Abstract
Strawberry halves, with and without osmotic pre-treatment, were dried at 40 °C air temperature, with and without microwave application (0.2 W/g). Changes in volume, pectic fractions, mechanical behaviour and glass transition temperature due to dehydration treatments were evaluated. Microwave application resulted in an increased water soluble pectic fraction as a consequence of both protopectin and oxalate soluble pectic fraction reductions. This solubilisation leads us to obtain dried samples with a more rigid, firmer structure which, indeed, showed greater glass transition temperature values. Nevertheless, these samples are softer when rehydrated, thus indicating a greater structural damage. The sugar presence induced by an osmotic dehydration pre-treatment seems to reinforce the solid cellular matrix, which is evident from the mechanical behaviour of rehydrated samples. So, the greatest structural damage caused by microwaves may be reduced by an osmotic dehydration pre-treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Most fruits are consumed when fresh, but their market availability is commonly limited by the short shelf life and seasonal production throughout the year. This is the case of strawberry, a crop of which 95% is cultivated from March to June in the south of Spain (Huelva). Additionally, fresh strawberries have a short shelf life, even less than a week [1], mainly due to fungal attack and mechanical damage during distribution. In this sense, preservation methods that lengthen the shelf-life of strawberries, increasing their market availability, such as freezing, freeze-drying, dehydration, etc., are very useful [2, 3]. Nevertheless, all of them induce modification of fruit quality attributes (texture, colour and flavour) to a different extent.
Drying is an old widely used preservation technique, which greatly affects final product quality. Dehydrated product quality not only depends on the drying treatment and final water content, but also on raw material characteristics, pre-treatments and storage conditions. Several works [4–7] suggest microwave application in combination with air drying in order to reduce the process time improving the product quality. Nevertheless, a better understanding of the physical changes that products undergo during microwave drying is needed to design a more efficient drying method. On the other hand, pre-treatments such as osmotic dehydration prior to air drying may contribute to preserve some quality attributes such as colour and texture, due to the fact that the product dehydration occurs at moderate temperatures and water elimination occurs without phase changes [6, 8, 9]. Any of these drying procedures (osmotic dehydration, microwaves and air) will imply important changes in the product but a combination of them could imply some improvement in final product quality [10, 11].
During any technological transformation process, irreversible structural changes take place in the product, and these cause appreciable alterations of texture that may be interpreted by means of some physical parameters measured in mechanical tests [12]. The mechanical properties are closely related to the physical state, glassy or rubbery, achieved by the product phases [13, 14] and to the structure developed as a result of induced deformations (shrinkage(swelling) in cells and intercellular spaces and of ruptures of cellular bonds taking place throughout the drying process. Previous studies also showed that the drying process induces changes in the pectic substances due to the structural modification of the cell wall, which are also related to textural changes in dehydrated products [15–17]. In the case of strawberry, the changes induced on cross-links between calcium and adjacent polyuronide chains may be relevant as they represent a key factor in the maintenance of fruit firmness [2].
In this work, the effect of drying treatment: air drying or combined air-microwave drying, with and without pulsed vacuum osmotic dehydration pre-treatment, on some structure related properties of strawberry (var. Camarosa) was analysed. Changes which occur in the structural polymers (pectic fractions), glass transition temperature of the liquid phase present in dried fruit and the mechanical properties (puncture test) of the fresh, dried and rehydrated product were analysed.
Materials and methods
Materials
Strawberries (var. Camarosa) were purchased in a local market and visually selected according to homogeneity in size (about 35 mm longest axis), shape (malformed pieces were excluded), and redness (fruits surface completely red). After washing, the strawberry samples were cut longitudinally into two halves and each one identified. One of these was used for measurements in the fresh sample and the other one was submitted to drying treatments and afterwards analysed.
Drying experiments applying hot air and microwaves were carried out on fresh and pre-osmodehydrated samples. An osmotic dehydration pre-treatment was carried out with 55 °Brix sucrose solution at 25 °C, under stirring conditions, by applying one vacuum pressure pulse (50 mbar) for 5 min at the beginning of the process and then restoring the atmospheric pressure, keeping the samples immersed for 3 h more. These pre-treated samples were noted as PVOD. A mass ratio 1:20 for fruit sample-osmotic agent was used. After PVOD process, samples were taken out of the osmotic solution and gently blotted (with a paper towel) to remove adhering osmotic solution.
Drying treatments
Strawberry halves with or without PVOD pre-treatment were dried by air drying (AD) and by combined air-microwave technique (AD+MW), using a modified household microwave oven with a mode stirrer and coupled to a balance, where air temperature and velocity and microwave incident power may be controlled [18, 19]. For the experiments, air stream passed through the oven at 2.6 m/s inside the cavity, relative humidity ranged between 35–45%, air temperature was 40 °C and microwave incident power 0.2 W/g fruit sample. Strawberry halves (15 pieces) were placed cut side up on drier grid to favour the mass transfer, which occurs mainly through the cut surface of the fruit. All samples were dried to 10 g water/100 g sample, which was controlled through the continuous monitoring of sample mass and taking into account the initial moisture content. The required drying time was (37±4) h for AD samples, (41±1) h for PVOD-AD samples, (9±1) h for AD+MW samples and (11±1) h for PVOD-AD+MW samples. The greater drying time required in PVOD pre-treated samples could be attributed to the presence of sugar in the fruit surface that could induce a surface hardening.
Each drying treatment was carried out on 15 halves and it was repeated four times to obtain 60 dried halves in each case, in order to analyse the mechanical properties, pectin fractions, glass transition and volume.
Analytical determinations
Fresh and osmodehydrated samples were characterized as to water content, by vacuum drying at 60 °C and 50 mmHg until constant weight was achieved [20], and soluble solid content (°Brix) by using a refractometer (ABBE ATAGO 89553 of Zeiss) at 20 °C. Water activity was measured at 25 °C by using a dew point hygrometer (Aqualab CX-2) and pH was determined with a pH-meter (MicropH 2001, Crison).
Uronic acid content was determined as an indicator of pectin content in each fraction isolated: water soluble pectin (WSP), oxalate soluble pectin (OSP) and total pectin (TP). These fractions were obtained by selective extraction of homogenized samples, according to the method described by [17, 21]. Extraction (eight per drying treatment) was carried out from both fresh and dried samples. Two strawberry halves were used for each extraction. To make the homogenisation of dried samples easier, these were previously rehydrated with exactly the same amount of water that was lost during drying. After soaking for 10 h at 5 °C (long enough to ensure the maximum water gain), mechanical properties were measured as described below. Then, rehydrated samples, together with the remaining water, were homogeneized for pectin analysis with an Ultra-Turrax (8000 rpm for 2 min). Generally, the water extraction solubilizes loosely bound pectins from the cell wall, whereas the oxalate extraction us to obtain the ionically bound pectin enriched fraction [22]. Galacturonic acid (GalA) content in the different fractions was determined colorimetrically at 520 nm (16 replicates per treatment), as described by [23], by using the m-hydroxydiphenyl method. Results were expressed as g GalA/100 g of initial fresh sample (even in the dried samples). The difference between TP and the sum of WSP and OSP was taken into account to estimate the amount of non-extractable pectin (NXP), which was the protopectin fraction.
Mechanical properties of fresh, dried and rehydrated samples were analysed at 25 °C by using a puncture test with a Universal Texture Analyzer (TA.XT2, Stable Micro Systems). A cylindrical 2 mm diameter punch was used at a penetration rate of 1.5 mm/s until total sample penetration. For each drying treatment, this test was carried out on 20 halves of fresh strawberry and the corresponding twenty dried halves. The 16 rehydrated halves used to pectin fractions analysis were previously characterized as to mechanical properties.
Glass transition temperature of dried samples (10 halves for each drying treatment) was measured by differential scanning calorimetry (DSC 220CU, Seiko Instruments) after equilibration of samples to 0.33 water activity, that ensure the homogenous water content in the samples of 10 g/100 g sample, according to the water sorption data previously reported [3]. To this end, about 10 mg of each sample were placed in aluminium open pans (P/N SSC000C008) and stored in a hermetic chamber together with a jar containing a saturated solution of MgCl2 (relative humidity/0.33, [3]). Samples were weighted periodically and equilibrium was assumed when weight differences ≤0.001 g were detected. At that moment, pans were sealed and analysed. Heating rate of samples in the calorimeter was 5 K/min and the analysis was carried out from −40 to 80 °C. Midpoint of the observed transition was considered. The water content of each sample after the thermal analysis was confirmed by drying in a vacuum oven at 60 °C till constant weight. To this end pans were previously holed and a AND ER-182A balance (sensitivity 0.00001 g), was used.
Sample volume change during each drying treatment was determined in 14 strawberries by measuring volume of both fresh (V o) and dried (V f) samples (in the respective halves from the same fruit piece) through the volume displacement using a pycnometer at 20 °C. Water and toluene were used as reference liquid in fresh and dried samples, respectively. Volume reduction of samples was calculated as ΔV=(V o−V f)/V o. In these experiments, the mass of the two strawberry halves involved in each determination was measured and only data with coefficient of variation of mass halves lesser than 3% were considered.
Statistical analysis
Analyses of variance (ANOVA) were carried out to study the effect of process conditions on the measured properties of the samples, by using the Statgraphics Plus software version 5.1 [24].
Results and discussion
Strawberries selected for the study showed mean values of water content (0.912±0.013) g/g sample, °Brix 7.8±1.4, pH 3.6±0.2 and water activity 0.991±0.003. Due to the osmodehydration, the sample water content decreased to 0.863±0.009 and °Brix increased up to 10.1±0.7.
Changes in pectic composition due to drying treatment
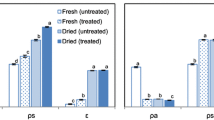
Table 1 shows the galacturonic acid content, referred to 100 g of initial fresh sample, analysed in the total pectin and in the different pectic fractions isolated from samples submitted to the different drying treatments and from fresh sample. GalA content analysed in TP extracted from fresh strawberry was in the range established by several authors [22, 25] for this fruit. The oxalate soluble pectins represent the major pectic fraction in the fresh sample, which is not usual in other fruits such as apple [17]. The lower degree of esterification of strawberry pectins is responsible for the greater amount of oxalate soluble pectins. This pectin fraction can bind calcium forming a cross-link structure [26], and it is reported to come from the middle lamella region [27]. The minor fraction was the protopectin, which is present in fresh sample as (0.09±0.04) g GalA/100 g fresh samples.
No significant differences (P 0.05) in total pectin content were observed between fresh samples and those submitted to the different drying treatments, the mean value being (0.54±0.07) g GalA/100 g fresh sample. Nevertheless, GalA content in the different pectic fractions was affected by drying process, which provoked a significant (P<0.05) WSP increase and NXP decrease in all treatments, as well as a significant OSP decrease, except for AD samples, when compared to fresh strawberry levels. These results suggest that the treatments induce changes in the protopectin present in the fresh sample that contribute to an increase in the WSP fraction and affect the calcium low methoxyl pectin bonding, thus helping to change the solubility of part of the initial OSP fraction of fresh fruit. Obtained results for AD samples indicate that calcium linking to polysaccharide chain was not notably affected in this case.
Changes in WSP and OSP fractions due to treatments were especially important when microwaves were applied. These results may be related with a greater sample heating reached under microwave conditions that may promote greater changes in pectin solubilisation. PVOD pre-treatment significantly affects pectin solubility (increase of WSP and decrease of OSP) when no microwave was applied. As a result of the vacuum step, impregnation of intercellular spaces of sample tissue with osmotic solution occurs, which seems to favour the pectin solubilisation during subsequent drying process. Nevertheless, the effect of impregnation was negligible when microwaves were applied, probably due to the predominant influence of product heating on the pectin solubility/structure changes. Similar effects were observed in apple tissue when submitted to drying treatments applying microwaves and impregnation pre-treatments [17].
Mechanical properties
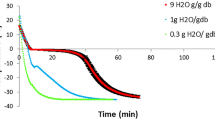
Figure 1a shows an example of force-penetration depth curves obtained from the puncture tests carried out on dried samples. Only one fracture peak could be observed, regardless of drying conditions. Parameters obtained from these curves were: the maximum force required to punch the sample (F max), the deformation at this point (d) and the slope (S i) of the curve in the linear zone prior to fracture point. The peak of maximum force is related to the product resistance to fracture or sample firmness [6] and the slope of the linear part of force-penetration depth curves is related to sample resistance to deformation (rigidity).
Table 2 shows the values of mechanical parameters obtained for the different dried samples. PVOD pre-treated samples and samples obtained with microwave application showed significantly (P<0.05) higher values of slope and maximum force but a similar d value (P>0.05). Both impregnation-osmotic pre-treatment and microwave application promote the dried sample mechanical resistance and rigidity. In fact, samples obtained by combined PVOD-AD+MW showed the highest values of all force-dependent parameters.
Differences in mechanical behaviour of the dried samples must be related with the differences induced in the composition of the soluble water phase and in the solid matrix during treatments. The increase in soluble pectin during drying, and in sugar content in PVOD pre-treatments, in the sample aqueous phase modifies its consistency. On the other hand, alteration of cell bonding zones and cell structure changes the solid matrix resistance. In this sense PVOD pre-treatment reinforce the dried sample mechanical resistance as well as the microwave application.
The different impact of treatments on cellular structure may be better observed in rehydrated samples [17]. The influence of drying conditions on the shape of the force-penetration depth curves obtained for the rehydrated samples can be observed in Fig. 1b and c. Fresh sample (before drying) was also considered in this case in order to compare the mechanical response of rehydrated strawberry with the initial fruit behaviour. In the fresh sample, two fracture peaks, related with the resistance (firmness) offered by the strawberry epidermis (skin) and the pulp, respectively, to punch advance were observed. In rehydrated samples, the two peaks were only observed for PVOD pre-treated samples (Fig. 1b) whereas only one peak with very low force values were observed in AD treated samples (Fig. 1c). For rehydrated samples, the obtained average force values (F) at the rupture peaks and the corresponding deformation (d) are summarized in Table 2. From these parameters, a greater resistance to fracture and recuperation of the height of rehydrated PVOD samples than non pre-treated ones was observed. The negative effect of microwave on mechanical resistance of rehydrated samples was observed in both cases. This seems to indicate that PVOD pre-treatment preserves the fruit matrix structure much better whereas microwave application provokes a greater structural alteration. So, some interactions between impregnated sugar and cellular matrix components may be responsible for the observed behaviour. In fact, it has been reported in a previous work [28] that cells protected by sugars exhibited less damage to the middle lamella and less severe shrinkage during drying. The less marked shrinkage of PVOD pre-treated samples was also observed in this work, the sample volume reduction after drying in these cases being 86–87% for PVOD samples compared to 89–90% for non pre-treated dried samples.
Calorimetric analysis
Calorimetric analysis allowed us to determine the T g of the product, and therefore the critical point when the change from a glassy to a rubbery state takes place. Figure 2 shows, as an example, one of the thermograms obtained by DSC for each kind of dried samples. In an idealized way, a fruit can be considered as a multiphase system: water and water soluble compounds forming a liquid aqueous phase and water compatible non-soluble compounds with different hydration levels. Taking into account the ratio in which water soluble-insoluble compounds are present in the strawberry tissue (about 8:1), the observed transitions must be assigned to the solutes present in this water soluble phase (sugars, soluble pectin, etc.) [3]. In this sense, PVOD pre-treatment, which increases the sugar content of the samples, and also the drying treatments, which promote the pectin solubilisation, will change the average molecular weight of solutes in the aqueous phase. This change has relevant repercussions on the T g values of the product liquid phase, since T g increases as the average molecular weight of the system increases [29, 30].
Mean T g values for samples from different drying treatments are shown in Table 3. Significant differences were detected among samples dried with different treatments. Differences may be explained taking into account the final composition of dried samples. Table 3 shows the water and soluble solid fraction determined in the samples submitted to DSC analysis, as well as the WSP fraction. Soluble solid fraction was determined from the soluble solid fraction in the liquid phase (°Brix/100) of osmodehydrated or fresh samples and applying the corresponding mass balances for drying operation. As can be observed in Table 3, samples osmotically pre-treated showed a significantly (P<0.05) greater soluble solid content, due to the external sucrose gain. Nevertheless, the ratio x WSP/x s was lower in PVOD-AD samples and PVOD-AD+MW samples (0.025±0.004 and 0.023±0.003, respectively), than in AD (0.034±0.003) or AD+MW (0.042±0.004) ones. From these data a lower average molecular weight could be expected for soluble compounds of liquid phase in PVOD pre-treated samples. This fact agrees with the lower T g values observed for them.
Conclusions
PVOD pre-treatment and changes in pectin solubility which occur during drying affect both the average molecular weight of solutes present in the fruit aqueous phase and the cell bonding forces supporting the cellular structure. These two factors have a great impact on T g and mechanical response of dried and rehydrated product. All samples with 10% water content obtained from the different treatments are at rubbery state at the usual temperatures of storage and commercialisation, which inhibits the sample fracture during their handling but increase sample deterioration rate. In this sense the maximum stability was obtained for microwave treated samples which showed the greater T g values. These samples are also the most firm and rigid when dried, although their structure becomes seriously affected during drying which give rise to a very soft texture after rehydration. Samples PVOD treated showed the lowest T g values and so a greater instability, but their structure was much better preserved during drying and they showed the best mechanical behaviour after rehydration.
References
Lara I, García P, Vendrell M (2004) Postharvest Biol Tec 34:331–339
Suutarinen J, Honkapää K, Heiniö R, Autio K, Mokkila M (2000) LWT-Food Sci Technol 33:188–201. DOI 10.1006/fstl.2000.0638
Moraga G, Martínez-Navarrete N, Chiralt A (2004) J Food Eng 62:315–321
Feng H, Tang J (1998) J Food Sci 63:679–683
Funebo T, Ahrné L, Kidman S, Langton M, Skjoldebrand C (2000) J Food Eng 46:173–182
Prothon F, Ahrné L, Funebo T, Kidman S, Langton M, Sjoholm I (2001) LWT-Food Sci Technol 34:95–101. DOI 10.1006(fstl.2000.0745
Torringa E, Esveld E, Scheewe I, Van Den Berg R, Bartels P (2001) J Food Eng 49:185–191
Wrolstad R, Skrede G, Lea P, Enersen G (1990) J Food Sci 55:1064–1065, 1072
Torreggiani D, Forni E, Guercilena I, Maestrelli A, Bertolo G, Archer G, Kennedy J, Bone S, Blond G, Contreras-Lopez E, Champion D (1999) Food Res Int 32:441–446
Aguilera JM, Chiralt A, Fito P (2003) Trends Food Sci Tech 14:432–437
Fito P, Chiralt A (2003) Food Sci Technol Int 9:151–156
Alonso J, Canet W (1994) J Sci Food Agric 66:1–7
Roos YH, Roininen K, Jouppila K, Tuorila H (1998) Int J Food Prop 1:163–180
Martínez-Navarrete N, Moraga G, Talens P, Chiralt A (2004) Int J Food Sci Technol 69:555–562
Forni E, Torreggiani D, Battiston P, Polesello A (1986) Carbohydr Polym 6:379–393
Torreggiani D, Forni D, Maestrelli A, Quadri F (1998) Influence of osmotic dehydration on texture and pectic composition of kiwifruit slices. In: Proceedings of the 11th international drying symposium (IDS’98), Halkidiki, Greece, pp 930–937
Contreras C, Martín ME, Martínez-Navarrete N, Chiralt A (2005) LWT-Food Sci Technol 38:471–477. DOI 10.1016/j.lwt.2004.07.017
Martín ME, Martínez-Navarrete N, Chiralt A, Fito P (2003) Alimentación. Equipos y Tecnología 181:101–107
Martín ME, Fito P, Martínez-Navarrete N, Chiralt A (1999) Combined air-microwave drying of fruit as affected by vacuum impregnation treatments. In: Proceedings of the 6th Conference of Food Engineering, Dallas, TX, pp 465–470
AOAC (1980). Official methods of analysis (13th ed.). Association of official analytical chemists, Washington DC
Yu L, Reitmeier C, Love M (1996) J Food Sci 61:844–846
Rosli H, Civello P, Martínez G (2004) Plant Physiol Biochem 42:823–831
Kinter P, Van Buren J (1982) J Food Sci 47:756–764
Statgraphics Plus 5.1 for Windows (2000) Statistical Graphics Corporation. StatPoint, Inc., Virginia, USA
Kader A (1992) Quality and safety factors: definition and evaluation for fresh horticultural crops. In: postharvest technology of horicultural crops, University California Special Publication 3311, DANR, Oakland, pp 185–189
Sirisomboon P, Tanaka M, Fujita S, Akinaga T, Kojima T (2001) J Food Compos Anal 14:83–91
May C (1997) Pectins. In: Imeson A (ed) Thickening and gelling agents for food. Blackie Academic professional, pp 230–261
Tregunno N, Goff H (1996) Food Res Int 29:471–479
Rao M (1998) Phase(State Transitions in Foods. Chemical, structural and rheological changes. Marcel Dekker, Inc., New York
Roos Y (1995) Phase transitions in food. Academic Press, Inc., New York
Acknowledgements
The authors thank to the Ministerio de Ciencia y Tecnología and to the Fondo Europeo de Desarrollo Regional (FEDER) for the financial support throughout the project AGL2005-05994.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Contreras, C., Martín-Esparza, M.E., Martínez-Navarrete, N. et al. Influence of osmotic pre-treatment and microwave application on properties of air dried strawberry related to structural changes. Eur Food Res Technol 224, 499–504 (2007). https://doi.org/10.1007/s00217-006-0345-6
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00217-006-0345-6