Abstract
Purpose
Hilsa (Tenualosa ilisha) is a commercially significant fish, which is popular as seafood. Although it has a high market value, salted hilsa is usually spoiled by various microorganisms and their metabolic activities that lead to the deterioration of fish quality. Therefore, it is necessary to control the physicochemical and microbiological characteristics of high-quality hilsa products by standardizing the process parameters during their production.
Methods
Hilsa filets were heat-treated at 75 °C for 5 min and compared with high-pressure-processed samples at different temperatures (40, 50, and 60 °C), pressures (300, 400, and 500 MPa), and hold duration (5, 10, and 15 min).
Results
The physical, chemical, and microbiological analyses of high-pressure-processed (500 MPa/5 min/50 °C) hilsa fish curry in retort pouch were found to be the most suitable treatment to retain the physicochemical parameters and reduce the microbial load. Moreover, the high-pressure-processed product also received higher sensory scores for texture and color (8.3 and 7.3 out of 10, respectively) compared with the heat-processed curry.
Conclusions
All the high-pressure treatments showed retention of quality attributes in terms of physical, chemical, and microbiological characteristics of ready-to-eat hilsa curry. This study presents an effective high-pressure processing approach to produce the best quality ready-to-eat hilsa product.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Hilsa (Tenualosa ilisha) is a popular fish in South Asia due to its delicious taste (Chouhan et al. 2015). Moreover, hilsa is highly beneficial for human health due to its high essential fatty acid, protein, vitamin, and mineral content (Ganguly et al. 2018). Hilsa curry, prepared with mustard oil and mustard seed paste, is a very popular dish due to its distinctive flavor and texture. Hilsa is rich in omega-3 polyunsaturated fatty, eicosapentaenoic, and docosahexaenoic acids (Mohanty et al. 2012). The consumption of these fatty acids reportedly controls cholesterol and insulin levels (Quazi et al. 1994) and assures an optimal essential nutrient intake (Salam et al. 2005). Hilsa is a commercially important traded seafood with high lipid content, which also influences its market value (Nowsad 2007). However, hilsa is highly perishable (Herbert and Shewan 1976) and usually spoiled by various microorganisms due to their high metabolic activities, leading to the deterioration of fish quality (Nowsad 2010) and reduced product shelf life. Traditionally, hilsa is preserved through salting, smoking, or drying (Hossain et al. 2012). However, these approaches might lead to undesirable changes in the nutritional quality of the product and require the addition of preservatives.
Thermal treatment is a predominant method to achieve the microbial inactivation. It is efficient, cost-effective, and readily available, though the high-temperature treatment might cause quality degradation in color and texture, which in turn could reduce consumer acceptance. Hilsa filet thermal treatment causes protein denaturation, which further reduces water holding capacity and stricken muscle fibers, subsequently leading to a harder and more compact tissue texture (Harris and Shorthose 1988).
Consumer interest for safe, minimally processed, stable foods with less additives and good maintenance of product appearance, flavor, color, texture, and dietary characteristics has been the main impetus of business use of non-thermal food processing methods, such as high-pressure processing (HPP). During HPP, the pressure is transmitted rapidly and consistently throughout a system irrespective of the shape of the packaging or volume of the product. The commercial impact of HPP is growing rapidly in the food industry, owing to its microorganism inactivation efficacy and operation at low temperatures. Furthermore, pre-packed products can be HPP-treated irrespective of their size or shape, which represents an added advantage for processing. Compared with conventional techniques, the HPP treatment extends shelf life and improves microbial food safety without affecting the flavor, color, and nutritional quality. Hicks et al. (2009) conducted a survey to assess the willingness of the consumers to pay more for ready-to-eat HPP products. The results showed that approximately 40% of the population were willing to pay more for the HPP-processed ready-to-eat products. Additional data and scientific studies show that the consumer base has further increased for HPP-treated products. Mengden et al. (2015) studied the effect of HPP on smoked and fresh fish filets. They reported that HPP treatments at 600 MPa for 5 min resulted in microbiologically safe fish samples throughout the storage period. They also observed that there was no further microbial growth during refrigerated storage. However, the sensory characteristics such as appearance, color, and texture reportedly vary in different fish samples mainly due to protein denaturation (Matser et al. 2000). Conventionally processed food products, packed in metal cans, are popular worldwide. Nevertheless, a significant impediment of metal can utilization is the taste conferred to the product upon storage. To overcome this problem, the retort pouch packing technique during HPP is gaining importance due to its safe and environment-friendly properties. Furthermore, they are flexible and suitable for in-pack processing and shelf-stable pouches (Awuah et al. 2007). The retort pouch has been generally perceived as an alternative to metal can for increasing packed food stability (Gopal et al. 2001; Dhanapal et al. 2010).
The present study was undertaken to investigate the effect of thermal and high-pressure processing treatments on physicochemical and microbiological characteristics, and standardize the process parameters for obtaining high-quality ready-to-eat hilsa curry.
Materials and Methods
Raw Material Collection and Ingredient Preparation
Hilsa fish samples with an average weight of 600–700 g were procured from the local fish market of Kharagpur, West Bengal, India. Samples were transported under chilled (ice storage) conditions. The samples were skinned, gutted, fileted, washed, and cut into pieces of an equal weight of 55–60 g. Next, ingredients were mixed properly and cooked under a mild-heating condition for 5 min. Ingredient quantity and cooking time or procedure may vary from region to region, person to person, or time to time depending on taste. The standard regional Bengali recipe was followed while sample cooking during the experiment (Appendix 1). A similar recipe was used by Gopal et al. (2001) to study the shelf life of traditional Kerala style fish curry during storage in the indigenous retort pouch. In contrast, local Bengali people were the target consumers in the present study; some changes were thus made during preparation according to the local recipe. However, every possible safeguard was taken care of to retain the nutritional quality of the cooked product.
Thermal Processing
Hilsa filets and pre-cooked ingredients were mixed properly and then filled into a retort pouch sized 18 × 10 cm. All pouches weighed 100 ± 5 g and were sealed using a vacuum sealing machine to remove the air entrapped inside the pouch. Based on preliminary experiments, the packed hilsa was thermally treated at different temperatures (75–121 °C) for varying durations (5–15 min). From preliminary trials, the treatment at 75 °C for 5 min was selected and further compared with the HPP-treated samples. Similarly, the treatment was recommended previously by Chouhan et al. (2015).
After the treatments, the samples were then immediately cooled to an ambient temperature using ice-cold water. After completion of thermal processing, the samples were stored in the refrigerator at 4 °C for further analysis.
High-Pressure Processing
The sealed retort pouches including the samples were covered with low-density polyethylene (LDPE) pouches of an 80-μm thickness, following which, vacuum sealing was repeated. A high-pressure food processor batch model, i.e., an HPP machine (S-IL-100-250-09-W, Stansted Fluid Power Ltd., Essex, UK) was used for the treatment of the packed samples. The samples were loaded into the vessel and treated at 300, 400, and 500 MPa for holding times of 5, 10, and 15 min at the process temperatures of 40, 50, and 60 °C, respectively. All procedure parameters were set through a PC interface with programmable logic controller (PLC). After the treatment, the samples were stored in a refrigerator at 4 °C temperature for further proximate analysis and comparison study with thermally treated samples.
Determination of the Microbiological and Physicochemical Characteristics
Microbiological Analysis
The microbiological analysis was performed according to the standard APHA protocol ( 2001). The total plate count (TPC) analysis was performed by mixing 10 g of aseptically cut sample with 90 mL of (N. Saline) diluents. This resulted in a 10-fold diluted sample. For further dilutions, 1 mL from the 10−1 dilution was mixed with 9 mL of diluents (10−2 dilution). One milliliter of the suitable dilution was plated on tryptone glucose agar (TGA). Enterobacteriaceae are a family of Gram-negative, non-spore-forming bacteria, including foodborne pathogens such as Salmonella spp., pathogenic Escherichia coli, and Shigella spp. Suitable dilution (1 mL) was plated on Violet Red Bile Glucose Agar (VRBGA) and incubated at 37 °C for 36–48 h, and then red small (2–4-mm diameter) colonies were counted and considered as Enterobacteriaceae colonies. Both results were expressed as log colony-forming unit per gram (CFU/g) of the sample.
pH Measurement
For this experiment, 5-g sample was homogenized with 25 mL of distilled water for 1 min, then the pH was measured using a pH meter (pH meter CL 46+, Toschon Industries Pvt. Ltd., India) by inserting a pH probe into the sample homogenate.
Texture Profile Analysis
The texture of the treated sample was assessed using a texture analyzer (TA.XT2, Stable Micro Systems, Surrey, UK) equipped with a 25-kg load cell. Samples were balanced at room temperature for 30 min before the analysis. Hilsa filets were compressed by 30% of their original height using a cylindrical probe of 6-mm diameter with a test speed of 2 mm/s (Chouhan et al. 2015).
Free Fatty Acid Content
The free fatty acid (FFA) content of the hilsa sample was determined using the method proposed by Kirk and Sawyer (1991). Hilsa sample (2 g) was blended with 4 g of anhydrous sodium sulfate and 45 mL of chloroform and filtered, and then 10 mL of ethanol was added to the solution. Finally, the solution was titrated against 0.01-N NaOH using phenolphthalein as an indicator.
Experimental Design and Statistical Data Analysis
The experimental design and data analysis for studying the effect of high-pressure processing parameters on the selected response variables were performed using the statistical software Design-Expert 7.0.0 version. The analysis of variance (ANOVA) test was performed to assess the influence of independent variables on the selected responses. A full-factorial experimental design was used for the present study (Appendix 2), and for optimization of independent variables (pressure 300, 400, 600 MPa; dwell time 5, 10, 15 min; processing temperature 30, 40, 50, 60 °C). The numerical optimization was performed based on the maximization of free fatty acid, pH, microbial inactivation, and minimization of hardness using the Design-Expert software.
Standardization of Process Parameters
The standardization of the process to find the optimum high-pressure processing condition for ready-to-eat hilsa curry based on the responses, microbial inactivation, texture, free fatty acid content, and pH was performed using the numerical optimization method described by Chakraborty et al. (2014). The goal of the optimization was the maximization of microbial inactivation, free fatty acid, and pH, and minimization of hardness.
Sensory Analysis
Sensory analysis of thermally and HPP-processed ready-to-eat hilsa curry was performed by a panel of 15 semi-trained members; subsequently, ASTM standards for sensory evaluation were followed. The one-to-nine point hedonic scale was used for the assessment, where 1 represents “extremely disliked” and 9 represents “extremely liked.” The tested attributes were color, aroma, taste, mouthfeel, and texture (hardness). The panelists were initially informed about the quality attributes of the hilsa curry and trained with conventionally prepared hilsa curry samples. Each member of the panel was asked to give a score to every attribute separately. The average score of each attribute was taken for final evaluation.
Results and Discussion
Effect of and High-Pressure Processing on the Quality of Hilsa Curry
Microbiological Analysis
Thermal treatment reduces the growth of microorganisms, which could affect consumer health and food properties. According to the international commission of microbiological standard for foods (FSSAI 2012), the maximum acceptable limit of microbial count in the ready-to-eat product is less than 3 log CFU/g. The initial microbial load of the control sample was 5.87 log CFU/g. The higher microbial burden measured in hilsa might be the aftereffect of contaminated water, utilized for culture and development, and the absence of appropriate handling and storage following harvesting (Chouhan et al. 2015). Thermally treated samples showed a significant (P < 0.05) reduction of microbial load by 2.98 to 2.84 log cycles compared with control, and this reduction was the greatest at the highest temperatures and longest holding times. Nevertheless, a significant reduction was found during pressure treatment, when the microbial load reduced by 0.78 ± 0.15 to 0.83 ± 0.10 log CFU/g at 500 MPa for 5- and 10-min holding time at 50 °C. This reduction of microorganisms is due to the direct effect of varying morphology, biochemical reactions, genetic mechanism, and microorganism cell membrane or wall (Hoover et al. 1989). The cell membrane is believed to be an essential target for high-pressure inactivation of microscopic organisms, as it affects permeability and in turn disturbs transport mechanisms, leading to the absence of nutrients, pH alterations, and ultimately cell death (Smelt 1998; Chouhan et al. 2015). The inactivation of the microbial load varied with pressure, holding time, and temperature. High pressure and temperature are more effective than high pressure and holding time. Total plate counts for varying pressure and holding time for different temperatures are shown in Fig. 1.
Effect on pH
The pH detection is one of the most commonly used physical quality rheostat methods for seafood products, which is pretentious by the free hydrogen and hydroxyl ion concentration variations due to changes in the food oxidation-reduction stability caused by microorganic or enzymatic activities (Varlik et al. 2000; Kaur et al. 2013). The hilsa filet pH showed a slight increase compared with the control after thermal processing, over both temperature and processing time (Fig. 2). The heat-induced pH increase is initiated by the decline in the number of acidic groups in muscle proteins as the proteins unfold (Hamm and Deatherage 1960). The high-pressure treatment might lead to the release of the muscle constituents such as basic amino acids, which might have led to a pH increase (Ramirez-Suarez and Morrissey 2006). Furthermore, the pH might increase due to a decrease in available acidic groups due to conformational changes (Angsupanich and Ledward 1998). It was observed that high-pressure-treated samples had higher pH values compared with the control and thermally treated samples. The initial pH of high-pressure-treated products at 300 MPa for 15 min was 6.49 ± 0.10. However, the pH was recorded within the range of 7.87 ± 0.10 at a pressure of 500 MPa for 15-min holding time. This pH increase coupled with an increasing pressure might be due to conformational changes induced by the pressure treatment associated with protein denaturation, unfolding, and basic amino acid exposure (Yamamoto et al. 1994; Kaur et al. 2013).
Effect on Free Fatty Acids
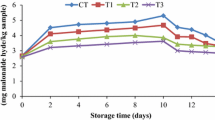
Textural changes lead to the upgradation of protein denaturation and also affect the flavor by increasing lipid oxidation, leading to off-flavor (Shewfelt 1981). Protein denaturation takes place during thermal processing, concurrently increasing lipid oxidation. Therefore, thermally treated samples showed higher free fatty acid content compared with untreated samples. High-pressure-treated samples showed increased free fatty acid composition compared with control and thermally treated samples (Fig. 3). It was demonstrated that the amount of free fatty acids released from high-pressure-treated samples increases with increasing pressure, and this free fatty acid accumulation also accelerates protein denaturation (Makri 2009). The above-described finding shows the protein content reduction trend of the samples with increased pressure treatment. The applied pressure treatment did not affect the hydrolysis mechanism, which leads to the production of free fatty acids. Similar results were reported by Kaur et al. (2013) and Chevalier et al. (2001) for black tiger shrimp (Penaeus monodon) and turbot (Scophthalmus maximus), respectively, with increasing pressure. Hilsa filets treated at 500 MPa for 15 min showed a significant (P < 0.05) increase in free fatty acid content, showing similarities with the work reported by Angsupanich and Ledward (1998), which described that the major effect of pressure above 400 MPa on cod muscles is increased lipid oxidation due to the release of free metal ions.
Texture Profile Analysis
Hardness is the most basic textural characteristics of meat and seafood, which rely on the connective tissue comprising of principal collagen and the myofibrils made of myosin and actin (Erdoğdu and Balaban 2000; Chouhan et al. 2015). Though, it was observed that hardness increased with temperature and processing time, where similar changes in hardness were reported by Bhattacharya et al. (1993) for pacific chum salmon, thermally processed at 60–100 °C. These changes in texture might be due to the denaturation and shrinkage of myofibrillar proteins (Harris and Shorthose 1988). The thermal denaturation of fish muscle proteins was also observed between 40 and 80 °C by Bell et al. (2001), where the temperature had a minor influence on hilsa filet springiness and cohesiveness. However, HPP-treated samples indicated significantly (P < 0.05) higher hardness esteems compared with control and thermally treated samples. The hardness values of the treated samples increased with pressure, temperature, and holding time. The appearance did not change when the sample was pressurized at low pressure (< 300 MPa) and temperature. Hardness exhibited growing drift with augmented pressure intensity, and the samples treated at 400 and 500 MPa were 5.5 and 6 times harder than control samples immediately after high-pressure processing (Fig. 4). Similar findings have been reported by Chouhan et al. (2015), who described that after increasing the pressure to 250 and 350 MPa, the hardness of high-pressure-treated samples increase by 1.6 and 1.7 times, respectively, compared with the control samples. The use of high hydrostatic pressure instigates either local or global changes in protein structure, ultimately leading to denaturation (Mozhaev et al. 1996). The extracellular space diminishes when pressure increases, in connection with muscle compaction and conceivable protein gel network formation (Hurtado et al. 2001). Jantakoson et al. (2012) reported myosin denaturation followed by subsequent formation of a network structure stabilized by a hydrogen bond. In this study, both the tissue toughening and softening were caused by the denaturation of myofibrillar proteins, collagen gelation, and solubilization, respectively. Furthermore, the textural quality correlated to some extent with moisture content, which decreased with increasing temperature.
Standardization of the Process Parameters
The standardization of process parameters for ready-to-eat hilsa curry has been performed to assure the microbial food safety and quality features in terms of the maximum microbial inactivation, higher hardness value with better gumminess, moderately acceptable free fatty acid, and minimum pH at the alkaline scale. However, after the numerical optimization of the process parameters for ready-to-eat hilsa curry and validation of the optimized conditions by further performing experiments to validate the process parameters, the obtained optimum processing condition was 500-MPa pressure for a 5-min holding time and 50 °C processing temperature. The obtained corresponding responses were 2.317g/100-g free fatty acid, 0.408 log CFU/g total microbial plate count, 176.42-g hardness, and 7.39 pH. In order to validate the optimized conditions, the experiments were performed and minimal variation (< 10%) was observed compared with the optimized conditions. Further experiments should be performed to address the storage stability of the optimized process conditions for the shelf life assessment of high-pressure-processed ready-to-eat hilsa curry.
Sensory Evaluation
The sensory evaluation experiment showed that the performance of both the samples (high-pressure-processed and thermally processed fish) is very good (Fig. 5). All the quality attributes were found between “moderately like” to “extremely like.” The texture and color of the high-pressure-processed food got a maximum score of 8.3 and 7.3, respectively, compared with the thermally processed product. A similar result has been reported by Mor-Mur and Yuste (2003), showing that pressurized products are preferred compared with conventionally pasteurized products, due to their better appearance, taste, and texture. Mengden et al. (2015) studied the sensory characteristics of mild smoked rainbow trout filets and fresh European catfish fillets. They reported that the pressure-treated trout filet samples were brighter, whereas in the case of catfish filets, the difference was insignificant.
Conclusion
Hilsa (Tenualosa ilisha) is one of the popular seafood with an abundance of macro- and micronutrients and plays an important role in providing essential nutrients. Nevertheless, due to the high rate of its perishability, hilsa requires preservation. Conventional methods, such as thermal treatment, are possible preservation approaches, but they have a detrimental effect on the quality of the final product. Therefore, new technologies, such as high-pressure processing, are gaining popularity in the seafood industry. During the physical, chemical, and microbiological analyses of processed hilsa fish curry in retort pouches, processing using the high-pressure treatment with under 500 MPa for 5 min at 50 °C was found the most suitable to retain all the physical, chemical, and microbiological parameters at the optimum level. Moreover, high-pressure-processed products received the highest sensory scores for texture and color compared with the thermally processed curry. This study demonstrates the effective use of high-pressure processing to obtain the best quality of ready-to-eat hilsa market product.
References
Angsupanich, K., & Ledward, D. A. (1998). High pressure treatment effects on cod (Gadus morhua) muscle. Food Chemistry, 63(1), 39–50. https://doi.org/10.1016/S0308-8146(97)00234-3.
APHA. (2001). In F. P. Downes & K. Ito (Eds.), Compendium of methods for the microbiological examination of foods. Washington: American Public Health Association.
Awuah, G. B., Ramaswamy, H. S., & Economides, A. (2007). Thermal processing and quality: principles and overview. Chemical Engineering and Processing: Process Intensification, 46(6), 584–602. https://doi.org/10.1016/j.cep.2006.08.004.
Bell, J. W., Farkas, B. E., Hale, S. A., & Lanier, T. C. (2001). Effect of thermal treatment on moisture transport during steam cooking of Skipjack Tuna (Katsuwonas pelamis). Journal of Food Science, 66(2), 307–313. https://doi.org/10.1111/j.1365-2621.2001.tb11337.x.
Bhattacharya, S., Choudhury, G. S., & Studebaker, S. (1993). Hydrothermal processing of Pacific Chum salmon: effect on texture & in-vitro digestibility. Journal of Food Quality, 16(4), 243–251. https://doi.org/10.1111/j.1745-4557.1993.tb00110.x.
Chakraborty, S., Rao, P. S., & Mishra, H. N. (2014). Effect of pH on enzyme inactivation kinetics in high-pressure processed pineapple (Ananas comosus L.) puree using response surface methodology. Food and Bioprocess Technology, 7(12), 3629–3645. https://doi.org/10.1007/s11947-014-1380-0.
Chevalier, D., Le Bail, A., & Ghoul, M. (2001). Effects of high pressure treatment (100–200 MPa) at low temperature on turbot (Scophthalmus maximus) muscle. Food Research International, 34(5), 425–429. https://doi.org/10.1016/S0963-9969(00)00187-3.
Chouhan, A., Kaur, B. P., & Rao, P. S. (2015). Effect of high pressure processing and thermal treatment on quality of hilsa (Tenualosa ilisha) fillets during refrigerated storage. Innovative Food Science and Emerging Technologies, 29, 151–160. https://doi.org/10.1016/j.ifset.2015.03.016.
Dhanapal, K., Reddy, G. V. S., Nayak, B. B., Basu, S., Shashidhar, K., Venkateswarlu, G., & Chouksey, M. K. (2010). Quality of ready to serve tilapia fish curry with PUFA in retortable pouches. Journal of Food Science, 75(7), 348–354. https://doi.org/10.1111/j.1750-3841.2010.01762.x.
Erdoğdu, F., & Balaban, M. O. (2000). Thermal processing effects on the textural attributes of previously frozen shrimp. Journal of Aquatic Food Product Technology, 9(4), 61–84. https://doi.org/10.1300/J030v09n04_07.
FSSAI (2012), Microbiology of foods, microbiological testing lab manual 14.
Ganguly, S., Mahanty, A., Mitra, T., Mohanty, S., Das, B. K., & Mohanty, B. P. (2018). Nutrigenomic studies on hilsa to evaluate flesh quality attributes and genes associated with fatty acid metabolism from the rivers Hooghly and Padma. Food Research International, 103, 21–29. https://doi.org/10.1016/j.foodres.2017.10.017.
Gopal, T. K. S., Vijayan, P. K., Balachandran, K. K., Madhavan, P., & Iyer, T. S. G. (2001). Traditional Kerala style fish curry in indigenous retort pouch. Food Control, 12(8), 523–527. https://doi.org/10.1016/S0956-7135(01)00058-5.
Hamm, R., & Deatherage, F. E. (1960). Change in hydration, solubility & protein charges of muscle proteins during heating of meat. Journal of Food Science, 25(5), 587–610. https://doi.org/10.1111/j.1365-2621.1960.tb00004.x.
Harris, P. V., & Shorthose, W. R. (1988). Meat texture. In R. A. Lawrie (Ed.), Developments in meat science (pp. 245–296). London: Elsevier Ltd..
Herbert, R. A., & Shewan, J. M. (1976). Roles played by bacterial and autolytic enzymes in the production of volatile sulphides in spoiling North Sea cod (Gadus morhua). Journal of the Science of Food and Agriculture, 27(1), 89–94. https://doi.org/10.1002/jsfa.2740270114.
Hicks, D. T., Pivarnik, L. F., McDermott, R., Richard, N., Hoover, D. G., & Kniel, K. E. (2009). Consumer awareness and willingness to pay for high-pressure processing of ready-to-eat food. Journal of Food Science Education, 8(2), 32–38. https://doi.org/10.1111/j.1541-4329.2009.00069.x.
Hoover, D. G., Metrick, C., Papineau, A. M., Farkas, D. F., & Knorr, D. (1989). Biological effects of high hydrostatic pressure on food microorganisms. Food Technology (Chicago), 43(3), 99–107.
Hossain, M., Adhikary, R. K., Mahbub, K. R., Begum, M., & Ul Islam, M. R. (2012). Effect of 10% concentrations of salt, garlic and coriander on the quality of smoked hilsa fish (Tenualosa ilisha). American Journal of Food Technology, 501-505. DOI: https://doi.org/10.3923/ajft.2012.501.505
Hurtado, J. L., Montero, P., Borderías, J., & Solas, M. (2001). High-pressure/temperature treatment effect on the characteristics of octopus (Octopus vulgaris) arm muscle. European Food Research and Technology, 213(1), 22–29. https://doi.org/10.1007/s002170100321.
Jantakoson, T., Kijroongrojana, K., & Benjakul, S. (2012). Effect of high pressure and heat treatments on black tiger shrimp (Penaeus monodon Fabricius) muscle protein. International Aquatic Research, 4(1), 1–12. https://doi.org/10.1186/2008-6970-4-19.
Kaur, B. P., Kaushik, N., Rao, P. S., & Chauhan, O. P. (2013). Effect of high-pressure processing on physical, biochemical, and microbiological characteristics of black tiger shrimp (Penaeus monodon). Food and Bioprocess Technology, 6(6), 1390–1400. https://doi.org/10.1007/s11947-012-0870-1.
Kirk, S., & Sawyer, R. (1991). Pearson’s composition and analysis of foods (No. Ed. 9). Longman Group Ltd.
Makri, M. (2009). Biochemical and textural properties of frozen stored (−22°C) gilthead seabream (Sparus aurata) fillets. African Journal of Biotechnology, 8(7), 1287–1299.
Matser, A. M., Stegeman, D., Kals, J., & Bartels, P. V. (2000). Effects of high pressure on colour and texture of fish. International Journal of High Pressure Research, 19(1-6), 109–115 https://doi.org/10.1080/08957950008202543.
Mengden, R., Röhner, A., Sudhaus, N., & Klein, G. (2015). High-pressure processing of mild smoked rainbow trout fillets (Oncorhynchus mykiss) and fresh European catfish fillets (Silurus glanis). Innovative Food Science & Emerging Technologies, 32, 9–15. https://doi.org/10.1016/j.ifset.2015.10.002.
Mohanty, B. P., Paria, P., Mahanty, A., Behera, B. K., Mathew, S., Sankar, T. V., & Sharma, A. P. (2012). Fatty acid profile of Indian shad Tenualosa ilisha oil and its dietary significance. National Academy of Science Letters, 35(4), 263–269. https://doi.org/10.1007/s40009-012-0042-x.
Mor-Mur, M., & Yuste, J. (2003). High pressure processing applied to cooked sausage manufacture: physical properties & sensory analysis. Meat Science, 65(3), 1187–1191. https://doi.org/10.1016/S0309-1740(03)00013-5.
Mozhaev, V. V., Hermans, K., Frank, J., Masson, P., & Balny, C. (1996). High pressure effect on protein structure & function. Proteins: Structure, Function, and Genetics, 24(1), 81–91. https://doi.org/10.1002/(SICI)1097-0134(199601)24:1<81::AID-PROT6>3.0.CO;2-R.
Nowsad, A. K. M. (2007). Participatory training of trainers: a new approach applied in fish processing. Bangladesh Fisheries Research Forum, pp.213.
Nowsad, A. K. M. A. (2010). Post-harvest loss reduction in fisheries in Bangladesh: a way forward to food security. Final report PR, 5(08), 171.
Quazi, S., Mohiduzzaman, M., Mostafizur Rahaman, M., & Keramat Ali, S. M. (1994). Effect of hilsa (Tenualosa ilisha) fish in hypercholesterolemic subjects. Bangladesh Medical Research Council Bulletin, 20(1), 1–7.
Ramirez-Suarez, J. C., & Morrissey, M. T. (2006). Effect of high pressure processing (HPP) on shelf life of albacore tuna (Thunnus alalunga) minced muscle. Innovative Food Science & Emerging Technologies, 7(1-2), 19–27. https://doi.org/10.1016/j.ifset.2005.08.004.
Salam, K. A., Hossain, A. K. M., Motahar, A. H. M., Alam, K., Pervin, F., & Absar, N. (2005). A comparative analysis on physio-chemical characteristic of oil extracted from six different parts of hilsa fish (Hilsa ilisha). Pakistan Journal of Biological Sciences, 8(6), 810–815. https://doi.org/10.3923/pjbs.2005.810.815.
Shewfelt, R. L. (1981). Fish muscle lipolysis-a review. Journal of Food Biochemistry, 5(2), 79–100. https://doi.org/10.1111/j.1745-4514.1981.tb00663.x.
Smelt, J. P. P. M. (1998). Recent advances in the microbiology of high pressure processing. Trends in Food Science and Technology, 9(4), 152–158. https://doi.org/10.1016/S0924-2244(98)00030-2.
Varlik, C., Baygar, T., Özden, Ö., Erkan, N., & Mol, S. (2000). Sensory evaluation & determination of some physical & chemical characteristics of shrimp during gold storage. Turkish Journal of Veterinary and Animal Sciences, 24(3), 181–186.
Yamamoto, K., Yoshida, Y., Morita, J. I., & Yasui, T. (1994). Morphological & physicochemical changes in the myosin molecules induced by hydrostatic pressure. The Journal of Biochemistry, 116(1), 215–220. https://doi.org/10.1093/oxfordjournals.jbchem.a124496.
Funding
The first author thanks the Ministry of Human Resource Development, Govt. of India, for GATE scholarship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Appendices
Appendix 1. Ingredients per kilogram used for hilsa curry preparation (Gopal et al. 2001)
Ingredients | Quantity | Unit |
|---|---|---|
Dried mustard seeds soaked in little warm water | 200 | g |
Green chili chopped | 10 | g |
Nigella seeds | 2 | g |
Mustard oil | 50 | mL |
Red chili powder | 5 | g |
Turmeric powder | 15 | g |
Pepper powder | 5 | g |
Curd | 50 | mL |
Salt | 20 | g |
Water | 150 | mL |
Appendix 2. Full-factorial experimental design indicating the independent and dependent variables
Sl. No. | Pressure (MPa) | Dwell time (min) | Temperature (°C) | Total plate count (TPC) CFU/mL | Free fatty acid (g/100g) | Hardness (g) | pH |
|---|---|---|---|---|---|---|---|
1 | 300 | 5 | 30 | 3.365 | 1.41 | 81.4 | 6.21 |
2 | 400 | 5 | 30 | 3.1072 | 1.41 | 105.5 | 6.252 |
3 | 500 | 5 | 30 | 2.79 | 1.53 | 111.43 | 6.28 |
4 | 300 | 10 | 30 | 3.25 | 1.47 | 114.3 | 6.24 |
5 | 400 | 10 | 30 | 3.057 | 1.44 | 120 | 6.27 |
6 | 500 | 10 | 30 | 2.53 | 1.62 | 142.3 | 6.36 |
7 | 300 | 15 | 30 | 3.08 | 1.49 | 116.2 | 6.25 |
8 | 400 | 15 | 30 | 2.705 | 1.49 | 122.8 | 6.32 |
9 | 500 | 15 | 30 | 2.2 | 1.75 | 192.5 | 6.42 |
10 | 300 | 5 | 40 | 3.287 | 1.46 | 100.6 | 6.25 |
11 | 400 | 5 | 40 | 2.87 | 1.7 | 142.5 | 6.4 |
12 | 500 | 5 | 40 | 1.55 | 2.415 | 181 | 6.37 |
13 | 300 | 10 | 40 | 3.11 | 1.54 | 134.2 | 6.3 |
14 | 400 | 10 | 40 | 2.38 | 2 | 193.9 | 6.44 |
15 | 500 | 10 | 40 | 1.14 | 2.44 | 226.6 | 6.5 |
16 | 300 | 15 | 40 | 3.041 | 1.66 | 160.4 | 6.35 |
17 | 400 | 15 | 40 | 2.36 | 2.1 | 211.5 | 6.53 |
18 | 500 | 15 | 40 | 0.411 | 2.51 | 252.4 | 6.68 |
19 | 300 | 5 | 50 | 3.08 | 2.17 | 128.2 | 6.26 |
20 | 400 | 5 | 50 | 1.72 | 2.06 | 164.5 | 6.77 |
21 | 500 | 5 | 50 | 0.301 | 2.45 | 177 | 7.39 |
22 | 300 | 10 | 50 | 3 | 2.36 | 165.4 | 6.33 |
23 | 400 | 10 | 50 | 1.62 | 2.22 | 221.7 | 6.85 |
24 | 500 | 10 | 50 | 0.301 | 2.47 | 266.7 | 7.42 |
25 | 300 | 15 | 50 | 2.71 | 2.4 | 178.63 | 6.45 |
26 | 400 | 15 | 50 | 1.3801 | 2.35 | 289.3 | 7.14 |
27 | 500 | 15 | 50 | 0 | 2.67 | 256.11 | 7.45 |
28 | 300 | 5 | 60 | 2.62 | 2.35 | 142.3 | 6.43 |
29 | 400 | 5 | 60 | 1.04 | 2.12 | 163.6 | 7.06 |
30 | 500 | 5 | 60 | 0.204 | 2.66 | 189.8 | 7.44 |
31 | 300 | 10 | 60 | 2.55 | 2.41 | 166 | 6.46 |
32 | 400 | 10 | 60 | 0.477 | 2.32 | 243.4 | 7.21 |
33 | 500 | 10 | 60 | 0 | 2.71 | 270.2 | 7.45 |
34 | 300 | 15 | 60 | 2.38 | 2.44 | 198.4 | 6.52 |
35 | 400 | 15 | 60 | 0.397 | 2.55 | 280.6 | 7.34 |
36 | 500 | 15 | 60 | 0 | 2.8 | 296.5 | 7.51 |
Rights and permissions
About this article
Cite this article
Singha, K.K.R., Swami Hulle, N.R., Deb, S. et al. Physicochemical and Microbiological Characteristics of High-Pressure-Processed Ready-to-Eat Hilsa (Tenualosa ilisha) Curry. J. Biosyst. Eng. 45, 94–103 (2020). https://doi.org/10.1007/s42853-020-00049-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42853-020-00049-8