Abstract
The moisture adsorption isotherms of solar dehydrated mango and jackfruit were determined at temperatures ranging from 30 °C to 50 °C. The equilibrium moisture content (EMC) of mango and jackfruit increased sharply as the temperature increased at water activity (a w) above 0.6 and 0.8, respectively. However, there were no clear isothermal intersection points observed at higher a w and temperatures. The EMC of solar dehydrated jackfruit showed the isothermal characteristics between types II and III. In contrast, dehydrated mango followed the characteristic type III adsorption isotherms due to high total soluble solids content of 67.8 °Brix and total sugars of 14.21 g/100 g fresh mango. Estimated parameters and fitting ability of three isotherm models were also evaluated. The Guggenheim-Anderson-Boer (GAB) model gave the best fit to the experimental EMC data. The GAB monolayer moisture contents (m o) of mango and jackfruit ranged from 11.1–10.0 % and 4.7–3.4 %, respectively. Specific surface area of active binding sites (S) was calculated based on the m o values. The S value of dehydrated mango was 2.5 to 2.8 times larger than jackfruit. The maximum net isosteric heat (q s) of sorption of solar dehydrated mango and jackfruit were determined as 19.5 and 33 kJ mol−1, respectively, and q s decreased significantly at high moisture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mango (Mangifera indica) and jackfruit (Artocarpus heterophyllus) are seasonal tropical crops with perishable fruits subject to significant postharvest losses. Postharvest losses of mango and jackfruit are considered high, but no estimates were located in the literature. Both mango and jackfruit grow in homestead gardens and have high demand in local markets as a fresh fruit or vegetable. Mango and jackfruit are consumed as a cooked vegetable, in chutneys and pickles or as a dessert fruit, depending on the stage of maturity. There is greater demand for tropical fruits in export markets as value-added products like dehydrated chips and fruit bars (Mir and Nath 1995). Even though the tropical dehydrated fruit and vegetable industry has been growing rapidly in recent times, there is a greater need to improve drying, storage, and processing methods to minimize production costs and improve the quality of the dehydrated products. Product quality parameters such as texture, aroma, and taste are especially sensitive to the mode of drying. Solar drying or dehydration is one of the most economical ways of preserving these fruits. Compared with the open sun drying, solar drying significantly reduces drying time and improves product quality. However, variations in drying temperature due to varying intensity of solar radiation affects the rate of solar drying over the drying period (Lutz et al. 1987).
Tunnel-type solar dryers have been successfully adopted under different tropical and subtropical climatic conditions for drying and dehydration of various agricultural commodities without affecting their final quality (Lutz et al. 1987; Schirmer et al. 1996). Nevertheless, solar dying is slower and more difficult to control compared with mechanical drying techniques. Significant changes in ambient humidity and temperature during solar drying leads to irreversible biochemical reactions, structural changes, and physical modifications (Cernisev 2010) in food materials. Therefore, during dehydration and rehydration, products may behave differently due to changes in their microstructure properties (Tortoe and Orchard 2006). The control of moisture content in fruits and vegetables during processing and storage is very important because water activity (a w) and storage temperature play an important role in controlling the physico-chemical properties of the processed product. Therefore, information on the moisture sorption characteristics of dehydrated fruit is useful in designing and modeling of drying, aeration, and storage processes. The relationship between water activity (a w) or ERH and equilibrium moisture content (EMC) of a product is described by the moisture sorption isotherms. Each commodity has a unique set of isotherms, and these isotherms are useful in determining the lowest moisture content attainable under specific drying or storage conditions (Labuza et al. 1985; Sun and Woods 1994; Sun 1999; Singh et al. 2006). The Guggenheim-Anderson-de Boer (GAB) equation has been used successfully to predict EMC values for dehydrated fruits and vegetables for a wide a w range (Saravacos et al. 1986; Tsami et al. 1990a; Falade et al. 2004; Bellagha et al. 2008). In addition to the GAB equation, the Brunauer-Emmett-Teller, modified Henderson, modified Chung-Pfost, modified Halsey, modified Oswin, and several other equations have also been adopted to describe the moisture sorption characteristics of various dehydrated food materials (Hubinger et al. 1992; Kiranoudis et al. 1993; Sun 1999; Mir and Nath 1995; Falade et al. 2004; Singh et al. 2006; Yanniotis and Blahovec 2009).
The net isosteric heat of sorption (q s) is useful when estimating the energy requirements for dehydration processes. It is an indicator of the amount of latent heat of vaporization required to vaporize the “bound water” in the food (Tsami et al. 1990b). The heat of vaporization of bound water is higher than vaporization of pure water when food is dehydrated to low moisture contents.
At present, there is a lack of information on moisture sorption characteristics of solar dehydrated products. Therefore, the objectives of this study were to examine the physico-chemical properties and to determine the moisture adsorption isotherms for solar dehydrated mango and jackfruit. The data were further analyzed to estimate the q s of the fruits at three different storage temperatures.
Materials and Methods
Dehydration of Mango and Jackfruit
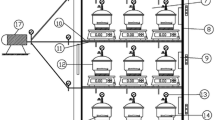
Unripe but mature jackfruit and firm-ripe mango (variety Karthakolomban) at the 50 % ripening stage (50 % yellow peel surface) was purchased from a local market. Mature jackfruit have fertilized perianths (bulbs) and containing fully mature brown seeds. Edible bulbs of jackfruits were removed, and the bulbs were cut into strips approximately 5 × 1 cm in dimension for processing. Mangoes were peeled and cut into 5 × 1 × 1 cm slices. The samples were dipped in a pretreatment solution of 2 % (w/w) ascorbic acid and 0.1 % (w/w) potassium metabisulfite for 5 min (Singh et al. 2006). Then, the samples were blanched in hot water at 90 °C for 3 min, to inactivate enzymes activity. The treated slices were allowed to drain for 15 min arranged in a thin layer and loaded into a hybrid tunnel solar dryer (Esper and Mühlbauer 1996). The dryer (18 m long and 2 m wide) consists of a flat plate solar collector (8 m) and a drying tunnel (10 m) fabricated as a single unit. The whole unit was covered with a 0.2 mm thick, UV-stabilized polyethylene sheet. The flat solar collector was coated with black paint to absorb solar energy. Temperatures inside the solar dryer were measured using K-type thermocouples. Air temperatures reached up to a maximum of 60 °C during the drying process. Products were dried at dryer temperatures of 47–56 °C and 23 ± 7 % r.h., when ambient temperature and r.h. were 32 ± 3 °C and 75 ± 5 %, respectively. Fruit slices were dried to final moisture content of about 10 % (d.b.) during 20 h of drying over 2.5 days (8 h of drying per day).
Characteristics of Fruits
Physico-chemical properties (fruit firmness, flesh color, total soluble solids (TSS), pH, titratable acidity (TA), total sugars, vitamin C, moisture, and a w) were measured in triplicate for selected fresh fruits and the solar dehydrated samples. Flesh color was measured using a color difference meter (2E 2000 Denshuku, Japan). Firmness of the fresh mango and jackfruit bulbs was tested by using fruit firmness tester (AST Everwell Co.). Water activity of the solar-dehydrated samples was measured using a w meter (Aqualab-3, USA). Dehydrated and fresh samples were ground separately using a Stein laboratory mill (model M-2) and laboratory mortar and pestle, respectively. Each sample (5 g) was vortexed with 5 ml distilled water, and the mixture was centrifuged (Kokushan H-1500 F) at 6,000 rpm for 10 min. The TSS of the supernatant was measured using a refractometer (PAL-1 Atago, Japan). The supernatant was further diluted with up to 80 ml of distilled water in order to measure pH, TA, total sugars, and vitamin C (Ranganna 1986; AOAC 1996; Illeperuma and Jayasuriya 2002).
Experimental Procedure for Moisture Sorption
Depending on initial a w of the solar-dehydrated samples, moisture sorption study was designed to start from the lowest possible a w level in the samples. Therefore, solar-dehydrated samples were further dehydrated in desiccators containing silica gel for 2 days at 30 °C prior to the experiment. Air-tight glass jars, containing ten saturated salt solutions were placed in temperature-adjusted incubators to provide constant r.h. values (Wexler 1999) ranging from 22 % to 99 % at three different temperatures of 30 °C, 40 °C, and 50 °C. Perforated plastic cups were attached to the glass jars, which were used as sample holders above the saturated salt solutions. Three 10 g desiccated samples of (solar-dehydrated) mango and jackfruit were placed separately inside the jars, and the glass jars were closed tightly. In order to prevent mold growth at high r.h. levels (>60 %), small pieces of cotton containing formalin were placed inside the sealed glass jars.
The EMC of solar-dehydrated mango and jackfruit was identified by the static–gravimetric method. Sample weights were taken every 3 days until nearly constant weights (±0.1 mg) were achieved after about 14 days. Moisture content of the samples was determined after sample had reached EMC. The initial moisture (in grams per 100 g dry matter) and EMC were obtained by drying the products in a vacuum oven at 70 °C temperature for 6 h.
Model Development
Three mathematical models, modified Henderson equation (Thompson et al. 1968), modified Chung-Pfost equation (Chung and Pfost 1976), and Guggenheim-Anderson-de Boer equation (Van den Berg 1984), were used to analyze the adsorption isotherms (Mir and Nath 1995; Sun 1999; Singh et al. 2006) of solar-dehydrated fruits. These are three-parameter models and include the effects of a w and T at EMC.
Modified Chung-Pfost Equation (MCPE)
Modified Henderson equation (MHEE)
Guggenheim-Anderson-de Boer equation
It has been shown that parameters K must be in the range of 0 < K ≤ 1, whereas parameter C essentially needs to be C > 0 (Blahovec 2004; Quirijns et al. 2005; Blahovec and Yanniotis 2008). The coefficients m o, C, and K are related to temperature and can be expressed using Arrhenius-type equations (Kiranoudis et al. 1993; Togrul and Arslan 2006; Blahovec and Yanniotis 2008; Bellagha et al. 2008).
Where \( g = {h_m} - {h_n}/R\,{\hbox{and}}\,i = {h_i} - {h_n}/R \)
Once the m o content was known, the S of active binding sites of dry matter (square meters per gram) could be determined from Eq. 7 (Togrul and Arslan 2006).
Where the constant 3.53 × 103 m2 g−1 = (Avogadro number × surface area of a water molecule)/molar weight of water.
Net Isosteric Heat of Moisture Sorption
Net q s (J mol-1) is the amount of energy required to remove water from the products in excess of the energy required for free water evaporation. The Clausisus-Clapeyron equation is used to calculate q s:
The value of q s is calculated from the slope of the linear regression line plotted between ln (a w) and 1/T at constant moisture (Tsami 1990b; Togrul and Arslan 2006).
Statistical Analysis
The statistical analysis of experimental data was determined using the SAS software package, applying an analysis of variance to estimate any statistically significant at p < 0.05 (SAS Institute USA 1990). One-way student t test was conducted for the physico-chemical data. The SAS-NLIN least-square estimation (SAS Institute 1990) was used to fit the experimental data to three sorption isotherm equations. The method used to evaluate the goodness-of-fit was based on R 2, RSS, SEE, and P. The P (percent) value has good ability to describe the goodness-of-fit and then SEE. The value of P and SEE is defined as:
Where, m i is the observed moisture content at i th experiment; m p is the predicted moisture content at i th experiment; df is degrees of freedom, and n is the number of observations. Lower values of P and SEE indicate a better goodness of fit (Sun 1999; Togrul and Arslan 2006).
Results and Discussion
Characteristics of Fruits
Physico-chemical characteristics of fresh and solar dehydrated samples are presented in Table 1. Characteristics of fresh fruits were evaluated during the selection of raw material quality for processing. Fruit firmness, TSS, pH, TA, total sugar, vitamin C, and color indicated that there was no significant variability (p > 0.05) within the selected mango or jackfruit samples. However, significant differences (p < 0.05) were observed between mango and jackfruit due to differences in the morphology and biochemical constituents of the two fruits. Ripe mango contained high levels of total sugars as indicated by TSS (Table 1) and was similar to the data reported for a similar mango variety (lleperuma and Jayasuriya 2002). Mature jackfruit is low in sugars but is a richer source of starch (Rahman et al. 1995) compared with mango. The relative lightness index “L” value was significantly changed (p < 0.05) during solar dehydration of fruits, indicating that the solar dehydration process may not replicate the results of other dehydration methods.
Moisture Sorption Isotherms
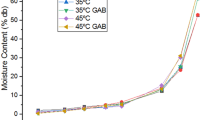
The experimental EMC data on adsorption isotherms at three different temperatures (30 °C, 40 °C, and 50 °C) and water activities of solar dehydrated mango and jackfruit are shown in Figs. 1 and 2, respectively. At a w < 0.6, the EMC of the two dehydrated fruits decreased significantly (p < 0.05) as the temperature increased from 30 °C to 50 °C. During solar dehydration, starch in jackfruit perianths may have undergone a pasting or partial gelatinization process which effectively traps water. Therefore, moisture adsorption may be significantly decreased with increasing temperature at low a w due to reduction in moisture diffusivity within the starch tissue (Sato et al. 2010). Hygroscopicity of dehydrated mango and jackfruit appears to have increased significantly (p < 0.05) as the temperature increased from 30 °C to 50 °C when a w > 0.6 and a w > 0.8, respectively. The dehydrated mango had a sticky and soft texture due to more moisture adsorption at higher a w values than jackfruit. The sugar contents of solar dehydrated mango and jackfruit, in terms of TSS, were increased to 67.8 and 23.5°Brix (d.b.), respectively, than TSS of fresh fruits. Therefore, the sharp increase in moisture content of dehydrated mango and jackfruit could be explained by the solubility of sugars in mango and swelling of starch in jackfruit at increasing temperature under constant a w. At low moisture, fruits contain sugars in amorphous state that are hygroscopic and tend to absorb large amount water at high a w and temperature as results of solubilization of sugars (Hayoglu and Gamli 2007). On the other hand, mango contains a more cellulosic and porous microstructure where more moisture can be retained in the mango due to surface tension. These results are also in agreement with observations of Hubinger et al. (1992), Isse et al. (1993), and Mir and Nath (1995) on moisture sorption isotherms of dehydrated mango and osmotically dehydrated mango. Similar isotherm behavior has also been reported for sugars and sugar containing dehydrated fruits by Saravacos et al. (1986), Verma and Narain (1990), Hubinger et al. (1992), Singh and Singh (1996), and Falade et al. (2004). Perianths of matured jackfruit contain 330 g of starch per kilogram of dry matter (Rahman et al. 1995). Therefore, sharp increases of moisture in dehydrated jackfruit could be related to swelling of starch on water uptake at a w > 0.8 and creation of a transient capillary structure along with an increase the number of active binding site (Aguerre et al. 1989).
Isotherms of dehydrated mango and jackfruit (Figs. 1 and 2) did not show any intersection points of the predicted adsorption data with increasing temperature at higher a w > 0.8. Isse et al. (1993) reported significant intersection isothermal point at a w > 0.5 for vacuum dehydrated mango. Other authors have shown that adsorption isotherms of many mechanically dehydrated fruits and sugar products intersect at a w between 0.5 and 0.8 with increasing storage temperature (Saravacos et al. 1986; Tsami et al. 1990a; Verma and Narain 1990; Mir and Nath 1995; Falade et al. 2004; Hayoglu and Gamli 2007). Therefore, moisture adsorption isotherms of solar-dehydrated fruits may have obtained different moisture sorption characteristics than other dehydrated fruits found in the literature.
Modeling of the Adsorption Isotherms
Six-parameter GAB model, three-parameter MHEE model, and MCPE model were analyzed for goodness of fit for the experimental sorption data of jackfruit and mango, and the estimated parameters are summarized in Table 2. The lowest estimates for P, RSS, SEE, and high R 2 were observed for the GAB model for both mango and jackfruit. Therefore, the six-parameter GAB equation fit the data more accurately over the range of a w and temperatures between 30 °C and 50 °C (Figs. 1 and 2). The values of P ≤ 5 % indicate an excellent fit, but P > 10 % is considered a poor fit for the equation (Sun 1999). The GAB model met this criterion while the other two models gave significantly higher P values (p < 0.05) except at 50 °C. Therefore, the GAB model was selected as the best model for both mango and jackfruit. In contrast, Isse et al. (1993) observed that the two-parameter Halsey’s equation was the most appropriate model to describe the moisture adsorption characteristics of vacuum-dried mango. Mir and Nath (1995) have shown that the GAB model can also be used to predict the sorption behavior of plain mango bars. The GAB model has been used successfully to explain the moisture sorption behavior of many foods (Tsami et al. 1990a; Singh and Singh 1996; Falade et al. 2004; Quirijns et al. 2005; Blahovec and Yanniotis 2008; Bellagha et al. 2008). Of the other equations, MHEE had better fit than MCPE equation for jackfruit. For mango, the MCPE was a better fit than the MHEE equation. The difference in the degree of suitability of moisture sorption models for the different fruits could be due to inherent differences in the fruits and vegetables particularly in relation to their biochemical composition (Tsami et al. 1990a; Chowdhury et al. 1997) and the method of dehydration. However, at 50 °C, all three models were well fitted to the experimental data as indicated by low P values.
Parameters C and K of the GAB equation were calculated using Eqs. 4 and 5, respectively. The parameter K increased from 0.721 to 0.724 while parameter C decreased from 1.1 to 1.08 with increasing temperature from 30 °C to 50 °C for dehydrated mango. For type III isotherms, generally, the value of C is in the range of 0 < C ≤ 2 without point of inflexion (Blahovec 2004; Blahovec and Yanniotis 2008). Therefore, based on the behavior of parameter C and K, dehydrated mango showed the characteristic nature of the type III adsorption isotherm. In contrast, the K value of jackfruit was 0.96, and the C value decreased from 35.7 to 34.3 with the increasing temperature from 30 °C to 50 °C. The adsorption isotherms of jackfruit have obtained characteristic type II isotherms according to GAB classification. If parameter C ≥ 2, the GAB equation gives a sigmoidal shape curve of type II with a point of inflection (Blahovec and Yanniotis 2008). Lewicki (1997) reported that GAB model describes well sigmoidal type II isotherms when parameters K and C are kept in the ranges of 0.24 < K ≤ 1 and 5.76 ≤ C ≤ ∞. However, our study found that the shape of the adsorption isotherms curves of solar-dehydrated jackfruit more or less resembles type III isotherms because the sigmoidal shape or inflection point is not clearly visible in the isotherms. The type III isotherm is referred as a “J-shape” isotherm which is a characteristic feature of foods that are rich in sugars or soluble solids and is undoubtedly related to multilayer moisture development (Tsami et al. 1990a; Falade et al. 2004; Hayoglu and Gamli 2007) due to the physical adsorption of moisture a w > 0.6. The type II isotherm is more related to moisture sorption of starchy foods (Sun and woods 1994; Yanniotis and Blahovec 2009). Therefore, solar-dehydrated jackfruit showed isothermal behavior between type II and type III because mature jackfruit contains both sugars and starch (Rahman et al. 1995; Chowdhury et al. 1997).
Monolayer Moisture Adsorption
The m o is the minimum moisture content covering the active hydrophilic binding site on the material and is necessary information for predicting maximum storage period with minimum quality loss (Labuza et al. 1985). The m o was calculated using the GAB model (Eq. 6). Table 3 indicates that the m o value decreased with increasing in temperature. For the temperature range 30–50 °C, the m o of mango and jackfruit varied from 11.14 % to 9.97 % and 4.74 % to 3.41 %, respectively. These values of m o were smaller than the data found in the literature for several dehydrated fruits and vegetables (Saravacos et al. 1986; Tsami et al. 1990a; Kiranoudis et al. 1993; Falade et al. 2004; Quirijns et al. 2005; Bellagha et al. 2008). Isse et al. (1993) and Singh and Singh (1996) reported very low GAB m o values of 3.15 % to 3.16 % (d.b.) for vacuum-dehydrated mango in the range of 26–50 °C. Solar dehydration is a much slower and uncontrolled process than vacuum dehydration and other dehydration techniques. Therefore, the differences in m o found for dehydrated mango could be caused by differences in drying techniques and physico-chemical composition of different varieties.
There was a considerable difference of m o between mango and jackfruit (~57–68 %) perhaps due to higher sugar content in firm-ripe mango than unripe jackfruit (Rahman et al. 1995; Illeperuma and Jayasuriya 2002). Fluctuation of temperature during solar dehydration may have a significant influence on the structure of the dehydrated fruits. Quirijns et al. (2005) reported a reduction of the total number of active sites for water binding as a result of physical or chemical changes in the existing porous structure of the products. This phenomenon can be further explained by using S (specific surface area) of active binding sites as given in Table 3. The total S available for hydrophilic binding in adsorption decreased with increasing temperature from 30 °C to 50 °C. The total S of active binding site for solar-dehydrated mango is 2.5 to 2.8 times larger than for solar-dehydrated jackfruit. The large S of active binding sites was attributed to the existence of many intrinsic micro-nanoporous structures of dehydrated fruits (Ngoddy and Bakker-Arkema 1970, 1972; Togrul and Arslan 2006).
Table 3 also gives the EMC for 75 % r.h., the average r.h. of Sri Lanka, for product a w in the range of 0.7–0.8. Solar-dehydrated mango has higher EMC at 75 % r.h. than jackfruits. Therefore, the solar-dehydrated mango slices are less storable than solar-dehydrated jackfruit. In comparison to other drying/dehydration methods for mango (Hubinger et al. 1992; Mir and Nath 1995), solar-dehydrated mango showed less storability at 75 % r.h.
The Isosteric Heat of Moisture Sorption
The values of net isosteric heat of sorption of water (q s) were estimated using Clausius-Clapeyron Eq. 8. The q s for different moisture contents are shown in Fig. 3. The figure demonstrates that q s increase continually and sharply with decreasing moisture content. The net q s can be expressed by a power function of moisture content. The functions for solar-dehydrated mango and jackfruit are q s = 102.2 M-1.21(R 2 = 0.98) and q s = 348.53 M-1.46 (R 2 = 0.94), respectively, where “M” represents the moisture content (percent d.b.) of the products. The dimensionless exponent factor of the power functions determines the rate that q s decreases with increasing moisture content whereas slop (scaling factor) of the equations represents the isosteric sorption heat (kilojoules per mole) of first water molecule or when extremely low moisture holds in the product. The net q s increased as the moisture content of the fruit was reduced from 80 % to 20 % (d.b.) for both solar-dehydrated mango and jackfruit, but the increase was shaper in the 20 % to 4 % moisture content range. Theory indicates that q s in dried fruits decrease when the moisture content is increased (Tsami et al. 1990b; Quirijns et al. 2005). The maximum q s values for solar-dehydrated mango and jackfruit were about 19.5 and 33 kJ mol−1, respectively. Values of maximum q s reported for dehydrated fruits and vegetables range between 100 and 15 kJ mol−1 and for starchy products, range between 55 and 25 kJ mol−1 (Tsami et al. 1990b; Kiranoudis et al. 1993; Quirijns et al. 2005). The q s was higher in solar-dehydrated jackfruit than mango possibly due to different physico-chemical and structural composition with regards to their sorption characteristics. The very high q s at low moisture content was an indication of strong water–starch component interactions in the solar dehydrated jackfruit. In solar dehydration, water molecules in starch may behave differently than other dehydration techniques. At low moisture content, moisture is adsorbed at the strongest binding sites on the active surface of the solids (Table 3) perhaps to the surface of nano-size capillaries (Ngoddy and Bakker-Arkema 1972). As moisture increases, the food material tends to swell and may open up new sites for moisture to bind in the food, resulting in low heat of sorption for high-moisture food products (Aguerre et al. 1989; Tsami et al. 1990b; Togrul and Arslan 2006).
Conclusion
The moisture adsorption isotherm curves of solar-dehydrated jackfruit showed the characteristic “J” shaped isotherm that falls between II and type III isotherm curves while mango showed a type III isotherm curve. These isotherm types were based on the temperature-dependent GAB constant values of K and C. Solar-dehydrated mango has higher monolayer moisture content and specific active surface areas than solar-dehydrated jackfruit, but both decreased with increase in temperature. The net isosteric heat of sorption was higher for solar-dehydrated jackfruit than mango possibly due to differences in their structural and physico-chemical compositions which determines the moisture sorption characteristic. Solar dehydration is an uncontrolled drying process; hence, it may significantly affect the structural properties of the products. Therefore, solar-dehydrated fruits showed low storage stability than other dehydrated fruits.
Abbreviations
- a, b, c :
-
Henderson and Chung-Pfost model parameters
- a w :
-
Water activity
- C K :
-
GAB model parameters
- c o k o :
-
Entropic accommodation factors
- d.b.:
-
Dry basis (%)
- df:
-
Degrees of freedom
- e :
-
Temperature dependence constant of monolayer (K)
- EMC:
-
Equilibrium moisture content (g/100 g dry matter)
- ERH:
-
Equilibrium relative humidity (%)
- g :
-
Monolayer and multiplayer water enthalpy difference (K)
- GAB:
-
Guggenheim-Anderson-Boer equations
- h i :
-
Molar sorption enthalpy of free water (J mol−1)
- h m :
-
Monolayer sorption enthalpy (J mol−1)
- h n :
-
Multilayer molar sorption enthalpy (J mol−1)
- i :
-
Multilayer and free water enthalpy difference (K)
- m :
-
Moisture content (g/100 g dry matter)
- M :
-
Moisture content (% d.b.)
- m a :
-
Temperature dependence of monolayer (K)
- MCPE:
-
Modified Chung-Pfost equation
- MHEE:
-
Modified Henderson equation
- m i :
-
Observed moisture content (g/100 g dry matter)
- m o :
-
Monolayer moisture content (g/100 g dry matter)
- m p :
-
Predicted moisture content (g/100 g dry matter)
- P :
-
Mean relative percentage deviation modulus (%)
- p :
-
Probability value
- q s :
-
Isosteric heat of sorption (J mol−1)
- R :
-
Universal gas constant (8.314 J mol−1 K−1)
- r.h.:
-
Relative humidity (%)
- R 2 :
-
Regression coefficient
- rpm:
-
Round per minute
- RSS:
-
Residual sum of squares
- S :
-
Specific surface area (m2 g−1)
- SD:
-
Standard deviation
- SEE:
-
Standard error of estimate
- T :
-
Absolute temperature (K)
- TA:
-
Titratable acidity (%)
- TSS:
-
Total soluble solids content (°Brix)
- w.b.:
-
Wet basis (%)
References
Aguerre, R. J., Suarez, C., & Viollaz, P. E. (1989). Swelling and pore structure in starchy materials. Journal of Food Engineering, 9, 71–80.
AOAC. (1996). Official method of analysis. Washington, DC: Association of Official Analytical Chemists.
Bellagha, S., Sahli, A., & Farhat, A. (2008). Desorption isotherms and isoseteric heat of three Tunisian date cultivars. Food and Bioprocess Technology, 1, 270–275.
Blahovec, J. (2004). Sorption isotherms in materials of biological origin mathematical and physical approach. Journal of Food Engineering, 65, 489–495.
Blahovec, J., & Yanniotis, S. (2008). GAB generalized equation for sorption phenomena. Food and Bioprocess Technology, 1, 82–90.
Cernisev, S. (2010). Effect of conventional and multistage drying processing on non-enzymatic browning in tomato. Journal of Food Engineering, 96, 114–118.
Chowdhury, F. A., Raman, M. A., & Mian, A. J. (1997). Distribution of free sugars and fatty acids in jackfruit (Artocarpus heterophyllus). Food Chemistry, 60, 25–28.
Chung, S. G., & Pfost, H. B. (1976). Summarizing and reporting equilibrium moisture data for grains (pp. 76–3520). St. Joseph: American Society of Agriculture Engineers.
Esper, A., & Mühlbauer, W. (1996). Solar tunnel dryer for fruits. Plant Research and Development, 44, 61–80.
Falade, K. O., Olukini, I., & Adegoke, G. O. (2004). Adsorption isotherm and heat of sorption of somatically pretreated and air-dried pineapples slices. European Food Research and Technology, 218, 540–543.
Hayoglu, I., & Gamli, O. F. (2007). Water sorption isotherms of pistachio nut paste. International Journal of Food Science and Technology, 43, 224–227.
Hubinger, M., Menegalli, F. C., Aguerre, R. J., & Suarez, C. (1992). Water vapor adsorption isotherms of guava, mango and pineapple. Journal of Food Science, 57, 1405–1407.
Illeperuma, C. K., & Jayasuriya, P. (2002). Prolonged storage of ‘Karthakolomban’ mango by modified atmosphere packaging at low temperature. The Journal of Horticultural Science and Biotechnology, 77, 153–157.
SAS Institute (1990) SAS language and procedures, version 6.1st edition. SAS Institute, Cary, NC.
Isse, M. G., Schuchmann, H., & Schubert, H. (1993). Divided sorption isotherm concept: An alternative way to describe sorption isotherm data. Journal of Food Process Engineering, 16, 147–157.
Kiranoudis, C. T., Maroulis, Z. B., Tsami, M., & Marinos-Kouris, D. (1993). Equilibrium moisture content and heat of desorption of some vegetables. Journal of Food Engineering, 20, 55–74.
Labuza, T. P., Kannane, A., & Chen, J. (1985). Effect of temperature on the moisture sorption isotherm and water activity shift of two dehydrated foods. Journal of Food Science, 50, 385–392.
Lewicki, P. P. (1997). The applicability of the GAB model to food water sorption isotherms. International Journal of Food Science and Technology, 32, 553–557.
Lutz, K., Muhlbauer, W., Muller, J., & Reisinger, G. (1987). Development of multi-purpose solar crop dryer for arid zones. Solar and Wind Technology, 4, 417–428.
Mir, A. M., & Nath, N. (1995). Sorption isotherms of fortified mango bars. Journal of Food Engineering, 25, 141–150.
Ngoddy, P. O., & Bakker-Arkema, F. W. (1970). A generalized theory of sorption phenomena in biological materials (Part I. The isotherm equation). Transactions of ASAE, 13, 612–617.
Ngoddy, P. O., & Bakker-Arkema, F. W. (1972). A generalized theory of sorption phenomena in biological materials. Part III—A pore size distribution function of the power law type for biological materials. Transactions of ASAE, 15, 1160–1164.
Quirijns, E. J., van Boxtel, A. J. B., van Loon, W. K. P., & van Straten, G. (2005). Sorption isotherms, GAB parameters and isosteric heat of sorption. Journal of the Science of Food and Agriculture, 85, 1805–1814.
Rahman, A. K. M., Huq, E., Mian, A. J., & Chesson, A. (1995). Microscopic and chemical changes occurring during the ripening of two forms of jackfruit (Artocarpus heterophyllus L.). Food Chemistry, 52, 405–410.
Ranganna, S. (1986). Hand book of analysis and quality control for fruit and vegetable products (2nd ed.). New Delhi: Tata McGraw-Hill Publishing Company Limited.
Saravacos, G. D., Tsiourvas, D. A., & Tsami, E. (1986). Effect of temperature on the water adsorption isotherms of sultana raisins. Journal of Food Science, 51, 381–383.
Sato, Y., Wada, Y., & Higo, A. (2010). Relationship between monolayer and multilayer water contents, and involvement in gelatinization of baked starch products. Journal of Food Engineering, 96, 172–178.
Schirmer, P., Janjai, S., Esper, A., Smitabhindu, R., & Muhlbauer, W. (1996). Experimental investment of the performance of the solar tunnel dryer for drying bananas. Renewable Energy, 7, 119–129.
Singh, P. C., & Singh, R. K. (1996). Application of GAB model for water sorption isotherms of food products. Journal of Food Processing and Preservation, 20, 203–220.
Singh, B., Panesar, P. S., Gupta, A. K., & Kennedy, J. F. (2006). Sorption isotherm behavior of osmoconvectively dehydrated carrot cubes. Journal of Food Processing and Preservation, 30, 684–698.
Sun, D.-W. (1999). Comparison and selection of EMC/ERH isotherm equations for rice. Journal of Stored Products Research, 35, 249–264.
Sun, D.-W., & Woods, J. L. (1994). The selection of sorption isotherm equation for wheat based on the fitting of available data. Journal of Stored Products Research, 24, 22–474.
Thompson, T. L., Peart, R. M., & Foster, G. H. (1968). Mathematical simulation of corn drying—A new model. Transactions of ASAE, 11, 582–586.
Togrul, H., & Arslan, N. (2006). Moisture sorption behaviour and thermodynamic characteristics of rice stored in a chamber under controlled humidity. Biosystems Engineering, 95, 181–195.
Tortoe, C., & Orchard, J. (2006). Microstructural changes of osmotically dehydrated tissues of apple, banana and potato. Scanning, 28, 172–178.
Tsami, E., Marinos-Kouris, D., & Maroulis, Z. B. (1990). Water sorption isotherms of raisins, currants, figs, prunes and apricots. Journal of Food Science, 55, 1594–1597.
Tsami, E., Maroulis, Z. B., Marinos-Kouris, D., & Saravacos, G. D. (1990). Heat of sorption of water in dried fruits. International Journal of Food Science and Technology, 25, 350–359.
Van den Berg, C. (1984). Description of water activity of food for engineering purposes by means of the GAB model of sorption. In B. M. Mckenna (Ed.), Engineering and Food (pp. 21–311). London: Elsevier Applied Science.
Verma, V. K., & Narain, M. (1990). Moisture absorption isotherms of jaggery. Journal of Stored Product Research, 26, 61–66.
Wexler, A. (1999). Constant humidity solutions. In D. R. Lide (Ed.), CRC handbook of chemistry and physics (pp. 15–25). New York: CRC Press.
Yanniotis, S., & Blahovec, J. (2009). Model analysis of sorption isotherm. LWT-Food Science and Technology, 42, 1688–1695.
Acknowledgment
We thank Jatal Mannapperuma (PGIA, University of Peradeniya, Sri Lanka) and Judy Johnson (USDA-ARS, Parlier, CA 93648, USA) for their valuable guidance and support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Prasantha, B.D.R., Amunogoda, P.N.R.J. Moisture Adsorption Characteristics of Solar-Dehydrated Mango and Jackfruit. Food Bioprocess Technol 6, 1720–1728 (2013). https://doi.org/10.1007/s11947-012-0832-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-012-0832-7