−17 mmHg, for epicatechin (25 °C) is 3.33 × 10−31 mmHg, and for quercetin (25 °C) is 4.24 × 10−17 mmHg. Moreover, van Galde et al. (2003) reported the retention of gallic acid and catechin after freeze-drying was identical (64 %) a result which in terms of “volatility” would be difficult to explain since there is a difference of six orders of magnitude between their vapor pressures.
On the contrary, in present work the retention of gallic acid, catechin, and epicatechin after freeze-drying of red wine + MD10 amounted nearly to 100 % (average for this three phenolics). Sanchez et al. (2011) also reported that after freeze-drying of red wine with MD10 total polyphenols retention (Folin–Ciocalteu method) amounted to 97.8 %. However, it has to be stressed that these values were obtained after freeze-drying of red wine with MD10 and not in regular red wine as done by van Galde et al. (2003). Addition of MD10 may had protected phenolics by generating micro-regions of reduced molecular mobility and diffusion rate, thus lowering chemical reaction rates (Buera et al. 2006 ; Li et al. 2011) which might enable polyphenols recovery.
Figure 1 shows the behavior of gallic acid, caftaric acid, quercetin 3-G, caffeic acid and resveratrol in WP equilibrated at a w = 0.33 and stored at 28 °C and 38 °C. It can be seen that phenolics concentration in WP remains practically constant, either at 28 °C or 38 °C during 70 days of storage. Similar results (not shown in the figure) were obtained at lower water activity (a w = 0.053).
Some authors reported data on the stability of these phenolics in other low-moisture powders. For example, Altiok et al. (2009) studied the properties of trans-resveratrol in chitosan microspheres produced by spray drying finding that the stability of trans-resveratrol when incorporated into the microspheres remained constant. The same was observed in present work for resveratrol freeze-dried encapsulated in MD. For gallic acid, Tandale (2007) used whey protein concentrate as carrier material and stored freeze-dried microencapsulated gallic acid at 25 °C in dark or under UV light. In both conditions the retention of microencapsulated gallic acid (at a w = 0.22 and 0.44) was above 90 % after 56 days storage. The stability of caftaric acid in a freeze-dried extract of a medicinal herb (Echinacea purpurea) was studied by Rattanadechsakul et al. (2007), who found that it remained relatively constant for at least 60 days at temperatures of 30 °C and 45 °C.
The stability of some of the aforementioned phenolics “in solution” (e.g., wine) has also been studied and may be compared with present data in WP. Giovanelli and Brenna (2007) detected minor changes (degradation) in gallic and caftaric acid when red wine bottles were stored at 30 °C for up to 75 days. Zafrilla et al. (2003) reported that changes in quercetin 3-G content in red wine stored at 20 °C (in the dark) were small, finding losses of 6.4 % and 9.2 %, after 30 and 60 days storage, respectively. Overall, these studies performed in wine might suggest that gallic acid, caftaric acid and quercetin 3-G might be relatively stable even when “in solution” (wine) during storage time (65/75 days) similar to those in present work with WP. It is to be noted, however, that studies in “liquid” wine were performed at lower temperatures (20 and 30 °C) than the highest temperature (38 °C) at which the phenolics proved to be stable in WP. Jeandet et al. (1995) investigated the influence of aging on the resveratrol content of wine through the analysis of wines of different ages and reported that resveratrol appeared relatively stable in wine. Ratola et al. (2004) and Cvejic et al. (2010) also indicated a good thermal/chemical stability of resveratrol in red wines.
Behavior of epigallocatechin content in WP during storage (Fig. 2) was different from the other catechins here studied (see Fig. 3) since after an initial pronounced loss of around 61 % epigallocatechin content appeared to level off during storage at studied conditions of aw and temperature.
In Fig. 3, the data referring to epicatechin gallate, catechin and epicatechin in WP equilibrated at a w = 0.33 and stored at 28 °C and 38 °C are presented. A small loss is observed for these catechins, and this loss increased significantly (p < 0.05) with increasing temperature from 28 °C to 38 °C. Nevertheless, at the highest a w/temperature losses ranged between 15 % and 25 % after 70 days of storage. Stability of catechins in the “dry” state has been studied in dry tea products. Friedman et al. (2009) reported changes for epicatechin and epicatechin gallate (among other catechins) during storage of eight commercial brands of tea leaves in the “dry” state (at 20 °C, in the dark). They concluded that even in the “absence of moisture” catechins content decreased with time. Unfortunately, they did not report the moisture content (or a w) of their tea leaves neither variations of moisture content during storage. These variations were likely to happen since the tea bags were stored in the original containers and without controlling ambient relative humidity. After 60 days of storage the average loss of epicatechin and epicatechin gallate amounted to 18 % and to 57 %, these values being an average of the eight tea brands studied. However, inspection of their data revealed a strong dispersion of data among different tea brands, suggesting some unknown influence on the stability of catechins. It is not known whether these differences might be due to (among other things) different moisture content of the tea bags, since moisture has an important effect on stability of reduced moisture food systems.
Li et al. (2011) studied the effect of temperature and relative humidity on the degradation kinetics of catechins (among them, epicatechin, epigallocatechin and epicatechin gallate) in spray dried green tea powder. They used a commercial brand and did not disclosure the ingredients in the product (besides from green tea extract); it may be mentioned that MD is sometimes used as carrier material for spray drying of tea extracts (Nadeem et al. 2011). Li et al. (2011) reported that catechin degradation was affected by an increase in water activity and temperature, temperature being a dominant factor. Interestingly, an inspection of their results at a w = 0.41 (at 60 °C) revealed that epigallocatechin exhibited the highest rate of degradation, as compared to epicatechin and epicatechin gallate, a behavior similar to that reported in present work (Figs. 2 and 3) for catechins but in WP at a w = 0.33. From the first-order degradation kinetic curves reported by Li et al. (2011), we calculated that after 50 days storage in green tea powder (60 °C, a w = 0.41) epigallocatechin experimented 40 % loss while for epicatechin gallate and epicatechin the losses were 23.7 % and 7.7 %, respectively. No explanation was advanced for the higher instability of epigallocatechin in green tea extract powder as compared to the other catechins. It is well known that catechins can undergo degradation, oxidation, epimerization and polymerization (Ananingsih et al 2011) and many factors could contribute to these chemical changes. Moreover, Wang et al. (2000) reported the loss of catechins in “liquid phase” of tea (i.e., green tea extracts) during storage at 50 °C. Their tabulated data for epigallocatechin, catechin and epicatechin content in tea extract were recalculated and plotted as % retention versus storage time (at 50 °C), finding that the epigallocatechin evidenced the highest loss.
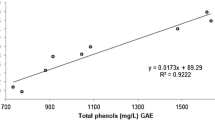
Changes in malvidin 3-G content in WP during storage are observed in Fig. 4. At 28 °C and a w = 0.053 malvidin 3-G did not show any loss during 70 days storage. However, increasing either the aw from 0.053 to 0.33 or the temperature from 28 °C to 38 °C resulted in losses which amounted to about 26 % at 70 days storage at the highest a w/temperature. These losses were also reflected in changes in the measurements of monomeric AC. For this purpose, a correlation between malvidin 3-G content (determined by HPLC) with monomeric AC determined by differential pH method was done. An acceptable linear correlation (r 2 = 0.852; n:12) was observed suggesting that chemical determination of monomeric anthocyanins is strongly related to malvidin 3-G content, which is known to be the major anthocyanin in Cabernet Sauvignon wines (Birse et al., 2004; Radovanovic and Radovanovic 2010).
Zafrilla et al. (2003) determined changes in phenolic compounds of bottle-stored red wine in the dark at 20 °C. Their data for malvidin 3-G and total anthocyanins were calculated as % retention and compared to present data for malvidin 3-G and total anthocyanins retention in WP stored at 28 °C and a w = 0.33. Figure 5 shows that malvidin 3-G and total anthocyanins content is much more stable in the dry matrix of WP (28 °C) than in regular red wine stored at 20 °C. Giovanelli and Brenna (2007) also reported that in red wine a sharp decrease in total anthocyanins occurred during storage at 30 °C for up to 75 days.
Comparison of malvidin 3-G retention in WP equilibrated at a w = 0.33 and stored at 38 °C with malvidin 3-G retention in regular red wine (calculated with data reported by Zafrilla et al. 2003) stored at 20 °C in the dark
The higher stability of anthocyanins in WP, as compared to regular red wine, may be attributed to the limited molecular mobility and diffusion rates associated with the low moisture content of WP (Buera et al. 2006). Li et al. (2011) reported similar results when comparing the degradation kinetics of catechins in green tea extract solution and green tea powder (at various a w) and found that powder products were much more stable than solutions.
The storage stability of spray-dried or freeze-dried encapsulated fruit anthocyanins has been studied in many works. Estupiñan et al. (2011) investigated the stability of anthocyanin freeze-dried powders from Andes berry during storage and concluded that addition of maltodextrin DE20 improved the color and stability of antioxidants present suggesting a protective enclosing of anthocyanin within an MD matrix. Laine et al. (2008) reported that a polyphenol-rich raspberry extract was stabilized by freeze-drying, with MDs (DE5–8 and DE18.5) as material coating and found the freeze-dried particles were stable over long periods and provided to polyphenols an effective protection against the oxidation phenomenon during their storage, whereas antioxidant activity remained identical. Osorio et al. (2010) also reported that the stability of anthocyanins was enhanced by spray drying encapsulation in MD and/or arabic gum matrixes. Tonon et al. (2010) studied anthocyanin stability of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carriers and found that temperature influenced anthocyanin stability in a negative way and the increase of water activity also resulted in higher degradation. This was attributed to the higher molecular mobility, which allows easier oxygen diffusion, thus accelerating the oxidation reactions.
Antioxidant Activity
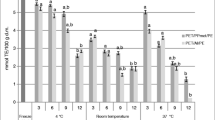
One of the important characteristics of polyphenolic compounds is their antiradical property. Different methods are used for the determination of antioxidant capacity, such as the chromogen free radical DPPH* assay (Kruma et al. 2010) and FRAP (Ou et al. 2002; Stratil, et al. 2008). Figure 6 shows changes in antioxidant activity of WP (determined with chromogen radical DPPH*) during storage at all studied conditions. A moderate decrease of antioxidant activity along storage time was observed, this decrease being enhanced by higher temperature and water activity. Further on, Fig. 7 shows the change in ferric reducing capacity (FRAP method) of WP during storage at different conditions of water activity and temperature which is in good agreement with results obtained by DPPH* method (Fig. 6). The antioxidant capacity as determined by FRAP method declined slightly during storage, the greatest loss being of only 14 % at 38 °C and a w = 0.33.
Several authors tried to correlate (with more or less success) the antioxidant activity and the polyphenolic contents of red wine. For example, a linear correlation between antioxidant capacity and the contents of total polyphenols have been reported by many authors (Avalos Llanos et al. 2003; Cioroi and Musat 2007; Anli and Vural 2009; Kruma et al. 2010). However, Zafrilla et al. (2003) reported a pronounced fall in the concentration of total phenols in red wine stored at 20 °C, but the antioxidant activity remained almost constant. These authors also failed to correlate the anti-radical activity with some individual phenolic compounds in red wine. These behavior may be attributed to two main facts, a) polyphenols usually identified by different authors represented only a small proportion of the several hundreds of phenolic compounds existing in red wine (Zafrilla et al., 2003) and b) many factors which influence hydrolysis, oxidation and condensation reactions may take place during wine storage (Kallithraka et al. 2009).
Without attempting to correlate changes in antioxidant capacity (either by DPPH* or FRAP method) with changes of phenolics in WP, it may be mentioned that the observed loss of antioxidant capacity after 70 days storage (at a w = 0.33 and 38 °C) was 18 % for DPPH* and 14 % for FRAP. These values were aligned with similar losses found in catechine (19.1 %), epicatechine (25.7 %) epicatechin gallate (13.6 %) and malvidin 3-G (26.1 %) for identical storage conditions.
Very recently, Munin and Edwards-Lévy (2011) presented a review on encapsulation of natural polyphenolic compounds for cosmetics, nutrition and health, and concluded that encapsulation is an interesting means to potentialize their activity (i.e., via spray drying or freeze-drying). They concluded that encapsulation provided a significant protection for polyphenols against drastic conditions such as oxidation and thermal degradation.
Present results on the stability of several red wine phenolics in a protective enclosing within an MD matrix (WP) of low moisture content, appears to be on line with above suggestion.
Conclusions
A free-flowing dealcoholized WP having a phenolic concentration about 3.6 times higher than the original liquid red wine (compared on the same weight basis) was obtained. Most phenolics remained stable in the MD matrix during the 70 days storage time at the a w (0.053 and 0.33) and temperatures (28 °C and 38 °C) considered. The WP remained free flowing at all conditions studied; undesirable physical changes (such as caking) were not visually observed.
Epigallocatechin was the only polyphenol which experienced an important decrease during storage (61 %). Even though no correlation was attempted, a moderate decline of the antioxidant activity (either measured by DPPH* or FRAP methods) was also observed along storage time. This was enhanced by higher temperature and water activity (38 °C and a w = 0.33).
This freeze-dried, highly stable wine product, with a polyphenol composition similar to that of red wine could be used as a healthy ingredient in alcohol-free powder drinks since encapsulation provided protection against conditions such as oxidation and thermal degradation. Future work goes on in this direction.
References
Altiok, E., Bayraktar, O., & Tihminlioglu, F. (2009). Stability of trans-resveratrol incorporated in chitosan microspheres. Biomedical Engineering Meeting, BIYOMUT 2009. 14th National. doi:10.1109/BIYOMUT.2009.5130340.
Ananingsih, V. K., Sharma, A., & Zhou, W. (2011). Green tea catechins during food processing and storage: a review on stability and detection. Food Research International. doi:10.1016/j.foodres.2011.03.004.
Anli, R. E., & Vural, N. (2009). Antioxidant phenolic substances of Turkish red wines from different regions. Molecules, 14, 289–297.
Avalos Llanos, K. R., Sgroppo, S. C., & Avanza, J. (2003). Actividad antioxidante y contenido en fenoles totales en vinos de origen nacional. FACENA, 19, 11–19.
Benzie, I. F. F., & Strain, J. J. (1996). The ferric reducing ability of plasma (FRAP) as a measurement of “antioxidant power”: the FRAP assay. Analytical Biochemistry, 239, 70–76.
Birse, M., Pollnitz, A., Kwiatkowski, M., Gockowiak, H., Parker, M., Eglinton, J., & Herderich, M. (2004). Anthocyanins, anthocyanin-derived pigments and the colour of red wine. Proceedings of the 12th World Congress of Food Science and Technology.
Brouillard, R., George, F., & Fougerousse, A. (1997). Polyphenols produced during red wine ageing. BioFactors, 6, 403–410.
Buera, M. P., Welti-Chanes, J., Lillford, P., & Corti, H. R. (2006). Water properties of food, pharmaceutical and biological materials. CRC, Taylor & Francis. United States of America.
Burin, V. M., Lima da Silva, A., Malinovsky, L. I., Rosier, J. P., Falcao, L. D., & Bordignon-Luiz, M. T. (2011). Characterization and multivariate classification of grapes and wines of two Cabernet Sauvignon clones. Pesquisa Agropecuária Brasileira, 46, 474–481.
Camussoni, G., & Carnevali, E. (2004). Determinación comparativa del contenido de polifenoles en vinos tintos de origen Argentino. Invenio, 7, 151–159.
Chranioti, C., & Tzia, C. (2012). Binary mixtures of modified starch, maltodextrin and chitosan as efficient encapsulating agents of fennel oleoresin. Food and Bioprocess Technology: 1–9, doi. 10.1007/s11947-012-0966-7.
Cioroi, M., & Musat, C. L. (2007). Investigations on the correlations between polyphenol content from red wines and their antioxidant capacity. Cercetari Agronomice in Moldova, 4, 35–42.
Cvejic, J. M., Djekic, S. V., Petrovic, A. V., Atanackovic, M. T., Jovic, J. M., Brceski, I. D., & Gojkovic-Bukarica, L. C. (2010). Determinatinon of trans- and cis-resveratrol in Serbian commercial wines. Journal of Chromtographic Science, 48, 229–234.
Díaz, B., Gomes, A., Freitas, M., Fernandes, E., Nogueira, D. R., González, J., Moure, A., Levoso, A., Vinardell, M. P., Mitjans, M., Domínguez, H., & Parajó, J. C. (2012). Valuable polyphenolic antioxidants from wine vinasses. Food and Bioprocess Technology, 5, 2708–2716.
Estupiñan, D.C., Schwartz, S.J. & Garzón, G.A. (2011). Antioxidant activity, total phenolics content, anthocyanin, and color stability of isotonic model beverages colored with andes berry (rubus glaucus benth) anthocyanin powder. Journal of Food Science, 76, s26–s34.
Fanzone, M., Peña-Neira, A., Jofré, V., Assof, M., & Zamora, F. (2010). Phenolic characterization of Malbec wines from Mendoza province (Argentina). Journal of Agricultural and Food Chemistry, 58, 2388–2397.
Favetto, G. J., Resnik, S. L., Chirife, J., & Ferro Fontán, C. (1983). Statistical evaluation of water activity measurements obtained with the Vaisala Humicap humidity meter. Journal of Food Science, 48, 534–537.
Friedman, M., Levin, C. E., Lee, S. U., & Kozukue, N. (2009). Stability of green tea catechins in commercials tea leaves during storage for 6 months. Journal of Food Science, 74, H47–H51.
Galmarini, M. V. (2010). Estudios sensoriales y fisicoquímicos del disacárido trehalosa en relación a su uso como ingrediente funcional en alimentos. PhD thesis, Facultad de Farmacia y Bioquímica, Universidad de Buenos Aires, Buenos Aires, Argentina.
Galmarini, M. V., van Baren, C., Zamora, M. C., Chirife, J., Bandoni, A., & Di Leo Lira, P. (2011). Impact of trehalose and other carbohydrates addition on aroma retention in freeze dried strawberry puree. International Journal of Food Science and Technology, 46(7), 1329–1336.
Gambelli, L. & Santaroni, G.P. (2004). Polyphenols content in some Italian red wines of different geographical origins. Journal of Food Composition and Analysis, 17, 613–618.
Giovanelli, G., & Brenna, O. V. (2007). Oxidative stability of red wine stored in packages with different oxygen permeability. European Food Research and Technology, 226, 169–179.
Giusti, M.M. & Wrolstad, R.E. (2001). Characterization and measurement of anthocyanins by UV-visible spectroscopy. In: Current Protocols in Food Analytical Chemistry (edited by R. Wrolstad, T. Acree, H. An, E. Decker, M. Penner, D. Reis, S. Schwartz, C. Shoemaker & P. Sporns). 1st edn. Pp. F1.2.1–F1.2.13, New York, NY: John Wiley & Sons, Inc..
Gorelik, S., Ligumsky, M., Kohen, R., & Kanner, J. (2008). A novel function of red wine polyphenols in humans: prevention of absorption of cytotoxic lipid peroxidation products. The FASEB Journal, 22, 41–46.
Hukkanen, T., Pölönen, S., Kärenlampi, O., & Kokko, H. (2006). Antioxidant capacity and phenolic content of sweet rowanberries. Journal of Agricultural and Food Chemistry, 54, 112–119.
International Organization of Vine and Wine (2009). Recueil international des methodes d’analyses. Indice de Folin–Ciocalteu. Method OIV-MA-AS2-10, 2009.
Jeandet, P., Bessis, R., Sbaghi, M., Meunier, P., & Trollat, P. (1995). Resveratrol content of wines of different ages: relationship with fungal disease pressure in the vineyard. American Journal of Enology and Viticulture, 46, 1–4.
Kallithraka, S., Salacha, M. I., & Tzourou, I. (2009). Changes in phenolic composition and antioxidant activity of white wine during bottle storage: accelerated browning test versus bottle storage. Food Chemistry, 113, 500–505.
Klopotek, Y., Otto, K., & Bohm, V. (2005). Processing strawberries to different products alters content of vitamin C, total phenolics, total anthocyanins and antioxidant capacity. Journal of Agricultural and Food Chemistry, 53, 5640–5646.
Kruma, Z., Karklina, D., Cinkmanis, I., & Rutkovska, O. (2010). Polyphenolic composition and free radical scavenging activity of red wines available in the Latvian market. Chemine Technologija, 54, 56–61.
Laine, P., Kylli, P., Heinonen, M., & Jouppila, K. (2008). Storage stability of microencapsulated cloudberry (Rubus chamaemorus) phenolics. Journal of Agricultural and Food Chemistry, 56, 11251–11261.
Li, N., Taylor, L. S., & Mauer, L. J. (2011). Degradation kinetics of catechins in green tea powder: Effects of temperature and relative humidity. Journal of Agriculture and Food Chemistry, 59, 6082–6090.
Marc, F., Davin, A., Deglene-Benbrahim, L., Ferrand, C., Baccaunaud, M., & Fritsch, P. (2004). Méthodes d’évaluation du potentiel antioxidant dans les aliments. Médecine Sciences, 20, 458–463.
Mazza, G., Fukumoto, L., Delaquis, P., Girard, B., & Ewert, B. (1999). Anthocyanins, phenolics, and color of cabernet Franc, merlot, and pinot noir wines from British Columbia. Journal of Agricultural and Food Chemistry, 47, 4009–4017.
Midgley, J. (1971). Drinking and attitudes towards drinking in a Muslim community. Quarterly Journal of Studies on Alcohol, 32(1-A), 148–158.
Munin, M., & Edwards-Lévy, F. (2011). Encapsulation of natural polyphenolic compounds; a review. Pharmaceutics, 3, 793–829.
Nadeem, H. S., Torun, M., & Ozdemir, F. (2011). Spray drying of the mountain tea (Sideritis stricta) water extract by using different hydrocolloid carriers. LWT- Food Science and Technology, 44, 1626–1635.
Nikfardjam Pour, M. S., Márk, L., Avar, P., Figler, M., & Ohmacht, R. (2006). Polyphenols, anthocyanins, and trans-resveratrol in red wines from the Hungarian Villány region. Food Chemistry, 98, 453–462.
Osorio, C., Acevedo, B., Hillebrand, S., Carriazo, J., Winterhalter, P., & Morales, A. L. (2010). Microencapsulation by spray-drying of anthocyanin pigments from Corozo (Bactris guineensis) fruit. Journal of Agricultural and Food Chemistry, 58, 6977–6985.
Ou, B., Huang, D., Hampsch-Woodill, M., Flanagan, J. A., & Deemer, E. K. (2002). Analysis of antioxidant activities of common vegetables employing Oxygen Radical Absorbance Capacity (ORAC) and Ferric Reducing Antioxidant Power (FRAP) assays: a comparative study. Journal of Agricultural and Food Chemistry, 50, 3122–3128.
Radovanovic, B., & Radovanovic, A. (2010). Free radical scavenging activity and anthocyanin profile of Cabernet Sauvignon wines from the Balkan region. Molecules, 15, 4213–4226.
Ratola, N., Fari, J. L., & Alves, A. (2004). Analysis and quantification of trans-resveratrol in wines from Alentejo Region (Portugal). Food Technology and Biotechnology, 42, 125–130.
Rattanadechsakul, P., Rattanadechsakul, J., Okonogi, S., & Yotsawimonwat, S. (2007). Stability of bioactive compounds and antioxidant activity of Echinaceaof purpurea extract. XVth International Workshop on Bioencapsulation, Vienna, Austria, Sept 6–8, pp. 1–4.
Renaud, S., & de Lorgeril, M. (1992). Wine, alcohol, platelets, and the French paradox for coronary heart disease. The Lancet, 339(8808), 1523–1526.
Ribereau-Gayon, P., & Stonestreet, E. (1968). Le dosage des anthocyanes dans le vin rouge. Bulletin de la Société Chimique de France, 47, 2649–2652.
Roos, Y. (1995). Phase transitions in foods. New York, USA: Academic Press.
Roos, Y., & Karel, M. (1991). Phase transitions of mixtures of amorphous polysaccharides and sugars. Biotechnology Progress, 7, 49–53.
Salas, E., Atanasova, V., Poncet-Legrand, C., Meudec, E., Mazauric, J. P., & Cheynier, V. (2004). Demonstration of the occurrence of flavanol–anthocyanin adducts in wine and in model solutions. Analytica Chimica Acta, 513, 325–332.
Sanchez, V., Baeza, R. I., Galmarini, M. V., Zamora, M. C., & Chirife, J. (2011). Freezedrying encapsulation of red wine polyphenols in an amorphous matrix of maltodextrin. Food and Bioprocess Technology. doi:10.1007/s11947-011-0654-z.
Singleton, V. L., & Rossi, J. A., Jr. (1965). Colorimetry of total phenolics with phosphomolybdic–phosphotungtic acid reagent. American Journal of Enology and Viticulture, 16, 144–158.
Soulat, T., Philippe, C., dit Sollier, C. B., Brezillon, C., Berge, N., Teissedre, P. L., Callebert, J., Rabot, R., & Drouet, L. (2006). Wine constituents inhibit thrombosis but not atherogenesis in C57BL/6 apolipoprotein Edeficient mice. British Journal of Nutrition, 96, 290–298.
Souquet, J. -M., Veran, F., Mané, C., & Cheynier, V. (2006). Optimization of extraction conditions on phenolic yields from the different parts of grape clusters. Quantitative distribution of their proanthocyanidins. XXIII International Conference on Polyphenols Winipeg (Manitoba, Canada), pp 245–246.
Stratil, P., Klejdus, B., & Kuban, V. (2006). Determination of total content of phenolic compounds and their antioxidant activity in vegetables – evaluation of spectrophotometric methods. Journal of Agricultural and Food Chemistry, 54, 607–616.
Stratil, P., Kubá, V., & Fojtová, J. (2008). Comparison of the phenolic content and total antioxidant activity in wines as determined by spectrophotometric methods. Czech Journal of Food Science, 26, 242–253.
Tandale, S. R. (2007). Microencapsulation of vitamin c and gallic acid in whey protein concentrate by spray and freeze drying — characterization and degradation kinetics. PhD thesis, Graduate Faculty of the University of Georgia, Athens, GA.
Tonon, R. V., Brabet, C., & Hubinger, M. D. (2010). Anthocyanin stability andantioxidant activity of spray-dried açai (Euterpe oleracea Mart.) juice produced with different carrier agents. Food Research International, 43, 907–914.
van Galde, P. H., van der Westelaken, M., Bruma, B. N., & van de Weil, A. (2003). Characteristics of piraltin, a polyphenol concentrate produced by freeze drying of red wine. Life Sciences, 74, 1159–1166.
Wang, L. F., Kim, D. M., & Lee, C. Y. (2000). Effects of heat processing and storage on flavanols and sensory qualities of green tea beverage. Journal of Agricultural and Food Chemistry, 48, 4227–4232.
Zafrilla, P., Morillas, J., Mulero, J. Cayuela, J., Martinez-Cacha, A., Pardo, F. & Lopez Nicolas, J.M. (2003). Changes during Storage in Conventional and Ecological Wine: Phenolic Content and Antioxidant Activity. Journal of Agricultural and Food Chemistry, 51 (16),4694–4700.
Acknowledgements
The authors acknowledge Facultad de Ciencias Agrarias, Pontificia Universidad Católica for financial support. One of the authors (MVG) acknowledges CONICET for a travel grant associated with this research. The authors are grateful to the technical team of GRAPPE research unit for their assistance and help in analyzing wine polyphenols.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Galmarini, M.V., Maury, C., Mehinagic, E. et al. Stability of Individual Phenolic Compounds and Antioxidant Activity During Storage of a Red Wine Powder. Food Bioprocess Technol 6, 3585–3595 (2013). https://doi.org/10.1007/s11947-012-1035-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-1035-y