Abstract
Freeze-drying is a dehydration technique which, from a sensory, nutritional and functional point of view, provides high-quality powder products. Nevertheless, both long processing times and high economic costs are required. In this study, pre-drying the samples using hot air or microwave has been considered in order to reduce the initial product’s water content thereby shortening the freeze-drying time so as to obtain high value products at a reduced cost. The effect of dehydration pre-treatments on the kinetics, antioxidant activity and solubility of freeze-dried kiwifruit products was evaluated. Nine different thin-layer semi-theoretical models were used to fit the drying data. According to the obtained results, the pre-treated samples exhibited higher drying rates than the fresh sample. In turn, the dehydration pre-treatments used did not affect the solubility or the antioxidant activity of the samples.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In recent years, consumer preferences seem to have changed towards a greater consumption of processed fruit products. Fruit juices, fruit purees and different forms of powdered fruit products are being marketed due to an increased demand for ready-to-eat foodstuffs. Thus, alternatives to conventional processing technologies are being explored to produce better quality products.
To date, dehydration is the method which has been most commonly used to prolong the product’s shelf-life. However, traditional drying conditions (high temperatures and long times) have been reported to cause a dramatic loss in food quality (Cano 1991; Vadivambal and Jayas 2007). Freeze-drying (FD) emerged as an alternative drying method which generates high-quality products with a very low moisture content, good sensory and nutritional properties and a good capacity for rehydration. However, since long processing times and high operation costs are necessary to obtain freeze-dried products with an adequate level of quality (Menlik et al. 2010), in the last few decades, studies have been carried out into how to reduce processing costs. Some of these studies have focused on abolishing the cost of vacuum generation, others have tried to reduce energy use by combining technologies during FD operation and others have tried to shorten the processing time by enhancing heat transfer (Pardo and Leiva 2010). In the present research work, the use of combined technologies is proposed. Both microwave drying (MW) and hot-air drying (HA) can be applied to remove part of the product’s water content prior to FD in order to produce high-quality, but low-cost, powder products (Huang et al. 2011; Zhang et al. 2006). In addition to the economic point of view, freeze-dried products are highly hygroscopic and are prone to suffering changes in their physical properties brought about by the environment and time. Therefore, adding solutes, such as arabic gum and tricalcium phosphate, has been shown to be necessary in order to increase the product stability by acting as a barrier to water adsorption. The main benefits associated with their use are (a) an increase in the glass transition temperature, (b) a reduction in the hygroscopicity, (c) an increase in the viscosity of the rehydrated product and (d) anti-caking properties (Cozic et al. 2009; Gabas et al. 2007; Ghosal et al. 2010; Jaya and Das 2009; Mosquera et al. 2010).
On the other hand, the mathematical modelling of the drying processes can play an important role in their design, control and optimization (Contreras et al. 2008; Panchariya et al. 2002; Simal et al. 2005). In the field of freeze-drying processing, there has, to date, been a lack of theoretical analysis, modelling and simulation of the processes. Fahloul et al. (2009), Daoussi et al. (2009), Xiang et al. (2004) and Zhai et al. (2003) have published some of the few results from research carried out in kinetics of freeze-drying. Thin-layer drying models that describe the drying phenomenon of agricultural materials can be classified into three categories: theoretical, semi-theoretical and empirical (Akpinar 2006; Panchariya et al. 2002). Theoretical models only take into account the internal resistance to moisture transfer, while the external resistance is considered by semi-theoretical and empirical models (Panchariya et al. 2002). Most of the semi-theoretical drying models are derived by simplifying general series solutions of Fick’s second law, which has been the most extensively investigated theoretical drying model (Doymaz 2004, 2006; Panchariya et al. 2002). These models are characterized by deriving a direct relationship between the average water content and the drying time, neglecting the fundamentals of the drying process and including parameters with no physical meaning (Panchariya et al. 2002; Simal et al. 2005). By reviewing papers mentioned in bibliography, it can be seen that semi-theoretical models such as the Newton, the Page, the Henderson and Pabis, the Lewis, the Logarithmic or the exponential model have been widely used to describe dehydration processes (Akpinar 2006; Contreras et al. 2008; Doymaz 2006; Togrul and Pehlivan 2003).
The aim of this work was to evaluate the advantages of applying combined drying technologies in order to produce a powder of kiwifruit, with added arabic gum and tricalcium phosphate. Conventional freeze-dried product was compared with the behaviour of samples obtained by applying microwave or convective drying as a pre-treatment. In each case, a kinetic study and some of the most important physicochemical and functional properties of freeze-dried products were the variables selected to compare the efficacy of the processes and the quality of the product. The most adequate thin-layer semi-theoretical model to fit the drying data was selected.
Material and Methods
Sample Preparation and Treatments
Kiwifruit (Actinida deliciosa var. Hayward) was purchased from a local market in Valencia (Spain). Fruit pieces (°Brix between 12.5 and 13.7) were peeled, washed with distilled water and triturated in a Thermomix (TM 21, Vorwerk, Spain).
Kiwifruit puree was freeze-dried to produce kiwifruit powder. The outline describing the experimental procedure is presented in Fig. 1. A kiwifruit puree layer (5 cm thickness) was placed in a standardised aluminium plate (15 cm diameter and 5 cm length). Samples were stored in a CVF 525/86 cryo freezer (−86 °C) (Ing. Climas, Spain) for 24 h before the freeze-drying in a Lioalfa-6 Lyophyliser (Telstar, Spain) at 0.026 mBar and −56.6 °C for 48 h. The shelf temperature was not controlled, and in order to facilitate the kinetic study, the secondary drying was not favoured by changing the temperature at the end of the process. Arabic gum (AG) (Sigma CAS: 9000-01-5, Spain) and tricalcium phosphate (TCP) (Sigma CAS: 7758-87-04, Spain) were added to the puree samples prior to freezing. The quantities used were 1 kg arabic gum/kg soluble solids of the liquid phase of the kiwifruit (Mosquera et al. 2012) and 0.02 kg tricalcium phosphate/kg soluble solids of the liquid phase of the kiwifruit (Cubero et al. 2002; Jaya and Das 2009). Two drying methods were used to pre-treat the kiwifruit puree prior to FD in order to reduce the initial water content: MW and HA. According to preliminary experiments (data not shown), the final water content was set at 65 g water/100 g sample prior to solutes addition and freeze-drying process. This moisture level led to a sample good in appearance with a noticeable water loss without implying an excessively long treatment time. To ensure this water content in the samples, its weight was recorded during the process. It allowed for the calculation of the water content at different drying times provided the initial moisture content is known (Eq. 1).
where:
- \( x_w^t \) :
-
Water mass fraction of sample at each drying time (in grams water per gram product)
- m 0 :
-
Initial kiwifruit puree mass (in grams)
- \( x_w^0 \) :
-
Initial water mass fraction in the kiwifruit puree (in grams water per gram product)
- m t :
-
Sample mass at different drying times (in grams)
- ∆m :
-
m t − m 0 (in grams)
Fresh kiwifruit puree (300 g) was placed in a standard size recipient (25 cm diameter and 3 cm depth) made of Teflon (Mecaplast, Spain) and aluminium for MW and HA drying, respectively. A household microwave (model 3038GC, Norm, China), set at a microwave power of 600 W, and an oven (model, 5141 AFW2, Moulinex, China), set at an air temperature of 40 °C and an air velocity of 1.5 m/s, were used to carry out the drying pre-treatments. The processing conditions for both technologies (microwave power (in watts) and air temperature (in degree Celsius)) were selected by taking into account the minimal loss of antioxidant capacity obtained in preliminary experiments (data not shown). The treated samples were immediately cooled in ice water until the puree reached 30 °C. In this way, the freeze-dried samples considered in the present study were the non-pre-treated (F) and the microwave (MWD) and hot-air (HAD) pre-dried purees (Fig. 1).
Freeze-Drying Kinetics: Mathematical Modelling
The freeze-drying kinetics of the fresh and pre-treated samples were evaluated. In this sense, the water content and water activity of freeze-dried samples were analysed at 4, 8, 12, 16, 20, 24, 30, 36, 42 and 48 h. The freeze-drying curves obtained from the experimental water content of the samples at different process times were fitted to nine different expressions proposed by earlier authors (Table 1), which were tested so as to select the best model with which to describe the freeze-drying process for pre-dried and non-pre-dried kiwifruit puree (Akpinar 2006; Doymaz 2004; Fahloul et al. 2009; Panchariya et al. 2002; Togrul and Pehlivan 2003).
Data Analysis and Model Evaluation
The goodness of the fit between the experimental and predicted data was assessed by using the adjusted regression coefficient (adjusted R 2) (Eq. 2), the root mean square error (RMSE) (Eq. 3) and the chi-square (χ 2) (Eq. 4). The higher the adjusted R 2 value and the lower the RMSE and X 2 values, the better the fit.
where
- m :
-
Number of observations
- j :
-
Number of model parameters
- SSQ:
-
Sum of squares
In Eqs. (3) and (4), M R, exp,i is the experimental dimensionless moisture ratio, M R, pre,i is the predicted dimensionless moisture ratio, n is the number of observations and z is the number of drying constants.
Analysis
All the samples were homogenized and analysed before and after being pre-treated and freeze-dried as described below. Each analysis was carried out in triplicate. The results are expressed as the average obtained values with standard deviation in brackets.
Physicochemical Properties
The water content (x w) was measured by drying the sample until constant weight was reached at 60 °C in a vacuum oven (AOAC 934.06, 2000). The soluble solids were determined by measuring the °Brix in a previously homogenised sample using a portable digital refractometer, Refracto 3PX, at 20 °C (Metler Toledo, Switzerland). The water activity (a w) was measured by using a dew point hygrometer (GBX FA-st lab, France).
Antioxidant Capacity Measurement
To determine the antioxidant capacity of kiwifruit puree, the DPPH• radical scavenging capacity of kiwifruit extracts was measured as described by Igual et al. (2010), with some modifications. Kiwifruit puree was appropriately diluted with methanol by mixing with external cooling for 30 s. The homogenate was centrifuged at 11,872×g for 10 min at 4 °C, and the supernatant was collected. Aliquots of the kiwifruit extract (0.03 mL) were combined with 3 mL of DPPH• 6.25 × 10−5 M in methanol. A Thermo Electron Corporation spectrophotometer (USA) was used to measure the change in absorbance at 517 nm and 25 °C until the reaction reached a plateau (time at the steady state). A control sample, where the kiwifruit puree extract was replaced by DPPH• 6.25 × 10−5 M in methanol, was used to measure the maximum DPPH• absorbance. The percentage of DPPH• was calculated as indicated in Eq. 5:
where:
- A C :
-
Absorbance of the control sample
- A S :
-
Absorbance of the kiwifruit puree, treated or untreated, at the time of the steady state
In order to express the antioxidant capacity in terms of millimolar Trolox, a calibration curve was prepared by measuring absorbance at 517 nm of different Trolox solutions in the range of 0.3–3 mM.
Solubility Measurement
The mass fraction of the dissolved solutes (DS) was evaluated so as to assess the sample solubility. This parameter has been successfully used by other authors to measure the quality of similar products (Fyfe et al. 2011; Schokker et al. 2011). Both fresh and pre-treated samples which were freeze-dried for 24 and 48 h were rehydrated following the methodology described by Mimouni et al. (2009), with some modifications. Distilled water was added to kiwifruit powder (30 g) in a 100-ml glass beaker until the same soluble solid content was reached as in the untreated kiwifruit puree. Stirring was performed at constant speed (600 rpm) using an electric overhead mixer (RET Basic, IKA, Spain) and four-bladed propeller stirrer 25 mm in diameter (MAGP, Labbox, Spain) for 1.5 h. Three different temperatures were considered: 20, 30 and 40 °C. At the end of each rehydration period, 3 g of the obtained suspension was sampled in triplicate to determine the total solid content (TS). In turn, samples of 15 g of the suspension were also transferred into 50 mL centrifugation tubes and centrifuged at 11,872×g for 10 min at 4 °C. The supernatant was then filtered (Whatman nº 1 filter paper) under vacuum. After that, 3 g of the filtrate was sampled in triplicate for the determination of the soluble solid content (SS). Both TS and SS were determined by oven drying (Vaciotem, J.P. Selecta, Spain) at 102 °C for 24 h. The mass fraction of the dissolved solutes was calculated by applying Eq. 6.
Statistical Analysis
Analyses of variance (ANOVA), with one (drying pre-treatment or added solutes) or two factors (kind of sample and temperature of rehydration), were run to study the effect of process variables (drying pre-treatment technology, time of freeze-drying and solutes addition) on the measured properties. Means were compared by a significant difference test, with a confidence level of 95 % (p < 0.05), using the Statgraphics Centurion XV software program (StatPoint Technologies, Inc., Warrenton, VA, USA). Additionally, non-linear regression analyses were carried out in order to estimate the kinetic parameters using the SPSS Statistics 19 software program (IBM SPSS, Inc., New York, NY, USA) and were based on the Levenberg–Marquardt estimation method.
Results and Discussion
Kiwifruit Puree Characterization: Effect of Solute Addition and Drying Pre-treatments
The antioxidant capacity and some physicochemical properties of fresh kiwifruit were determined in order to control the fruit which was used as raw material in the present study. The average obtained values (and standard deviation) were 84.6 (0.8) g water/100 g product, 13.1 (0.6) g soluble solids/100 g liquid phase of the product, water activity 0.982 (0.003) and a radical scavenging capacity equivalent of 14.5 mM of Trolox/g of kiwifruit extract or 6.9 (1.3) mM of Trolox/ml of kiwifruit extract. These values are close to those reported by other authors (Antunes et al. 2010; De Ancos et al. 1999; Du et al. 2009; Fúster et al. 1994; Zolfaghari et al. 2010).
Kiwifruit puree samples were analysed after being pre-treated in order to check that the obtained water content levels before adding solutes were close to 65 g water/100 g product (Table 2). Both drying pre-treatments and the addition of solutes led to important variations in the physicochemical properties of the puree. In both cases, samples showed the expected reduction in moisture level and water activity and a rise in ºBrix (p < 0.05).
As regards the effect of the MW or HA drying technology used on the time necessary to reach the set moisture level (65 g water/100 g product), the MW treatment was much more effective than HA drying (Table 2), since it shortened the processing time by 92 %. Time reduction is often considered one of the main advantages of microwave drying technology, a fact which is frequently related to its high penetrative power and its volumetric heating (Clary et al. 2007; Maskan 2001; Vadivambal and Jayas 2007; Zhang et al. 2006).
Both MW and HA pre-treatments led to a significant reduction in the antioxidant capacity of kiwifruit puree samples (p < 0.05). Nevertheless, a residual antioxidant capacity of 82 % (6) was detected in MW pre-dried samples compared with one of 66 % (3) in the case of HA pre-dried ones. The greater duration of HA pretreatments (Table 2) could justify this difference. High temperatures favour the reactions that contribute to antioxidants compounds degradation; the greater the exposure time, the greater the degradation. Consequently, it can be assumed that microwave drying leads to a better antioxidant capacity conservation if compared with conventional technologies, such as hot-air drying.
Freeze-Drying Kinetic: Effect of Drying Pre-treatments
The contribution of microwave and hot-air drying pre-treatments to the kiwifruit freeze-drying kinetic was evaluated. Figure 2 shows the water activity and the water content of the fresh and pre-treated kiwifruit samples during the freeze-drying process. Along with the obtained data, the main moisture reduction took place during the first 12 or 24 h of the freeze-drying process for pre-treated MWD or HAD samples and for the fresh kiwifruit sample, respectively. From that moment onwards, the secondary drying is started and only a slight water content variation of the samples was observed, reaching a final water content level at the end of the entire process (48 h) of 1.07 (0.06), 5.280 (0.007) and 4.5 (0.1) g water/100 g product in F, MWD and HAD samples, respectively. These results underline the fact that, although a shorter time was necessary to reduce the water content in pre-treated samples, at the end of the FD treatment, the water content of MWD and HAD samples was not as effectively reduced as in the F sample. Water activity data corroborated the same behaviour (Fig. 2). This can be linked to the cryoprotective role played by the partial dehydration of the samples, in which a smaller amount of ice per gram of the water present is formed during the freezing step prior to freeze-drying (Fabra et al. 2009). In fact this cryoprotective effect is confirmed in kiwifruit samples when the glass transition temperature of the maximally cryoconcentrated matrix is analysed, this being −52, −58 and −60 °C for F, MWD and HAD samples, respectively (own data). Although these values correspond to kiwifruit without solutes added, a similar tendency in the actual samples could be expected.
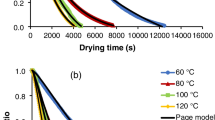
To analyse the freeze-drying kinetic of the samples, the moisture ratio was calculated (as indicated in Table 1) and plotted vs. time to obtain freeze-drying curves. Nine different models were used to fit experimental data, and the values of the corresponding constants appear in Table 3. Of these, the model with the highest adjusted R 2 value and the lowest RSME and χ 2 values would be the one that best explained the thin-layer drying characteristics. In this study, the adjusted R 2 and RMSE values were taken as the primary selection criteria. The results of the statistical computation undertaken to assess the consistency of the evaluated models are presented in Table 4. As can be observed, MWD samples showed lower adjusted R 2 and higher RMSE than F and HAD samples for all the studied models. This could be connected to the greater and more heterogeneous structural damage occurred due to microwave application (Contreras et al. 2007). Even though all the models were shown to fit the experimental data appropriately, the models which best described the freeze-drying process for F, MWD and HAD puree were the Page, logarithmic and Midilli–Kucuk models, respectively. These three models coincided very closely with the experimental data, as can be deduced from the RMSE and χ 2 values shown in Table 4, and can be considered to provide an appropriate description of the freeze-drying process of kiwifruit puree with or without the application of a drying pre-treatment. The experimental and predicted freeze-drying curves provided by the three selected models are presented in Fig. 3. Other authors have found that these three models adequately explain the drying kinetic of apricot, apple, strawberry, potato, pumpkin and corn when they were subjected to freeze-drying, microwave or hot-air drying processes (Akpinar 2006; Contreras et al. 2008; Doymaz and Pala 2003; Igual et al. 2012; Togrul and Pehlivan 2003).
Freeze-drying curves for kiwifruit puree samples. Experimental and predicted moisture ratio (Table 1) by using the model which fitted the best for fresh (experimental ( ) Page’s model (
) Page’s model ( )), microwave pre-dried (experimental (
)), microwave pre-dried (experimental ( ), logarithmic model (
), logarithmic model ( )) and hot-air pre-dried (experimental (
)) and hot-air pre-dried (experimental ( ), Midilli–Kucuk model (
), Midilli–Kucuk model ( ))
))
Page’s model was chosen from the three mentioned above to compare the samples’ drying constants. In the first place, this model has shown itself to be suited to describe the drying kinetics of a wide number of products of vegetable origin, such as kiwifruit, apple, apricot, mint leaves, strawberry and grapes (Contreras et al. 2008; Doymaz and Pala 2003; Igual et al. 2012; Karathanos and Belessiotis 1999; Simal et al. 2005; Therdthai and Zhou 2009). In second place, with respect to the other two selected models, it is the one that requires the lowest number of constants to describe the kinetics of the process. According to Pina-Pérez et al. (2007), models should be as simple as possible to ensure they are not only easy to use but easy to fit and in order to avoid errors associated with overparameterization. The kinetic parameters obtained from the fit of the Page model (Table 3) were within the range commonly observed for different agricultural products (Contreras et al. 2008; Igual et al. 2012; Fahloul et al. 2009; Karathanos and Belessiotis 1999; Prabhanjan et al. 1995). The n parameter was introduced in the Page model to overcome the shortcoming of the exponential model and represents an empirical modification of the processing time (Doymaz 2004; Simal et al. 2005). According to Simal et al. (2005), some authors have related the value of this parameter to the existence of an outer skin of the dried product, and it has been reported to be higher for products with a thicker skin, while others concluded that it was a function of air velocity and the initial water content. In our case, the n value was affected by the application of drying pre-treatments and was higher in the fresh sample. On the other hand, the k parameter has traditionally been linked to the drying rate of the process, so that the higher the k value, the higher the drying rate and, consequently, the shorter the process time (Karathanos and Belessiotis 1999; Contreras et al. 2008). According to Karathanos and Belessiotis (1999), the value of the k parameter can be linked to the food composition, porosity and the existence of an outer skin. In this study, the k value was found to be higher for both microwave and hot-air pre-treated samples than for the non-pre-treated puree. This indicates that the application of drying pre-treatments led to a faster freeze-drying of the samples, especially those subjected to MW drying, whose k value was six times higher than that of the fresh sample and 1.4 times higher than that of the HAD sample. This fact could be explained by the structure loss suffered by the cell walls which probably happens as a consequence of the application of a drying pre-treatment, which can reduce the water loss resistance (Maskan and Gögus 1998). This effect is greater when the MW treatment is applied due to the higher temperature reached in the sample and in the inner part of its structure. This leads to internal water evaporation which increases the porosity of the samples that also contributes to the greater structural damage (Contreras et al. 2007).
Effect of the Combined Drying Treatments on the Quality of the Kiwifruit Powder Products
The quality of the different kiwifruit powder products was evaluated through their solubility and their subsequent antioxidant activity. A review of the papers mentioned in bibliography points to the fact that solubility is the most widely assessed quality attribute of food powder (Marques et al. 2009; Troygot et al. 2011), and it depends directly on the drying technology used during product processing (Agnieszka and Andrzej 2010). On the other hand, the antioxidant capacity is considered as a relevant quality parameter in processed fruit since it is normally connected to the desirable properties associated with the regular intake of vegetable products (Beekwilder et al. 2005; Lu et al. 2011).
Figure 4 shows, at different temperatures, the mass fraction of the dissolved solutes of the samples which were freeze-dried for 24 h (a) and 48 h (b). At 20 °C, the samples freeze-dried for 24 h showed greater solubility than those freeze-dried for 48 h. Even though statistically significant differences were found in the DS values due to the rehydration temperature and the type of sample (p < 0.05), the results did not show a clear trend and the significance could be explained by the highly reduced variability of the obtained data. On the whole, powder products that were freeze-dried for 24 h showed excellent solubility (0.88–0.93), which did not seem to be influenced either by the temperature or the application of a pre-treatment. However, in the case of samples freeze-dried for 48 h, lower DS values were generally achieved (0.72–0.92). In this case, significant differences were observed in DS values (p < 0.05) in terms of the rehydration temperature, sample and the interaction of both factors. Generally, these samples exhibited lower solubility at 20 °C which increased when higher temperatures were used. Hence, from the rehydration point of view, it would be recommended to freeze-dry the kiwifruit puree for 24 h instead of 48 h. Taking these results into consideration, the effect of temperature rehydration as well as pre-treatment application on the antioxidant capacity was exclusively studied in samples that were freeze-dried for 24 h, as shown in Fig. 5. The antioxidant capacity of the kiwifruit puree rehydrated at 20 °C was found to be significantly lower than that analysed when the rehydration was carried out at a higher temperature (p < 0.05). This fact could be linked to the greater availability of the bioactive compounds at higher temperatures (Turkmen et al. 2005). Despite the fact that the antioxidant capacity of the samples was reduced because of pre-treatment application, at none of rehydration temperature were any significant differences found between the freeze-dried samples (p < 0.05). This fact can be explained by the higher stability of phenolic compounds in the pre-treated samples. Although drying pre-treatments might be responsible for the deterioration of a fraction of bioactive compounds, they can also inactivate enzymes related to the loss of antioxidant compounds, such as peroxidase and polyphenoloxidase (Dewanto et al. 2002; Turkmen et al. 2005).
Conclusion
The use of a freeze-drying process in combination with other drying technologies, especially microwave, can be considered a good alternative means of reducing the operating costs of freeze-drying without causing serious damages to the rehydration and antioxidant capacity of the product. However, some attention has to be paid to the processing conditions of the pre-drying treatments in order to obtain enough dried powder products. Freeze drying for just 24 h and rehydrating at 30 °C may be recommended as a means of increasing the mass fraction of dissolved solutes and the antioxidant capacity of the obtained product.
References
Agnieszka, C., & Andrzej, L. (2010). Rehydration and sorption properties of osmotically pretreated freeze-dried strawberries. Journal of Food Engineering, 97, 267–274.
Akpinar, E. K. (2006). Determination of suitable thin layer drying curve model for some vegetables and fruits. Journal of Food Engineering, 73, 75–84.
Antunes, M. D. C., Dandlen, S., Cavaco, A. M., & Miguel, G. (2010). Effects of postharvest application of 1-mcp and postcutting dip treatment on the quality and nutritional properties of fresh-cut kiwifruit. Journal of Agricultural and Food Chemistry, 58, 6173–6181.
Beekwilder, J., Jonker, H., Meesters, P., Hall, R. D., Van der Meer, I. M., & Vos, C. H. R. (2005). Antioxidants in raspberry: on-line analysis links antioxidant activity to a diversity of individual metabolites. Journal of Agricultural and Food Chemistry, 53, 3313–3320.
Cano, P. (1991). HPLC separation of chlorophyll and carotenoid pigments of four kiwi fruit cultivars. Journal of Agricultural and Food Chemistry, 39, 1786–1791.
Clary, C. D., Mejia-Meza, E., Wang, S., & Petrucci, V. E. (2007). Improving grape quality using microwave vacuum drying associated with temperature control. Journal of Food Science, 72(1), 23–28.
Contreras, C., Martín-Esparza, M. E., Chiralt, A., & Martínez-Navarrete, N. (2008). Influence of microwave application on convective drying: effects on drying kinetics, and optical and mechanical properties of apple and strawberry. Journal of Food Engineering, 88, 55–64.
Contreras, C., Martín-Esparza, M. E., Martínez-Navarrete, N., & Chiralt, A. (2007). Influence of osmotic pre-treatment and microwave application on properties of air dried strawberry related to structural changes. European Food Research and Technology, 224, 499–504.
Cozic, C., Picton, L., Garda, M., Marlhoux, F., & Le Cerf, D. (2009). Analysis of arabic gum: study of degradation and water desorption processes. Food Hydrocolloids, 23, 1930–1934.
Cubero, N., Monferrer, A., & Villalta, J. (2002). Aditivos alimentarios. Madrid: A. Madrid Vicente.
Daoussi, R., Vessot, S., Andrieu, J., & Monnier, O. (2009). Sublimation kinetics and sublimation end-point times during freeze-drying of pharmaceutical active principle with organic co-solvent formulations. Chemical Engineering Research and Design, 87, 899–907.
De Ancos, B., Cano, M. P., Hernández, A., & Monreal, M. (1999). Effects of microwave heating on pigment composition and color of fruit purees. Journal of the Science of Food and Agriculture, 79, 663–670.
Dewanto, V., Wu, X., Adom, K., & Liu, R. (2002). Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. Journal of Agricultural and Food Chemistry, 50, 3010–3014.
Doymaz, I. (2004). Effect of pre-treatments using potassium metabisulphide and alkaline ethyl oleate in the drying kinetics of apricots. Biosystems Engineering, 89(3), 281–287.
Doymaz, I. (2006). Drying kinetics of black grapes treated with different solutions. Journal of Food Engineering, 76, 212–217.
Doymaz, I., & Pala, M. (2003). The thin-layer drying characteristics of corn. Journal of Food Engineering, 60, 125–130.
Du, G., Li, M., Ma, F., & Liang, D. (2009). Antioxidant capacity and the relationship with polyphenol and vitamin C in Actinidia fruits. Food Chemistry, 113, 557–562.
Fabra, M. J., Talens, P., Moraga, G., & Martínez-Navarrete, N. (2009). Sorption isotherm and state diagram of grapefruit as a tool to improve product processing and stability. Journal of Food Engineering, 93, 52–58.
Fahloul, D., Lahbari, M., Benmoussa, H., & Mezdour, S. (2009). Effect of osmotic dehydration on the freeze drying kinetics of apricots. Journal of Food, Agriculture and Environment, 7, 117–121.
Fúster, C., Préstamo, G., & Cano, M. P. (1994). Drip loss, peroxidase and sensory changes in kiwi fruit slices during frozen storage. Journal of the Science of Food and Agriculture, 64, 23–29.
Fyfe, K. N., Kravchuk, O., Le, T., Deeth, H. C., Nguyen, A. V., & Bhandari, B. (2011). Storage induced changes to high protein powders: influence on surface properties and solubility. Journal of Food Science and Agriculture, 91, 2566–2575.
Gabas, A. L., Telis, V. R. N., Sobral, P. J. A., & Telis-Romero, J. (2007). Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. Journal of Food Engineering, 82, 246–252.
Ghosal, S., Indira, T. N., & Bhattacharya, S. (2010). Agglomeration of a model food powder: effect of maltodextrin and gum Arabic dispersions on flow behavior and compacted mass. Journal of Food Engineering, 96, 222–228.
Huang, L., Zhang, W., Mujumdar, A. S., & Lim, R. (2011). Comparison of four drying methods for re-structured mixed potato with apple chips. Journal of Food Engineering, 103, 279–284.
Igual, M., García-Martínez, E., Camacho, M. M., & Martínez-Navarrete, N. (2010). Effect of thermal treatment and storage on the stability of organic acids and the functional value of grapefruit juice. Food Chemistry, 118, 291–299.
Igual, M., García-Martínez, E., Martín-Esparza, M. E., & Martínez-Navarrete, N. (2012). Effect of processing on the drying kinetics and the functional value of dried apricot. Food Research International, 47, 284–290.
Jaya, S., & Das, H. (2009). Glass transition and sticky point temperatures and stability/mobility diagram of fruit powders. Food Bioprocess Technology, 2, 89–95.
Karathanos, V. T., & Belessiotis, V. G. (1999). Application of thin-layer equation to drying data of fresh and semi-dried fruits. Journal of Agricultural Engineering Research, 74, 355–361.
Lu, X., Wang, J., Al-Qadiri, M., Ross, C. F., Powers, J. R., Tang, J., & Rasco, B. A. (2011). Determination of total phenolic content and antioxidant capacity of onion (Allium cepa) and shallot (Allium oschaninii) using infrared spectroscopy. Food Chemistry, 129, 637–644.
Marques, L. G., Prado, M. M., & Freire, J. T. (2009). Rehydration characteristics of freeze-dried tropical fruits. LWT—Food Science and Technology, 42, 1232–1237.
Maskan, M. (2001). Drying, shrinkage and rehydration characteristics of kiwifruits during hot air and microwave drying. Journal of Food Engineering, 48, 177–182.
Maskan, M., & Gögus, F. (1998). Sorption isotherms and drying characteristics of mulberry (Morus alba). Journal of Food Engineering, 37, 437–449.
Menlik, T., Özdemir, M. B., & Kirmaci, V. (2010). Determination of freeze-drying behaviours of apples by artificial neural network. Expert Systems with Applications, 37, 7669–7677.
Mimouni, A., Deeth, H. C., Whittaker, A. K., Gidley, M. J., & Bhandari, B. R. (2009). Rehydration process of milk protein concentrate powder monitored by static light scattering. Food Hydrocolloids, 23, 1958–1965.
Mosquera, L. H., Moraga, G., & Martínez-Navarrete, N. (2010). Effect of maltodextrin on the stability of freeze-dried borojó (Borojoa patinoi Cuatrec.) powder. Journal of Food Engineering, 97, 72–78.
Mosquera, L. H., Moraga, G., & Martínez-Navarrete, N. (2012). Critical water activity and critical water content of freeze-dried strawberry powder as affected by maltodextrin and arabic gum. Food Research International, 47, 201–206.
Panchariya, P. C., Popovic, D., & Sharma, A. L. (2002). Thin-layer modeling of black tea drying process. Journal of Food Engineering, 52, 349–357.
Pardo, J. M., & Leiva, D. A. (2010). Effects of different pre-treatments on energy consumption during freeze drying of pineapple pieces. Interciencia, 35(12), 934–938.
Pina-Pérez, M. C., Rodrigo-Aliaga, D., Saucedo-Reyes, D., & Martínez-López, A. (2007). Pressure inactivation kinetics of Enterobacter sakazakii in infant formula milk. Journal of Food Protection, 70(10), 2281–2289.
Prabhanjan, D. G., Ramaswamy, H. S., & Raghavan, G. S. V. (1995). Microwave-assisted convective air drying of thin layer carrots. Journal of Food Engineering, 25, 283–293.
Schokker, E. P., Church, J. S., Mata, J. P., Gilbert, E. P., Puvanenthiran, A., & Udabage, P. (2011). Reconstitution properties of micellar casein powder: effects of composition and storage. International Dairy Journal, 21, 877–886.
Simal, S., Femenia, A., Garau, M. C., & Roselló, C. (2005). Use of exponential, Page’s and diffusional models to simúlate the drying kinetics of kiwi fruit. Journal of Food Engineering, 66, 323–328.
Therdthai, N., & Zhou, W. (2009). Characterization of microwave vacuum drying and hot air drying of meant leaves (Mentha cordifolia Opiz ex Fresen). Journal of Food Engineering, 91, 482–489.
Togrul, I. T., & Pehlivan, D. (2003). Modelling of drying kinetics of single apricot. Journal of Food Engineering, 58, 23–32.
Troygot, O., Saguy, I. S., & Wallach, R. (2011). Determination of characteristic curve from water sorption isotherms. Journal of Food Engineering, 105, 408–415.
Turkmen, N., Sari, F., & Velioglu, S. (2005). The effect of cooking methods on total phenolics and antioxidant activity of selected green vegetables. Food Chemistry, 93, 713–718.
Vadivambal, R., & Jayas, D. S. (2007). Changes in quality of microwave treated agricultural products. Biosystems Engineering, 98, 1–16.
Xiang, J., Hey, J. M., Liedtke, V., & Wang, D. Q. (2004). Investigation of freeze-drying sublimation rates using a freeze-drying microbalance technique. International Journal of Pharmaceutics, 279, 95–105.
Zhai, S., Taylor, R., Sanches, R., & Slater, N. K. H. (2003). Measurement of lyophilisation primary drying rates by freeze-drying microscopy. Chemical Engineering Science, 58, 2313–2323.
Zhang, M., Tang, J., Mujumdarc, A. S., & Wang, S. (2006). Trends in microwave related drying of fruits and vegetables. Trends in Food Science and Technology, 17, 524–534.
Zolfaghari, M., Sahari, A., Barzegar, M., & Samadloiy, H. (2010). Physicochemical and enzymatic properties of five kiwifruit cultivars during cold storage. Food Bioprocess Technology, 3, 239–246.
Acknowledgments
The authors thank the Ministerio de Educación y Ciencia and the Generalitat Valenciana for the financial support given throughout the Projects AGL 2010-22176 and ACOMP/2012/161, respectively.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Benlloch-Tinoco, M., Moraga, G., del Mar Camacho, M. et al. Combined Drying Technologies for High-Quality Kiwifruit Powder Production. Food Bioprocess Technol 6, 3544–3553 (2013). https://doi.org/10.1007/s11947-012-1030-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-1030-3



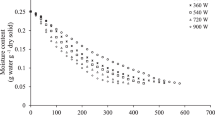


 ), MWD (
), MWD ( ) and HAD (
) and HAD ( )) during freeze-drying
)) during freeze-drying

 ), MWD (
), MWD ( ) and HAD (
) and HAD ( ) samples at different temperatures (20, 30 and 40 °C) and freeze-drying times (24 h (a) and 48 h (b))
) samples at different temperatures (20, 30 and 40 °C) and freeze-drying times (24 h (a) and 48 h (b))
 ), MWD (
), MWD ( ) and HAD (
) and HAD ( ) samples after 24 h of freeze-drying and at different rehydration temperatures (20, 30 and 40 °C)
) samples after 24 h of freeze-drying and at different rehydration temperatures (20, 30 and 40 °C)