Abstract
Purpose
The aim of this study was to determine the experimental moisture sorption isotherm of green pepper and okra, assess the performance of literature sorption models describing their behavior, and specify bound water properties.
Methods
Isotherms were determined through the static gravimetric method at 30, 40, 50, and 60 °C. Twenty models were studied and fittings were done through nonlinear regression using MATLAB R2015b. GAB and Caurie models were used to determine energy constants and bound water properties respectively. Structural parameters were determined according to Kelvin and Halsey equations.
Results
All the experimental isotherm were of sigmoid shape. For both samples, Peleg, Enderby, and Guggenheim Anderson de Boer (GAB) models gave the best validation statistic criteria. R-squared (R2) values were all greater than 0.926 and root-mean-square error (RMSE) values less than 0.04 for both substrates. The GAB energy constants varied with temperature between 6.00 to 51.75 and 34.23 to 176.78 for Cg constants, and 0.70–0.99 and 0.75–0.93 for Kg constants, respectively, for okra and green pepper. The Cg energy constant globally decreased with increasing temperature. The monolayer moisture content Wm, varied with temperature between (0.0740–0.1044) kg kg−1 and (0.0760–0.1522) kg kg−1 respectively for okra and green pepper. Green pepper bound water property values were globally higher than those of okra and both were successfully modeled according to the equilibrium moisture content and according to the temperature. Analysis based on the IUPAC classification revealed a macroporous structure of the two materials.
Conclusions
The hygroscopic equilibriums, physicochemical and microbiological stability conditions of green pepper and okra, were determined, for given environmental temperature values. The results obtained from the present study would serve in understanding the water state and mechanism of water sorption, defining drying and storage conditions of the tested vegetables, as well as designing corresponding dryers and packaging.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Okra (Abelmoschus esculentus L. Moench) and sweet green pepper (Capsicum annum L. Moench) are vegetable crops grown in the tropical, subtropical, and warm areas of the world. They are herbaceous plants whose fruits are considered vegetables (Mallikarjunaiah 2009). Originated from tropical Africa or Asia, okra is an annual plant of the Malvaceae family (Purseglove 1987). Its fruits are rich in fiber, protein, and essential amino acids; they are a good source of minerals for human diet (Ca, Na, Cu, Mn, Zn, Mg, P, Fe, and K) and source of provitamin A, and vitamins B, C, and E (Makhadmeh and Ereifej 2007; Olivera et al. 2012; Kouassi et al. 2013; Petropoulos et al. 2018). They are appreciated for their viscosity, and their medicinal properties, but also according to the color or the length of the capsule (Olivera et al. 2012). There is a wide variety of okra around the world and their physico-chemical characteristics are significantly influenced by genotype and harvest stage (Makhadmeh and Ereifej 2007; Olivera et al. 2012; Saha et al. 2016; Petropoulos et al. 2018). In Cameroon, cultivated varieties are not listed. Regarding green pepper, it is an annual plant of the Solanaceae family, native from South America, then probably domesticated in Mexico. Sweet green pepper is the world’s second most important solanaceous vegetable after tomato (Sahu and Tiwari 2007). This vegetable is a great source of fiber, K, Ca, P, Mg, provitamin A, and vitamins B and C; it also presents some medicinal properties (Jancik 1936; Van Duyn and Pivonka 2000; Pérez-López et al. 2007; Po et al. 2018). Several cultivated varieties have been mentioned in the literature (Terry Kelley and Boyhan 2009); however, there are no references mentioning the typology of those grown in Cameroon.
In the African and Asian continents, okra and sweet green pepper occupy a prominent place in the human diet. They are eaten raw or cooked, or dried and used in the form of powder for seasonings (Sahu and Tiwari 2007). However, after harvest, these two vegetables deteriorate relatively quickly (few days) due to their intrinsic properties and especially climatic and environmental conditions not always favorable (Charrier et al 1997; Shivhare et al. 2000; Heard 2002). Indeed, their moisture contents vary between 88 and 94% and they are usually, after harvest, stored at room temperature around 25–35 °C and in an environment with relative humidity between 70 and 100% (Heard 2002). In Africa in general, and in Cameroon in particular, their post-harvest conservation remains a permanent problem, as the artisanal techniques employed lead not only to a significant loss of their nutritive values, but also to their instability over time and to economic losses. The annual production of okra in Cameroon rose from 34,120 tons in 2002 to 60,384 tons in 2010 and 90,780 tons in 2016 (INS 2015; INS 2017). As for pepper, Cameroon’s annual production rose from 7,982 tons in 2002 to 37,307 tons in 2012 and to 58,903 tons in 2016 (INS 2015; INS 2017). Given the ever-growing production of these two vegetables, it is important to master the techniques of their conservation in order to give an added value when marketing.
Regarding conservation techniques, several authors have shown the need to determine the sorption isotherm of food materials (Al-Muhtaseb et al. 2002; Martinez-Monteagudo and Salais-Fierro 2014; Polachini et al. 2016). Indeed, they are experimental curves that show the evolution of the equilibrium water content of the product as a function of the relative humidity of the air surrounding the product, at constant temperatures. They give information on the mechanism of water sorption, the intensities of interactions between food components and water, and the distribution of water molecules as well as their functional availability in biochemical and biological substances (Touati 2008; Ahmat et al. 2014). The exploitation of sorption isotherm is necessary for the design and optimization of drying equipment, the determination of the drying limit at given air conditions, the estimation of the microbiological and physico-chemical storage stability, and the determination of the product moisture changes that may occur during storage or the choice of conditioning material (Erbas et al. 2005; Simal et al. 2007; Vega-Gálvez et al. 2007; Moreira et al. 2009).
Several models of fruit and vegetable sorption isotherm are cited in the literature and differ essentially in the number of parameters involved and in the empirical, semi-empirical, or theoretical nature of the model (Chirife and Iglesias 1978; Touati 2008; Wanjiru Gichau et al. 2019). These models make it possible to predict the hygroscopic behavior, the states and characteristics of the water in the product under consideration, depending on the progress of drying or during storage. They also give an indication of the evolution of the structure of the product through the variation of the pore radius and the bound water monolayer thickness (Ahmat et al. 2014).
Although green pepper and okra are vegetables of considerable economic value, their processing has not received due attention. Moreover, experimental moisture sorption isotherm as well as empirical, semi-empirical, or theoretical models to describe okra and green pepper behaviors remain limited (Kiranoudis et al. 1993; Fahrettin and Maskan 1999; Shivhare et al. 2000; Kaymak-Ertekin and Sultanoglu 2001). Some studies are conducted with temperatures not more than 50 °C (Fahrettin and Maskan 1999; Vega-Gálvez et al. 2007); therefore, they do not involve the usual ranges of drying temperatures. Other studies are conducted on pretreated or transformed materials (Shivhare et al. 2000; Sahu and Tiwari 2007). From all these past studies, information relative to the properties of bound water are limited, while several authors have mentioned the need to study the influence of temperature on the material characteristic quantities such as the number of monolayers, the percentage of bound water, the water content of the monolayer, the density of sorbed water and the specific surface area of sorption (Sahu and Das 2010; Choudhury et al. 2010; Ngono Mbarga et al. 2017). These parameters help to understand the moisture behavior in a particular material under different storage conditions. To our knowledge, no literature has been published on okra or green pepper from Cameroon, mentioning the behavior of their equilibrium moisture content according to environmental conditions. Therefore, the objectives of this work were to determine the experimental sorption isotherm of Cameroon okra and sweet green pepper at 30, 40, 50, and 60 °C, to evaluate the performance of literature sorption models, to classify them based on statistical parameters and to determine the bound water properties of these materials.
Materials and Methods
Experimental Device
The desorption isotherm of okra and sweet green pepper were determined by the static gravimetric method, based on the use of saturated salt solutions conferring, for a given temperature and at equilibrium, a precised relative humidity to the environment (Pahlevanzadeh and Yazdani 2005; Ahmat et al. 2014). The device used consists of a 53 liters temperature controlled oven (Memmert GmbH, Schwabach, Germany), with temperature range from 20 to 220 °C and absolute uncertainty of 0.5 °C. The tested temperatures were 30, 40, 50, and 60 °C. The 1-l-capacity jars of Bioblock brand used are equipped with a vacuum system creating a partial vacuum to accelerate the sorption process (Fig. 1). The jars were nine in number, providing nine experimental points. Each jar contained a quarter of different saturated salt solution (KOH, LiCl, CH3COOK, MgCl2, K2CO3, NaBr, CuCl, NaCl, KCl). This group of salts allows the obtention of a wide range of relative humidity, between 5 and 84% (Ahmat et al. 2014). Each jar had a sample holder above the salt solution, on which a cup containing a fresh mass of sample was placed.
(a) A view of the thermostated oven used for experiments. (b) From Ahmat et al. (2014) A schematic diagram of the sorption jar
Preparation of the Raw Material
Fresh okra and sweet green pepper pods, of uniform maturity, were purchased at the local market of Ngaoundere, in the Adamawa region of Cameroun in the month of July. Fig. 2 shows a picture of the samples. They were washed with tap water and drained. Using a knife, the stalks were cut and the sepal whorls hand-removed. Pods with wounds and bruises were discarded. Concerning physical characterization, they were weighed, their length and diameter measured using a Mitutoyo digital caliper, and their moisture content determined. These physical quantities were, respectively, for okra and green pepper pods, (17.64 ± 5.35) and (67.25 ± 17.67) g for their weight; (7.11 ± 1.12) and (4.86 ± 1.20) cm for their length; (1.73 ± 0.40) and (4.86 ± 0.78) cm for their diameter; and (94.2 ± 0.2) and (92.0 ± 0.2) % wet basis for their moisture content. Values are means ± standard deviations of measurements from 50 fruits. Pods were sliced (7–10 g each) and placed in a plastic cup. The mass of the sample was small enough to have a very weak influence on the hygrometric conditions of the atmosphere (almost stable saturation of the solution), but large enough for their mass to be measured, once equilibrium was reached, with the weighing scale whose precision was 1/100. The plastic cup was then placed on a grid disposed about 15 mm above the surface of the salt solution in order to avoid the immersion of the sample in the salt solution, as suggested by Trujillo et al. (2003). To avoid mold growth in the medium, samples were treated in a 10% (m/V) sodium benzoate solution. Three samples of the tested product were placed in each cup in order to obtain an average mass at equilibrium.
Monitoring of Sorption Experiments
The monitoring of the sorption in the experimental device was carried out by weighing the samples (without removing them from their cup and taking into account the weight of the tare) using a Sartorius electronic balance (precision at 1/100), every 24 h, until equilibrium. This was considered attained when the difference between two consecutive measurements of the mass of the sample was less than or equal to 0.01 g. At equilibrium, the water activity (aw) of the sample is identical to the relative humidity of the air (Eq. (1)).
The dry masses (Ms) were obtained after desiccation of the samples resulting from the desorption process, in an oven at 105±0.5 °C for 24 h. The equilibrium moisture content of the product was calculated as presented by Eq. (2).
All samples were run in triplicate.
Determination of the Biochemical Composition of Okra and Green Pepper
The total moisture and ash content were determined using the AOAC method (AOAC 1990). Protein content and total nitrogen were determined by mineralization of samples according to the Kjeldahl method (AFNOR 1982) and thereafter, nitrogen was assayed by the method of Devani, described by Sáez-Plaza et al. (2013). The total sugar content was determined by spectrophotometric method of Dubois et al. (1956) and total lipid content was determined by the Russian method described by Bourely (1982) based on the extraction of lipids using Soxhlet by hexane. The vitamin C content of substrates was determined using the 2,6-dichloro-indophenol titrimetric method (AOAC 2007). All samples were run in triplicate.
Modeling Sorption Curves
Numerous sorption models, empirical, semi-empirical, or theoretical, have been successfully used to describe water sorption isotherm of various food materials in a given range of water activity (Chirife and Iglesias 1978; Erbas et al. 2005; Ngono Mbarga et al. 2017). As several different mechanisms that depend on the material structure and composition are involved during sorption of water, sorption isotherm may be described by many different equations (Chirife and Iglesias 1978; Al-Muhtaseb et al. 2002). The experimental equilibrium moisture content (Xe) data for okra and green pepper were adjusted by testing twenty different models found in the literature (Table 1), among which eight models involving two parameters, ten other models having three parameters and the last two models involving four parameters. Eight of these models involve the effect of temperature. However, there is usually no scientific basis to justify the inclusion of the temperature effect in those models (Aguirre-Loredo et al. 2017). The aim was to determine the models that best describe the sorption isotherm of our products. For the adjustments, a tenth point (aw, Xe) at the origin (1E-05, 1E-05) was added to the experimental data, to take into account the absence of moisture in the product at equilibrium with a virtual zero-vapor pressure in the environment (Kouhila et al. 2002; Mayor et al. 2005).
Determination of the Properties of Bound Water
Caurie model (Caurie 1981) named Caurie2 in this work (Eq. (3)), based on monomolecular adsorption, allows the determination of some characteristics of bound water and the specific surface area of sorption (Singh et al. 2006). Similarly, the parameters of the GAB isotherm model (Eq. (4)) have a physical meaning in terms of the sorption processes (Timmermann et al. 2001).
Several authors (Menkov et al. 1999; Ayranci and Duman 2005; Erbas et al. 2005; Ahmat et al. 2014; Ngono Mbarga et al. 2017) use the linear form of isotherm equations to determine the properties of bound water. The linear model constants are then obtained through linear regression. For Caurie2, the linear form is generally expressed as presented in Eq. (5), where C and X0 correspond respectively to k1 and k2 of Eq. (3) and are respectively the constant related to the density of sorbed water and the monolayer moisture content.
For GAB model, the linear form is generally written as presented in Eq. (6), which necessitates the introduction of the correct value of Kg.
In this equation, Wm, Kg, and Cg respectively correspond to k1, k2, and k3 in Eq. (4) and are respectively the monolayer moisture content and energy constants. They are exponential functions of the inverse absolute temperature as presented in Eqs. (7)–(9) (Gabas et al. 2007).
where W0 is a constant and K0 and C0 are the entropic accommodation factors. The heats of sorption of the monolayer (hm) and of the multilayer (hn) were calculated as given in Eqs. (10) and (11).
where Lv is the average of the pure water enthalpy of vaporization, in the temperature range 30 to 60 °C. The average value is 43.12515 kJ mol−1, calculated using Popiel and Wojtkowiak (1998) table.
Each regression method uses a specific algorithm leading to some differences in model parameters values (Al-Mahasneh et al. 2012). In our calculations, statistical parameters associated to nonlinear regressions were always better than those obtained through linear regressions. Consequently, in the present work, the statistical criteria were privileged in the choice of the regression model. The parameters were therefore estimated by nonlinear regression as presented in the following section.
The number of monolayers of sorbed water, N, was directly calculated for each temperature (Eq. (12)) using the Caurie2 (Eq. (3)) parameters k1 and k2, estimated by nonlinear regression.
The percentage of bound water (bw) was calculated as shown in Eq. (13). The specific surface area of sorption, A, was calculated as presented in Eq. (14), assuming that the magnitude of k1 in the Caurie’s model is equivalent to the density of sorbed water in the monolayer (Singh et al. 2001).
where d =3.673 ∙ 10−10m is the diameter of water molecules.
The Kelvin equation (Eq. (15)) allows the determination of the critical pore radius rc (nm). This equation applies primarily to the condensation region of the isotherm (Singh et al. 2001; Miyata et al. 2003):
However, the Kelvin equation does not take into account the thickness of layers formed on the porous surface prior to condensation. In order to describe the thickness of the precondensation film on the pore walls, the Halsey equation (Eq. (16)) can be used to predict the thickness of the layer, t (nm), formed on the porous surface at a given water activity (Singh et al. 2001; Miyata et al. 2003).
Therefore, pore radius Rp (nm) is calculated as presented in Eq. (17) (Singh et al. 2001).
Statistical Analysis
The modeling of sorption isotherm requires the statistical methods of regression analysis and correlation. The model parameters were estimated by nonlinear regression using Newton’s least squares method to minimize the sum of squares of the residuals between experimental and predicted equilibrium moisture contents (Xexp and Xpre respectively). In literature, the statistics used to compare models vary from one author to another, which makes it difficult to compare values among several authors. To cover the maximum statistics used in literature and allow further comparisons, the models in this work were compared based on two-dimensional statistics, namely the root-mean-square error (RMSE) which measures the standard deviation of the residuals and the mean absolute error (MAE), and five nondimensional statistics, namely the mean relative error (MRE), the coefficient of determination (R2) which measures the proportion of variability in the response explained by the regression model, the adjusted R-squared (\( {R}_{\mathrm{adj}}^2 \)) which takes into account the effects of the number of model parameters, the percentage of deviation (Pd), and the normalized root-mean-square error (NRMSE) expressed as a percentage (Willmott et al. 1985). They were calculated using MATLAB R2015b software as presented by Eqs. (18)–(24). A model was considered to be acceptable if, for all the tested temperatures, the RMSE and MAE values were close to zero, the R-squared (R2) and adjusted R-squared (\( {R}_{\mathrm{adj}}^2 \)) values greater than 0.9, and the MRE, Pd, and NRMSE values below 10% (Kaymak-Ertekin and Gedik 2004; Ahmat et al. 2014). Moreover, the model should fit the experimental points in the range of tested water activity and present an acceptable shape.
This statistic is also known as the standard error of the estimate, where p is the model number of parameters. It is defined as the square root of the mean square error (MSE).
Three decision criteria, Cmax, Cmin1, and Cmin2, calculated as shown in Eqs. (25)–(27), were used to assess the performance of sorption isotherm models and classify them. Cmax is non-dimensional and takes into account the statistics which contribute positively to the description of data by the model, whereas Cmin1 (having the dimension of Xe) and Cmin2 (non-dimensional) involve terms that quantify the errors committed using the model. The statistical parameters involved in these three equations are average values over the tested temperatures. The models were first ranked by the value of Cmax, secondly by the value of Cmin1, and thirdly by the value of Cmin2. The best model being the one with the highest value of Cmax and the lowest values of Cmin1 and Cmin2.
Results and Discussion
Physico-chemical Characterization of Biological Materials
The physico-chemical compositions of okra and green pepper are presented in Table 2. This table shows that these two vegetables are rich in water with a dry matter mainly composed of carbohydrates (66.2% and 70.0% respectively), followed by lipids (17.9% and 12.8% respectively) and ash (9.8% and 11.4% respectively). Protein occupies the last place of macronutrients, with a dry matter content of 5.9% and 5.6% respectively. Vitamin C plays a vital role in the human health, as it is involved in many physiological processes in the body. It is the consumption of fruits and vegetables that allow people to cover their daily vitamin C requirements. The results show that this vitamin is present in both vegetables. However, its concentration in okra samples is almost three times higher than that in pepper samples, thus attributing better antioxidant properties to okra. Globally, the obtained results show a statistically significant difference at the 95.0% confidence level, on each of the nutrient contents of the two substrates.
The multiple comparison test applied to sweet green pepper shows that the ash and protein contents are not significantly different at a 5% level. The results obtained are different from those presented by Faustino et al. (2007) whose lipid and ash fractions, based on the dry matter of the green pepper samples, are approximately two times lower than our respective values. The dry matter of their sample contains three times more protein than ours; however, the sugar contents are almost identical. Similar trends are observed with Jancik (1936) results. Carbohydrate, fat, and ash contents of our samples are double that of Po et al. (2018), whereas the protein and vitamin C contents are twice lower than theirs. Our sample’s vitamin C contents are also lower than the results presented by Topuz and Ozdemir (2007) and Zoran Ilić et al. (2014), whose vitamin C levels in green pepper vary between 63 and 147 mg/100 g Several authors (Jancik 1936; Martinez et al. 2007; Pérez-López et al. 2007; Zoran Ilić et al. 2014) reported that the variety, the growing conditions, and the stage of maturity as well as the postharvest storage conditions of green sweet pepper affect the fruit quality parameters and bioactive compounds.
The multiple comparison test applied to okra shows that ash, protein, lipid, and soluble sugar levels are not significantly different at a 5% level. The total sugar and ash contents of our okra sample are similar to those presented by Makhadmeh and Ereifej (2007) on seven varieties of okra and by Kouassi et al. (2013) on two varieties of okra. However, contrary to our samples, the protein contents of the samples of these two authors are on average seven times higher than their lipid contents. Similar trends are observed with EL-Nahry et al. (1978) and Nwachukwu et al. (2014) results. Petropoulos et al. (2018) reported that fruit size has a genotype-dependent impact on the chemical composition and nutritional value of okra pods. Al-Wandawi (1983) and UdayasekharaRao (1985) reported that the okra seed is a rich source of proteins, and these contents depend on the cultivar. Knowing that okra lipids and proteins are mainly localized at the grain level, the observed deviations from results obtained from literature, in the contents of these two constituents, could be due to the differences in cultivars used, climatic conditions, quality of the soil, and degree of maturity of the fruits (Mallikarjunaiah 2009). These latter could also explain the vitamin C contents four times higher in our samples, compared to those reported by Bergeret and Masseyeff (1958) on a variety of okra from Cameroon.
Experimental Desorption Curves
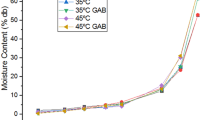
The experimental desorption curves of okra and pepper are presented in Fig. 3. The isotherms of the two substrates are all of type II. This one is the sigmoid form of isotherm, which present three zones and an asymptotic tendency when the water activity tends towards 1. This type is observed for very dispersed porous structure with condensation in the pores (capillary condensation) occurring before saturation of the material (Kouhila et al. 2002). The three zones, 1, 2, and 3, of type II isotherm, correspond respectively to the adsorption of monomolecular film of water, the adsorption of additional layers of water over the monolayer, and the zone where water is condensed into the pores of the material, followed by dissolution of the soluble material present (Aguirre-Loredo et al. 2017). According to IUPAC (International Union of Pure and Applied Chemistry) classification, there is a progressive thickening of the adsorbed layer characterizing a multimolecular adsorption (Brunauer et al. 1938; Wanjiru Gichau et al. 2019). According to several authors, most of agri-food product, and medicinal and aromatic plant isotherms, also exhibit this sigmoidal form of isotherm (Iglesias et al. 1986; Menkov et al. 1999; Kouhila et al. 2002; Goneli et al. 2010).
The experimental curves of the isotherm of okra and green pepper (Fig. 3) show that at a given water activity, the equilibrium moisture content increases with decrease in temperature. Similar results have been reported by several authors (Kouhila et al. 2002; Choudhury et al. 2010). Erbas et al. (2005) suggest that at lower temperatures, water molecules have a lower kinetic energy which is not enough to overcome the corresponding sorption energy. The effect of temperature on isotherm is more marked in pepper than in okra. For a given temperature and water activity in zones 1 and 2 of the sorption isotherm, the equilibrium water content observed for okra is always lower than that obtained with green pepper. The gap is even more marked at low temperatures. This phenomenon could be related to the difference in structure of the two materials and to the change in sorption properties of the material constituents during the sorption process, due to physical and chemical interactions induced by heating (Chirife and Iglesias 1978). Modeling experimental curves would allow for better understanding of the phenomenon.
Modeling and Classification of Desorption Isotherm
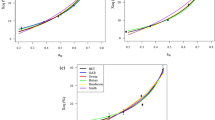
The twenty models of sorption isotherm presented in Table 1 were used to assess their ability to represent the experimental results of the desorption isotherm of okra and green pepper obtained at four different temperatures (30, 40, 50, and 60 °C). The classification of the twenty sorption isotherm models, based on the statistical parameters and the method previously described in the statistical analysis section, indicates that the four-parameter models occupy the first places. The first three models, in order of merit, are the Peleg, Enderby, and GAB models for okra and the Enderby, Peleg, and GAB models for green pepper (Table 3). These three models are compared to the experimental points in Fig. 4. It appears that for all the tested temperatures, the behavior of okra and pepper can suitably be described by these three models. Of the twenty tested models, in addition to these first three models, ten other models meet the acceptability criteria, these are the Caurie2, modified Hailwood and Horobin, Halsey, modified Halsey, Lespam for okra/Harkins and Jura for green pepper, modified Henderson, Langmuir, modified Luikov, Oswin, and modified Oswin models (Table 3). For each tested temperature, the statistical parameters of the validated models are presented in Tables 4 and 5, respectively, for okra and green pepper. The Lespam and Harkins & Jura models for the two substrates are also shown. The model constants are presented in Table 6 for both substrates.
In literature, the Peleg model has adequately described the moisture sorption isotherm of several food products (Kaya and Kahyaoglu 2005; Ouafi et al. 2015). However, unlike the GAB model, the Peleg model is a purely empirical model without a theoretical background. Several authors have mentioned that the GAB model is well suited to describe the sorption isotherm of most fruits and vegetables (Timmermann et al. 2001). It is therefore frequently used to determine important parameters which are useful in physicochemical descriptions, including the moisture content in the monolayer, which represents the amount of water required to form a monolayer on the surface, as well as energy constants (Timmermann et al. 2001). Fahrettin and Maskan (1999) successfully applied GAB model to describe the behavior of okra adsorption isotherm at temperatures between 10 and 30 °C. However, contrary to our results, they observed that the monolayer moisture content k1 (or Wm) decreases with increase in temperature and that the energy constants k2 (or Kg) and k3 (or Cg) increase with temperature. Taking into account their range of tested temperatures, this result would indicate the presence of an extremum around 30 °C. Kaymak-Ertekin and Sultanoglu (2001) showed that the GAB and the Oswin models are satisfactory in predicting the equilibrium moisture content of peppers, with orders of magnitude and evolution with the temperature of the constants k1 and k2, similar to our results. These authors also observed, with the red pepper, the presence of a maximum for the evolution of k3 (Cg) with temperature. However, our Cg values are on average ten times higher than theirs. The GAB model monolayer moisture contents obtained with red bell pepper by Vega-Gálvez et al. (2007) ranged from 0.07 to 0.10 (g g−1 db), similar to our results with sweet green pepper. Swami et al. (2005) attributed the decrease in the monolayer moisture content k1 (Wm) with the increase in temperature, to the decrease in the number of active sites, or total sorption ability of the material, due to physical and chemical changes induced by temperature.
According to Van Den Berg (1991), most dried food products are empirically observed to display their greatest stability at moisture contents comparable to the monolayer moisture content. According to the GAB model, the monolayer moisture contents vary with temperature between 7.40 and 10.44 (g/100 g db) for okra and between 7.60 and 15.22 (g/100 g db) for green pepper. Therefore, these two vegetables have an identical critical water activity value of 0.35 corresponding to an average moisture content of 7.5 (g/100 g db). Beyond this value, their stability would be compromised. This value is less severe than the average values of 3.7 (BET) and 4.9 (GAB) (g/100 g db) obtained by Sahu and Tiwari (2007) on osmotically dehydrated sweet pepper. The water content range for green pepper stability observed in literature varies between 7.0 and 45.0 (g/100 g db) (Kiranoudis et al. 1993; Zhang et al. 1996; Kaymak-Ertekin and Sultanoglu 2001) for temperatures between 10 and 60 °C. Vega-Gálvez et al. (2007) obtained a moisture content of the monolayer between 7.0 and 10.0 (g water/100 g db) with red bell pepper (Var Lamuyo), for temperatures between 10 and 30 °C. They observed no direct dependence on temperature.
An observation of the model-ranking Table 3 shows that the modified Caurie2, Oswin, and modified Oswin models have almost identical statistical results, justified by the identical form of the writing of their respective equations. The only difference lies in the adjusted R2 of the modified Oswin model, which has a third parameter to be determined, inducing a decrease in its adjusted R2 value. These three models are thus written, for a given temperature, in the general form presented by Eq. (28).
For the Caurie2 model, A = k1 ∙ k2 and B = 2 ∙ k1/k2 ; for the Oswin model, A = k1 and B = k2 and for the modified Oswin model, A = k1 + k2. T and B = k3.
Properties of Bound Water: Determination of Energy Constants
The models of the okra energy constants obtained, related to the water molecule layers, are presented in Eqs. (29)–(31):
The results indicate that temperature has a significant effect on the Cg energy constant. On the other hand, the two constants Wm and Kg evolve little with temperature. Wm tends to increase with increase in temperature. This phenomenon could be due to the effect of temperature on the gums and mucilaginous substances of okra, increasing their water retention properties.
Concerning green pepper, the models of the energy constants obtained are presented in Eqs. (32)–(34):
Here also, the constants Wm and Kg seem to be slightly influenced by the temperature with respective average values of 0.10239 ± 0.03493 (kg kg−1 db) and 0.86069 ± 0.07814. However, each of these constants evolves in the opposite direction of the variations of these parameters observed with okra. As for okra, the energy constant Cg of green pepper decreases significantly with increasing temperature between 40 and 60 °C. This effect of temperature could be attributed to structural modifications in the substrate, induced by changes in the crystallinity of polymers, cross-linking and denaturation of proteins, or non-enzymatic browning reactions (Iglesias et al. 1986). Since the Cg values are greater than 2, the isotherms are of type II (Taitano and Singh 2012) and confirm the sigmoid form observed above. For the two substrates and in the range of tested temperatures, the constant values related to multilayer properties, Kg, also known as the degree of freedom of water molecules (Aguirre-Loredo et al. 2017) were all close to but less than unity and varied between 0.70–0.99 and 0.75–0.93 respectively for okra and green pepper. These results confirm the particular characteristic of the GAB model (Timmermann et al. 2001; Taitano and Singh 2012). Table 7 presents the average values, over the range of tested temperatures, of Wm and Kg for okra and green pepper.
From Eq. (10) to Eq. (11), the heat of sorption for the first and subsequent layers of water molecules of okra and green pepper samples were calculated and are presented in Table 7. This table shows that the energy of sorption of water between the monolayer and the multilayer is greater for okra compared to that of green pepper; they are both positive and respectively equal to 52.19 and 38.44 kJ/mol. According to Kiranoudis et al. (1993), ΔH1 is expected to have a large positive value, due to the exothermic interaction of water vapor with primary sorption site of the material. They obtained estimated values for green pepper of 33.4 kJ/mol relatively close to the value obtained for our green pepper sample.
The removal of water from green pepper requires greater energies as we move from the zone of condensation of pure water to the area of the monolayer bound water. On the other hand, the positive value of the water sorption enthalpy between the condensing zone of the pure water and the upper layer of the okra multilayer (ΔH2) is linked to the fact that the energy constant, Kg, of okra decreases with temperature. This result indicates that the water sorption energy of the upper layers of okra is substantially equal to the latent heat of vaporization of pure water. In literature, the estimated values of ∆H2 are found to be positive for vegetables and negative in the case of fruits (Kiranoudis et al. 1993; Simal et al. 2007). Therefore, these results indicate that green pepper behaves like a fruit with regard to its sorption characteristics, while okra behaves like a vegetable. These behaviors are also noticed through the ∆H values of the two vegetables.
Modeling Bound Water Properties
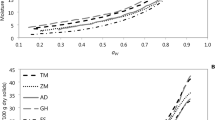
The Caurie2 model was used to determine these bound water properties. As illustrated in Fig. 5, the temperature effects on bound water properties are more pronounced with green pepper as compared to okra. In the range of tested temperatures, the number of monolayers is greater than 1 indicating that, for the two substrates, the sorption process is multilayered. Green pepper number of monolayers, percentage of bound water, water content of the monolayer, and specific surface area of sorption are in average higher than those of okra. This phenomenon is more accentuated at low temperatures. This result indicates a difference in structure of the two substrates. From 50 °C and above, these parameters converge to almost identical values, indicating that at high temperature, the bound water properties are not significantly influenced by the nature of the two substrates. The density of sorbed water (Caurie2 constant model, k1) globally decrease with the increase of temperature, with a more pronounced effect in the case of okra (Fig. 6).
For green pepper, the values of specific surface area of sorption, number of monolayers, percentage of bound water, and water content of the monolayer decrease with the increase in temperature and tend to attain a constant value at high temperatures. Similar results were found by Sahu and Das (2010) and Choudhury et al. (2010) on other substrates. On the contrary, the increase in temperature has a slightly positive effect on the okra’s specific surface area of sorption and on the sorbed water number of monolayers. However, the temperature effects on the other bound water properties are negligible. These results indicate that, for a better efficiency, the desorption process of okra substrates would require higher temperatures compared to those necessary for green pepper. Moreover, during desorption, it would be wise to find a compromise between high sorption surface area, low energy expenditure, and product quality.
Models of variation of bound water properties according to temperature have been determined (Table 8). These results are well-adjusted by second-order polynomial functions. However, with respect to Caurie2 constants with low values, such as k1 for green pepper and k2 for okra, a better adjustment could be obtained by third-order polynomial functions. Figs. 5 and 6 present the model fitting to the data.
Fig. 7 shows the evolution of the pore radius and the thickness of the bound water monolayer of the two substrates, as a function of the equilibrium water content of okra and green pepper. The ranges of variation of these two quantities are practically the same for the two plants and vary respectively from 300 to 8000 nm and from 0.4 to 1.1 nm. According to the IUPAC classification, we are in the presence of macropores. Since the thicknesses of the bound water monolayers are very small, less than 1.2 nm, the curves of the critical radius are similar to those of the pore radius for each substrate. Velázquez-Gutiérrez et al. (2015) reported that the pore radius of the mucilage extracted from chia seeds ranged from 0.87 to 6.44 nm. It can be seen from Fig. 7 that the pore radius and the thickness of the bound water monolayer of the two substrates increase with the equilibrium moisture content according to a second-order polynomial model, the parameters of which are presented in Table 9. Model parameters for the bound water monolayer thickness are also presented in the same table. The coefficients of determination of these models vary between 0.909–0.987 and 0.856–0.993 respectively for okra and green pepper. Hence, during desorption, there is a tightening of the pores of the substrate indicating a possible reduction in the volume of the material during drying. For a given equilibrium moisture content and for a given temperature, the pore radius and the thickness of the okra monolayer are always slightly higher than those of green pepper. Globally for both plants, for a given equilibrium water content, the increase in temperature causes a magnification of the pore radius and an increase in the thickness of the monolayer of bound water. Similar pore radius behaviors have been reported by Singh et al. (2001) and Igbeka and Blaisdell (1982) on meat products. However, in zone 1 of the sorption isotherm and in the case of okra, these quantities seem independent of the temperature and vary linearly with the equilibrium moisture content. The effect of temperature on the pore radius is more marked in zones 2 and 3 of the sorption isotherm, where for a given equilibrium moisture content, an increase in temperature induces an increase in pore radius and the thickness of the monolayer of bound water. This may be due to physical and/or chemical changes in the product induced by temperature. The difference in behavior between the two substrates would be closely related to their composition. The presence of mucilage and gums in okra is a key factor (Gunsanee 2011). The observed narrowing (shrinkage) suggests that prior to drying, a pretreatment of the substrates should be considered, to avoid severe shrinkage during drying.
Conclusion
The desorption isotherm curves of okra and green pepper were experimentally determined at four temperatures (30, 40, 50, and 60 °C) and were of type II, indicating a high hygroscopicity of the materials. Peleg, GAB, and Enderby models presented the best fits of the twenty tested models. The evaluation of the water content of the monolayer showed that the physico-chemical and microbiological stability of okra and green pepper are obtained at equilibrium moisture content of 8 and 10 (g/100 g db), respectively, corresponding to an optimal water activity of 0.35 for both vegetables. Bound water properties were determined and showed that substrate constituents play a key role in water sorption mechanism. The calculated energy constants indicate that okra has the characteristics of vegetables, while green pepper has those of fruits. The pore radius was found to decrease with decreasing moisture content for a given temperature, and to decrease with decreasing temperature for a given moisture content and for a particular product. For a given moisture content, okra’s pore radius were found to be slightly higher than those of green pepper. The determination of these parameters is of fundamental importance for good preservation of the product through drying, as well as the design of equipment for post-harvest preservation.
Abbreviations
- A :
-
specific surface area of sorption (m2 g−1)
- a w :
-
water activity (−)
- BET:
-
Brunauer, Emmett, and Teller
- bw:
-
percentage of bound water (%)
- C :
-
density of sorbed water in Caurie2 model (g ml−1)
- C g :
-
GAB energy constant (−)
- C 0 :
-
entropic accommodation factor (−)
- d:
-
water molecules diameter (d =3.673 ∙ 10−10 m)
- db:
-
dry basis
- Exp.:
-
experimental
- GAB:
-
Guggenheim Anderson de Boer
- h m :
-
heat of water sorption of the monolayer (kJ mol−1)
- h n :
-
heat of water sorption of the multilayer (kJ mol−1)
- HR:
-
air relative humidity (%)
- IUPAC :
-
International Union of Pure and Applied Chemistry
- k 1, k 2, k 3, k 4 :
-
model parameters
- K g :
-
GAB energy constant (−)
- K 0 :
-
entropic accommodation factor (−)
- L v :
-
average of the pure water enthalpy of vaporization (kJ mol−1)
- MAE:
-
mean absolute error (kg kg−1 db)
- M he :
-
thermodynamic equilibrium mass (g)
- Mod.:
-
modified
- MRE:
-
mean relative error (−)
- M s :
-
dry mass (g)
- N :
-
number of monolayers of sorbed water (−)
- n :
-
number of experimental points (−)
- NRMSE:
-
normalized root-mean-square error (%)
- Pd:
-
percentage of deviation (%)
- p :
-
number of model parameters (−)
- P vp :
-
partial pressure of water vapor in the air (Pa)
- P vs :
-
partial pressure of saturated vapor (Pa)
- R :
-
perfect gas constant (8.314462 J·mol−1·K−1)
- R 2 :
-
coefficient of determination (−)
- \( {R}_{\mathrm{adj}}^2 \) :
-
adjusted R squared (−)
- rc :
-
critical pore radius (nm)
- Rp :
-
pore radius (nm)
- RMSE:
-
root-mean-square error (kg kg−1 db)
- SSE:
-
sum of squares of errors
- SSR:
-
regression sum of squares
- SST:
-
total sum of squares
- t :
-
thickness of water monolayers formed on the porous surface (nm)
- T :
-
temperature in °C
- Ta:
-
absolute temperature in K
- V :
-
specific sorption volume (m3 g−1)
- V m :
-
standard molar volume (m3 mol−1)
- W m :
-
monolayer moisture content, GAB models (kg kg−1 db)
- W 0 :
-
constant (kg kg−1 db)
- X e :
-
Equilibrium moisture content of the product (kg kg−1 db)
- X i,exp :
-
the ith equilibrium moisture content of experimental equilibrium (kg kg−1 db)
- X i,pre :
-
the ith predicted equilibrium moisture content (kg kg−1 db)
- X 0 :
-
monolayer moisture content, Caurie2 model (kg kg−1 db)
- Y:
-
dependent variable
- ∆H 1 :
-
difference between monolayer and multilayer heats of sorption (kJ mol−1)
- ∆H 2 :
-
difference between the heat of vaporization of water and the heat of sorption of the multilayer (kJ mol−1)
- σ :
-
surface tension (N m−1)
References
AFNOR (1982). Recueil des normes françaises des produits dérivés des fruits et légumes, Première édition. Association Française de Normalisation, Paris, France.
Aguirre-Loredo, R. Y., Rodriguez-Hernandez, A. I., & Velazquez, G. (2017). Modelling the effect of temperature on the water sorption isotherms of chitosan films. Food Science and Technology, Campinas, 37(1), 112–118. https://doi.org/10.1590/1678-457X.09416.
Ahmat, T., Bruneau, D., Kuitche, A., & Waste, A. A. (2014). Desorption isotherms for fresh beef: an experimental and modeling approach. Meat Science, 96, 1417–1424.
Al-Wandawi, H. (1983). Chemical composition of seeds of two okra cultivars. Journal of Agricultural and Food Chemistry, 31(6), 1355–1358. https://doi.org/10.1021/jf00120a051.
Al-Mahasneh, M. A., Bani Amer, M. M., & Rababah, T. M. (2012). Modeling moisture sorption isotherms in roasted green wheat using least square regression and neural-fuzzy techniques. Food and Bioproducts Processing, 90(2), 165–170.
Al-Muhtaseb, A. H., Mcminn, W. A. M., & Magee, T. R. A. (2002). Moisture sorption isotherm characteristics of food products: a review. Transactions of the Institution of Chemical Engineers, 80(Part C), 118–128.
AOAC (1990). Agricultural chemicals; contaminants; drugs. in Kenneth Helrich (15th Ed.), Official methods of analysis, (1)9, CFR 318.19 (b), Virginia, USA: Association of Official Analytical Chemists.
AOAC (Ed.). (2007). Method 967.21 in Official methods of analysis (18th ed.). Gaithersburg: AOAC International.
Aviara, N. A., & Igbeka, J. C. (2015). Temperature dependence of cassava starch moisture sorption isotherms described using Modified Hailwood-Horrobin and other three parameter models. Faculty Seminar paper for Department of Agricultural and Environmental Resources Engineering, Faculty of Engineering, University of Maiduguri, Maiduguri.
Ayranci, E., & Duman, O. (2005). Moisture sorption isotherms of cowpea (Vigna unguiculata L. Walp) and its protein isolate at 10, 20 and 30°C. Journal of Food Engineering, 70, 83–91.
Bergeret, B., & Masseyeff, R. (1958). La vitamine C dans les fruits et legumes du Sud-Cameroun. Plant Food for Human Nutrition, 3, 192–201. https://doi.org/10.1007/BF01884050.
Bourely, J. (1982). Observations sur le dosage de l’huile des graines de cotonnier. Coton et Fibres Tropicales, XX.XVII(fasc. 2), 182–196.
Brunauer, S., Emmett, P. H., & Teller, E. (1938). Adsorption of gases in multimolecular layers. Journal of the American Chemical Society, 60(2), 309–319. https://doi.org/10.1021/ja01269a023.
Caurie, M. (1970). A practical approach to water sorption isotherms and the basis for the determination of optimum moisture levels of dehydrated foods. Journal of Food Science & Technology, 6, 85–93.
Caurie, M. (1981). Derivation of full range moisture sorption isotherms. In L. B. Rockland & G. F. Stewart (Eds.), Water Activity: Influences on Food Quality (pp. 63–86). New York: Academic Press.
Chirife, J., & Iglesias, H. A. (1978). Equations for fitting water sorption isotherms of foods: part I- a review. Journal of Food Technology, 13(1), 159–174.
Choudhury, D., Sahu, K., & Sharma, D. (2010). Moisture sorption isotherms, heat of sorption and properties of sorbed water of raw bamboo (Dendrocalamus longispathus) shoots. Industrial Crops and Products, 33, 211–216.
Chung, D. S., & Pfost, H. B. (1967). Adsorption and desorption of water vapour by cereal grains and their products. Part I. Heat and free energy changes of adsorption and desorption. Transactions of the ASAE, 10(549–551), 555.
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A., & Smith, F. (1956). Colorimetric method for determination of sugars and related substances. Analytical Chemistry, 28(3), 350–356.
El-Nahry, F. I., El-Ghorab, M. I., & Younes, R. (1978). Nutritive value of local varieties of fresh and sundried okra (Hibiscus esculentus) pods and seeds. Qualitas Plantarum, XXVIII(3), 227–231.
Erbas, M., Ertugay, M. F., & Certel, M. (2005). Moisture adsorption behaviour of semolina and farina. Journal of Food Engineering, 69(2), 191–198.
Fahrettin, G., & Maskan, M. (1999). Water adsorption and drying characteristics of okra Hibiscus Esculentus L. Drying Technology: An International Journal, 17(4&5), 883–894. https://doi.org/10.1080/07373939908917576.
Faustino, J. M. F., Barroca, M. J., & Guine, R. P. F. (2007). Study of the drying kinetics of green bell pepper and chemical characterization. Transactions of the Institution of Chemical Engineers. Part C, 85(C3), 163–170. https://doi.org/10.1205/fbp07009.
Gabas, A. L., Telis, V. R. N., Sobral, P. J. A., & Telis-Romero, J. (2007). Effect of maltodextrin and arabic gum in water vapor sorption thermodynamic properties of vacuum dried pineapple pulp powder. Journal of Food Engineering, 82, 246–252.
Goneli, A. L. D., Correa, P. C., Oliveira, G. H. H., & Botelho, F. M. (2010). Water desorption and thermodynamic properties of okra seeds. Transactions of the ASABE, American Society of Agricultural and Biological Engineers ISSN 2151-0032, 53(1), 191–197.
Gunsanee, S. (2011). Physical and Chemical Properties of okra (Abelmoschus esculentus (L.) Moench) gum and pectin and their application in food products. MS Thesis, Food and Nutrition for Development. Bangkok, Taiwan: Faculty of Graduate Studies, Mahidol University.
Halsey, G. (1948). Physical adsorption on non-uniform surfaces. Journal of Chemical Physics, 16(10), 931–937. https://doi.org/10.1063/1.1746689.
Harkins, W. D., & Jura, G. (1944). Surfaces of solids. XIII, A vapor method for the determination of the area of a solid without the assumption of a molecular area, and they are as occupied by nitrogen and other molecules on the surface of a solid. Journal of the American Chemical Society, 66, 1366–1381.
Heard, G. M. (2002). Microbiology of fresh-cut produce, Chap. 7, in Olusola Lamikanra (Ed.), Fresh-cut fruits and vegetables. Science, Technology, and Market (452 p.). Washington: CRC Press LLC.
Igbeka, J. C., & Blaisdell, J. L. (1982). Moisture isotherms of a processed product-Bologna. Journal of Food Technology, 17, 37–46.
Iglesias, H. A., & Chirife, J. (1976). Prediction of the effect of temperature on water sorption isotherms of food materials. Journal of Food Technology, 11, 910–917.
Iglesias, H. A., Chirife, J., & Ferro Fontan, F. (1986). Temperature dependence of water sorption isotherms of some foods. Journal of Food Science, 51(3), 551–553.
INS. (2015). Agriculture. Chapitre 14 in Annuaire Statistique du Cameroun. Institut National de la Statistique, Cameroun, Ministère de l’économie, de la planification et de l’aménagement du territoire, édition, 2015, 238–239.
INS (2017). Agriculture. Chapitre 13 in Annuaire Statistique du Cameroun. Institut National de la Statistique, Cameroun, Ministère de l’économie, de la planification et de l’aménagement du territoire, édition 2017, P 188.
Jancik, V.. (1936). Utilization and composition of green and red peppers (Capsicum annum). MS Thesis, University of Massachusetts Amherst https://scholarworks.umass.edu/theses/1638. Accessed 02 May 2020.
Kaya, S., & Kahyaoglu, T. (2005). Thermodynamic properties and sorption equilibrium of pestil (grape leather). Journal of Food Engineering, 71, 200–207.
Kaymak-Ertekin, F., & Gedik, A. (2004). Sorption isotherms and isosteric heat of sorption for grapes, apricots, apples and potatoes. Lebensmittel-Wissenschaft Technologie: Food Science Technology Science, 37, 429–438.
Kaymak-Ertekin, F., & Sultanoglu, M. (2001). Moisture sorption isotherm characteristics of peppers. Journal of Food Engineering, 47, 225–231.
Kiranoudis, C. T., Maroulis, Z. B., Tsami, E., & Marinos-Kouris, D. (1993). Equilibrium moisture content and heat of desorption of some vegetables. Journal of Food Engineering, 20, 55–74.
Kouassi, J. B., Massara, C.-C., Gnomblesson Tiahou, G., Monde, A. A., Essiagne Sess, D., & Tia Vama, E. (2013). Determination of physico-chemical properties of two varieties of okra traditionally dried. Journal of Food and Nutrition Sciences, 1(4), 38–42. https://doi.org/10.11648/j.jfns.20130104.12.
Kouhila, M., Kechaou, N., Otmani, M., Fliyou, M., & Lahsasni, S. (2002). Experimental study of sorption isotherms and drying kinetics of Moroccan Eucalyptus Globulus. Drying Technology, 20(10), 2027–2039.
Langmuir (1916). Journal of American Chemical Society, 46, 1361-1362.
Martinez, S., Curros, A., Bermudez, J., Carballo, J., & Franco, I. (2007). The composition of Arnoia peppers (Capsicum annuum L.) at different stages of maturity. International Journal of Food Sciences and Nutrition, 58, 150–161.
Mghazlia, S., Idlimamb, A., Mahrouza, M., Lahninea, L., Hidara, N., Ouhammoua, M., Mouhibc, M., Zantarc, S., & Bouchdoug, M. (2016). Comparative moisture sorption isotherms, modelling and isosteric heat of sorption of controlled and irradiated Moroccan rosemary leaves. Industrial Crops and Products, 88, 28–35. https://doi.org/10.1016/j.indcrop.2016.02.050.
Makhadmeh, I. M., & Ereifej, K. I. (2007). Geometric characteristics and chemical composition of okra (Hibiscus esculentus L.) grown under semi-arid conditions. International Journal of Food Properties, 7(1), 83–90. https://doi.org/10.1081/JFP-120022983.
Mallikarjunaiah, H. (2009). Performance of okra varieties under different nutrient management in areca garden. Thesis of the University of Agricultural Sciences, Bangalore, for the degree of Master of Science (Agriculture) in Horticulture, Bangalore. 74p.
Martinez-Monteagudo, S. I., & Salais-Fierro, F. (2014). Moisture sorption isotherms and thermodynamic properties of mexican mennonite-style cheese. Journal of Food Science and Technology, 51(10), 2393–2403.
Mayor, L., Moreira, R., Chenlo, F., & Sereno, A. M. (2005). Water sorption isotherms of fresh and partially osmotic dehydrated pumpkin parenchyma and seeds at several temperatures. European Food Research and Technology, 220, 163–167. https://doi.org/10.1007/s00217-004-1065-4.
Menkov, N. D., Paskalev, H. N., Galyazkov, D. I., & Kerezieva-Rakova, M. (1999). Applying the linear equation of correlation of Brunauer-Emmet-Teller (BET)-monolayer moisture content with temperature. Nahrung, 43(2), 118–121.
Miyata, T., Endo, A., Ohmori, T., Akiya, T., & Nakaiwa, M. (2003). Evaluation of pore size distribution in boundary region of micropore and mesopore using gas adsorption method. Journal of Colloid and Interface Science, 262, 116–125.
Moreira, R., Chenlo, F., & Torres, M. D. (2009). Simplified algorithm for the prediction of water sorption isotherms of fruits, vegetables and legumes based upon chemical composition. Journal of Food Engineering, 94(3), 334–343.
Ngono Mbarga, M. C., Bup Nde, D., Mohagir, A., Kapseu, C., & Elambo, N. G. (2017). Moisture sorption isotherms and properties of sorbed water of neem (Azadirichta indica A. Juss) kernels. Journal of Engineering Physics and Thermophysics, 90(1), 35–34. https://doi.org/10.1007/s10891-017-1536-7.
Nwachukwu, E. C., Nulit, R., & Rusea, G. (2014). Nutritional and biochemical properties of Malaysian okra variety. Advancement in Medicinal Plant Research, 16–19.
Olivera, D. F., Mugridge, A., Chaves, A. R., Mascheroni, R. H., & Viña, S. Z. (2012). Quality attributes of okra (Abelmoschus esculentus L. Moench) pods as affected by cultivar and fruit size. Journal of Food Research, 1(4), 224–235.
Oswin, C. R. (1946). The kinetics of package life III. Isotherm. Journal of Society of Chemical Industry, 65, 419–421.
Ouafi, N., Moghrani, H., Benaouad, N., Yassaa, N., Maachi, R., & Younsi, R. (2015). Moisture sorption isotherms and heat of sorption of Algerian bay leaves (Laurus nobilis). Maderas. Ciencia y tecnología, 17(4), 759–772. https://doi.org/10.4067/S0718-221X2015005000066.
Pahlevanzadeh, H., & Yazdani, M. (2005). Moisture adsorption isotherms and isosteric energy for almond. Journal of Food Process Engineering, 28, 331–345.
Peleg, M. (1993). Assessment of a semi-empirical four parameter general model for sigmoid moisture sorption isotherms. Journal of Food Process Engineering, 16(1), 21–37.
Pérez-López, A. J., López-Nicolas, J. M., Núñez-Delicado, E., Del Amor, F. M., & Carbonell-Barrachina, A. A. (2007). Effects of agricultural practices on color, carotenoids composition, and minerals contents of sweet peppers, cv. Almuden. Journal of Agricultural and Food Chemistry, 55, 8158–8164.
Petropoulos, S., Fernandes, A., Barros, L., & Ferreira, I. C. F. R. (2018). Chemical composition, nutritional value and antioxidant properties of Mediterranean okra genotypes in relation to harvest stage. Food Chemistry, 242, 466–474.
Po, L.G., Siddik, M., & Shahzad T. (2018). Chili pepper and paprika. 27. In Siddiq, M. and Mark, A. (Eds.), Uebersax, Second edition, Handbook of vegetables and vegetable processing, Vol II (pp. 633–660). John Wiley and Sons Ltd.
Polachini, T. C., Fachin, F., Betiol, L., Lopes-Filho, J. F., & Telis-Romero, J. (2016). Water adsorption isotherms and thermodynamic properties of cassava bagasse. Thermochimica Acta, 632, 79–85.
Popiel, C. O., & Wojtkowiak, J. (1998). Simple formulas for thermophysical properties of liquid water for heat transfer calculations (from 0°C to 150°C). Heat Transfer Engineering, 19(3), 87–101. https://doi.org/10.1080/01457639808939929.
Popovski, D., & Mitrevski, V., (2004). Some new four parameter models for moisture sorption isotherms. EJEAF Che. ISSN, 1579–4377.
Purseglove, J. W. (1987). Tropical crops-dicotyledons (pp. 368–370). New York: Edition Longman.
Sáez-Plaza, P., Navas, M. J., Wybraniec, S., Michałowski, T., & García Asuero, A. (2013). An overview of the Kjeldahl method of nitrogen determination. Part II. Sample Preparation, Working Scale, Instrumental Finish, and Quality Control. Critical Reviews in Analytical Chemistry, 43(4), 224–272. https://doi.org/10.1080/10408347.2012.751787.
Saha, S. R., Islam, A. F. M. S., Hasan, M. M., & Rob, M. M. (2016). Yield attributes and yield of okra as influenced by cultivars at Sylhet Region. Journal of the Bangladesh Agricultural University, 14(1), 13–16.
Sahu, K., & Das, H. (2010). Moisture sorption isotherm, properties of sorbed water and heat of sorption of sandesh – an indian milk product. Journal of Food Processing and Preservation, 34, 152–166.
Sahu, J. K., & Tiwari, A. (2007). Moisture sorption isotherms of osmotically dehydrated sweet pepper. International Journal of Food Engineering, 3(5), 15. https://doi.org/10.2202/1556-3758.1223.
Shivhare, U. S., Ahmed, J., & Singh, M. (2000). Equilibrium moisture content of bell pepper. International Journal of Food Properties, 3(3), 459–464.
Simal, S., Femenia, A., Castell-Palou, A., & Rosselló, C. (2007). Water desorption thermodynamic properties of pineapple. Journal of Food Engineering, 80(4), 1293–1301. https://doi.org/10.1016/j.jfoodeng.2006.10.001.
Simo Tagne, M. (2011). Contribution à l’étude du séchage des bois tropicaux au Cameroun : Aspects caractérisation, modélisation multi-échelle et simulation. Le cas des bois d’ayous (triplochiton scleroxylon) et d’ébène (diospyros crassiflora). Ph.D Thesis, University of Yaounde I, Faculty of Science, Department of Physics, Energy option, Cameroon.
Singh, R. R. B., Rao, K. H., Anjaneyulu, A. S. R., & Patil, G. R. (2001). Moisture sorption properties of smoked chicken sausages from spent hen meat. Food Research International, 34, 143–148.
Singh, R. R. B., Rao, K. H., Anjaneyulu, A. S. R., & Patil, G. R. (2006). Water desorption characteristics of raw goat meat: Effect of temperature. Journal of Food Engineering, 75, 228–236.
Smith, S. E. (1947). The sorption of water vapor by high polymers. Journal of the American Chemical Society, 69(3), 646–651.
Swami, S. B., Das, S. K., & Maiti, B. (2005). Moisture sorption isotherms of black gram nuggets (bori) at varied temperatures. Journal of Food Engineering, 67, 477–482.
Taitano, L. Z., & Singh, R. P. (2012). Moisture adsorption and thermodynamic properties of california grown almonds (Varieties: Nonpareil and Monterey). International Journal of Food Studies, 1, 31–75.
Terry Kelley, W., & Boyhan, G. (2009). Pepper history, scope, climate and taxonomy, cultural practices and varieties. Cooperative extension, Colleges of Agricultural and Environmental Sciences & Family and Consumer Sciences (Ed.), Commercial Pepper Production Handbook, Bulletin 1309 (pp. 3-7). Georgia: The University of Georgia.
Thompson, T. L., Peart, R. M., & Foster, G. H. (1968). Mathematical simulation of corn drying-a new model. Transactions of ASAE, 11(4), 582–586.
Timmermann, E. O., Chirife, J., & Iglesias, H. A. (2001). Water sorption isotherms of foods and foodstuffs: BET or GAB parameters? Journal of Food Engineering, 48, 19–31.
Topuz, A., & Ozdemir, F. (2007). Assessment of carotenoids, capsaicinoids and ascorbic acid composition of some selected pepper cultivars (Capsicum annuum L.) grown in Turkey. Journal of Food Composition and Analysis, 20, 596–602.
Touati, B. (2008). Etude théorique et expérimentale du séchage solaire des feuilles de la menthe verte (Mentha viridis). Ph.D Thesis. I.N.S.A., Lyon, France and TLEMCEN University, Algeria (Physique Energétique et Matériaux).
Trujillo, F. T., Pei, C. Y., & Tuan Pham, Q. (2003). Moisture sorption isotherm of fresh lean beef and external beef fat. Journal of Food Engineering, 60, 357–366.
UdayasekharaRao, P. (1985). Chemical composition and biological evaluation of okra (Hibiscus esculentus) seeds and their kernels. Qualitas Plantarum, Plant Foods for Human Nutrition, 35, 389–396.
Van den Berg, C. (1991). Food-water relations: progress and integration, comments and thoughts. In H. Levine & L. Slade (Eds.), Water Relations in Foods (pp. 21–28). New York: Plenum Press.
Van Duyn, M. A. S., & Pivonka, E. (2000). Overview of the health benefits of fruit and vegetable consumption for the dietetics professional: selected literature. Review. Journal of the American Dietetic Association, 100, 1511–1521.
Vega-Gálvez, A., Lemus-Mondaca, R., Fito, P., & Andrés, A. (2007). Moisture sorption isotherms and isosteric heat of red bell pepper (Var. Lamuyo). Food Science and Technology International, 13(4), 309–316. https://doi.org/10.1177/1082013207082530.
Velázquez-Gutiérrez, S. K., Figueira, A. C., Rodríguez-Huezo, M. E., Román-Guerrero, A., Carrillo-Navas, H., & Pérez-Alonso, C. (2015). Sorption isotherms, thermodynamic properties and glass transition temperature of mucilage extracted from chia seeds (Salvia hispanica L.). Carbohydrate Polymers. https://doi.org/10.1016/j.carbpol.2014.11.068.
Wanjiru Gichau, A., Kanensi Okoth, J., & Makokha, A. (2019). Moisture sorption isotherm and shelf-life prediction of complementary food based on amaranth-sorghum grains. Journal of Food Science and Technology, 57(3), 962–970. https://doi.org/10.1007/s13197-019-04129-2.
White, H. J., & Eyring, H. (1947). Textile Research Journal, 17, 523.
Willmott, C. J., Ackleson, S. G., Davis, R. E., Feddema, J. J., Klink, K. M., Legates, D. R., O’donnell, J., & Rowe, C. M. (1985). Statistics for the evaluation and comparison of models. Journal of Geophysical Research, 90(C5), 8995–9005.
Zhang, X. W., Lui, X., Gu, D. X., Zhou, W., Wan, R. L., & Liu, P. (1996). Desorption isotherms of some vegetables. Journal of the Science of Food and Agriculture, 70(3), 303–306.
Zoran Ilić, S., Ljubomir, Š., Mirecki, N., & M., & Fallik E. (2014). Cultivars differences in keeping quality and bioactive constituents of bell pepper fruit during prolonged storage. Journal of Advances in Biotechnology, 4(1), 313–318.
Acknowledgements
The authors thank the French Embassy through the Cooperation and Cultural Action Service (SCAC) program for the financial support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Kemmene Dapabko, S., Jiokap Nono, Y., Arebga, A.W. et al. Determination and Modeling Desorption Isotherms of Okra (Abelmoschus esculentus L. Moench) and Sweet Green Pepper (Capsicum annum L. Moench). J. Biosyst. Eng. 46, 60–80 (2021). https://doi.org/10.1007/s42853-021-00088-9
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s42853-021-00088-9