Abstract
Hexane extraction is the most common technique used to remove oil from full fat soy materials in the production of both soy oil and defatted soy. The popularity of hexane is based on its high oil extraction efficiency and its availability. The solvent, however, has some considerable economic, environmental, and safety drawbacks. A review of alternative soybean defatting techniques is carried out through the description of four main technological approaches including: (1) alternative organic (carbon-based) solvent extraction, (2) aqueous extraction, (3) supercritical carbon dioxide (SC-CO2) extraction, and (4) enzymatically aided extraction. Through detailed discussions of experimental results, the advantages and disadvantages of each approach are presented. The optimum oil yield for the various extraction techniques discussed ranges from as high as 26.0% for mixed organic solvent extraction of full fat soy flour to as low as ∼7% for some enzymatic treatments of full fat soy brokens extracted by mechanical pressing. An environmentally friendly, safe, and cost-efficient alternative technique has yet to be developed to replace hexane extraction. Current aqueous and SC-CO2 techniques show promise but require further research and development to ensure their practicality in terms of industrial processing.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
On the global scale, processed soybeans represent the largest source of protein feed and the second largest source of vegetable oil. Soy oil also shares 28% of the global trade in vegetable oil next to palm oil (32%) (USDA-FAS 2009). The increased consumption of soy has long hinged upon its known nutritional benefits; in the last two decades, the potential of soy to play a role in the prevention and treatment of chronic diseases has led to an increased interest in its processing and use in the development of food products (Messina 1997). Defatting (removal of oil) from soy is one of the primary steps involved in the value-added processing of soy. Traditional methods of extracting oil, such as mechanical and solvent extractions, all have their limitations. Mechanical extraction, or pressing, is limited in its applicability, particularly with low-oil content oilseeds such as soybean. Elevated temperatures employed during pressing can also have a deleterious effect on the quality of the extracted oil and residual meal (Sugarman 1956; Nelson et al. 1987).
In spite of its widespread use, solvent (hexane) extraction of soy material has many economic, environmental, and safety limitations. Economically, one of the main concerns is the stability of both hexane supply and price due to fluctuation in the fossil fuel market (Friedrich and List 1982; Lusas et al. 1990; Gandhi et al. 2003). As energy requirements of oilseed processing have grown to become a considerable cost, becoming the second most important production input after the seed (Lusas et al. 1995), the energy required to remove hexane from both the soy meal and soy oil has come under scrutiny. Coupled with these economic concerns, growing awareness of the deleterious effects of organic (carbon-based) solvents on environmental and human health has increased public scrutiny of environmental emissions from oil processing plants. A chronic exposure of hexane as low as 400 ppm in the air can cause multiple nerve disorders (polyneuropathy), vision impairment, hair loss, irritation to skin and lungs, fatigue, reproductive failure, and brain damage (NIOSH/OSHA/DOE Health Guidelines 1996). Increased emission restrictions, through such efforts as the 1990 amendments to the Clean Air Act of the USA, as well as the testing and determination of hexane as a hazardous air pollutant, have raised concern in terms of the current and long-term feasibility of hexane use (Lusas et al. 1991; Gandhi et al. 2003). On the whole, the vegetable oil sector, which includes a substantial input from soy and other plant oil processing, is principally responsible for the high volatile organic compound emission levels found in the food industry (Rosenthal et al. 1996). In addition, hexane is highly flammable, and as such safety precautions need to be taken during soy oil extraction as the risk of severe accidents is constantly present in many extraction plants (Rosenthal et al. 1996). Furthermore, although not sufficiently substantiated to force FDA regulations, a growing portion of the population is concerned by the potential toxicity of ingested residual hexane in foods consumed directly or through animal feeds (NIOSH/OSHA/DOE Health Guidelines 1996). Based on the economic, environmental, and safety reasons listed above, the search for alternative soy defatting techniques has long been called for (Rosenthal et al. 1996), and considerable progress has been made in developing various novel approaches.
As an immediate response, the industry switched to another hexane isomer (iso-hexane or 2-methyl pentane). Although more expensive, this solvent has very similar extraction properties and involves only slight capital investment. However, iso-hexane may be just a temporary replacement because it has very similar flammability, human and environmental toxicity to n-hexane. These two products are so similar that, most often in the literature, both isomers are simply called hexane.
This review paper focuses specifically on alternative techniques to hexane defatting. Previous reviews have focused on techniques applied to oilseeds in general (e.g., aqueous and enzymatic techniques) (Rosenthal et al. 1996; Dominguez et al. 1994) and alternative organic solvent extraction (Johnson and Lusas 1983; Lusas et al. 1990; Lusas and Gregory 1998). The primary objectives of this review are to focus on promising alternative approaches as they have been applied to processing of full fat soy materials alone and to highlight the strengths and weaknesses of these methods and their potential applicability in industrial scale soy processing. In certain cases, the aim of defatting soy material is for the processing and extraction of soy oil, whereas in other approaches the removal of soy oil is secondary to the production of low fat protein concentrates and isolates.
The review progresses from an initial discussion of the experimental results from pure hexane extraction after which it focuses on four major alternative approaches to soy material defatting. The first section deals with the use of organic solvents as alternative extraction mediums. The second section discusses aqueous extraction of oil from full fat soy materials. The third section details the development of supercritical extraction using carbon dioxide (SC-CO2) as a viable alternative approach to lipid removal, while the final section details the application of enzymatic treatment to techniques used for oil extraction from soybeans. Throughout these sections, the advantages and disadvantages of each technique are discussed to provide a practical guide for the implementation of alternative soy material defatting techniques. A final comparison of the alternative techniques presented herein allows for a discussion of current limitations, as well as provides possible future directions for the research and development of environmentally friendly, safe, and cost-efficient techniques to remove oil from full fat soy materials.
Equations and Terminology
This review paper attempts to summarize and compare experimental results from a variety of sources. It is, therefore, necessary to make a note of some of the common forms in which data are expressed. The most often cited measure of extraction efficiency is oil yield. Since the oil content of full fat soy is approximately 20%, as oil yield approaches this value, the more efficient is the oil extraction process. Oil yield is, therefore, defined as follows:
It is also possible to calculate the amount of oil which remains after extraction, i.e., the residual oil content. The lower the residual level of oil, the more efficient the extraction. This calculation is based on the percent ratio of the mass of oil in the final extracted soy meal as a function of the total mass of the starting material. Residual oil, thus, depends on the method used to measure the mass of oil in the final product. Hexane extraction under severe conditions is often used. As these extraction conditions may differ from one study to another, comparison between authors is to be made with caution.
Often, the notion of oil recovery is used, which involves two steps. The first step is an experimental determination of the total mass of oil present in the starting material. The second step involves the calculation of oil recovery as the percent ratio between the mass of oil extracted compared to the mass of oil originally present in the starting material. The closer the oil recovery is to 100%, the more efficient the extraction process. Note that this equation has similar limitations as Eq. 2 because of the need to measure the total mass of oil in the sample.
In some studies, the effect of different treatments are evaluated by calculating the percent change in oil yield, which involves the determination of the difference in total fat extracted using hexane before and after each given pre-treatment. The mass difference in extracted oil is reported as a percent of the initial oil extracted using hexane prior to the given treatment. Given that this calculation is a mass difference, an increase in oil extraction is indicated by a positive percent change, while a decrease in oil extraction is indicated by a negative percent change.
Another measure of the effect of alternative defatting techniques can be seen through the proximate analysis of soy protein isolates (SPI). In these cases, the residual oil/fat content in the SPI is an indication of the effectiveness of the defatting technique used, i.e., the lower the fat content, the better the process at removing soy oil. All fat content (percent) calculations are carried out on a dry basis.
Finally, in order to compare the optimum results for each defatting technique, the results need to be transformed onto the same scale and units, i.e., standardizing the results using theoretical calculations. This can be facilitated by setting the oil yield from a standard hexane extraction as a baseline and calculating any difference from this baseline using oil recovery as follows:
(or)
Hexane Extraction
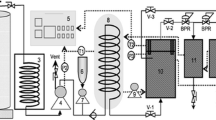
The major steps involved in soybean defatting using hexane are highlighted in Fig. 1 and include comminution, solvent extraction, meal desolventizing, separation of soy oil, and recovery of hexane solvent. Details of these processes are summarized elsewhere (Mustakas 1980; Serrato 1981). The main reason for the use of hexane is its ability to extract almost all of the oil present in soybeans, leaving a residual oil level in the meal of <1.0%. Wiese and Snyder (1987) found that full fat soy flour exposed to a continuous 4-min high performance liquid chromatography (HPLC) grade hexane extraction had an oil yield of 22.5%, while a comparable treatment of full fat soy flakes had an oil yield of 17.1%. This difference in oil yield is due to the fact that oil is extracted faster from flour than from flakes during a set time period. Nieh and Snyder (1991) also found an oil yield of 21.5% for a HPLC grade hexane batch extraction of full fat soy flour. However, in commercial practice, solvent extractors employ deep beds where the porosity of finely ground soy products would retard solvent flow and miscella (mixture of oil and solvent) percolation rates, whereas the coarse grits, flakes, and collets would enhance solvent flow and oil extraction rates (Williams 1995). Bargale et al. (2000) reported that a 7-h-long commercial hexane extraction process is effective to extract a maximum of 21% oil from ground soy grits, soy flakes, and expanded soy collets (50–100 mm length and 20 mm diameter) compared to oil extraction from soybeans that had not been ground which ranged from 15% to 19% during the same period. Moreover, the residual oil level in full fat expanded soy collets continuously extracted with hexane was 0.6% (Lusas 1997) indicating almost complete oil removal. Further to these findings, Gandhi et al. (2003) illustrated that n-hexane had the fastest batch extraction rate of soy oil from full fat soy flakes, in comparison to other pure solvents such as ethanol and heptane. As hexane is currently used throughout the soy oil processing industry, the experimental results discussed above provide a framework and baseline reference point against which to compare all other alternative defatting techniques. It should be mentioned here that in some papers petroleum ether is used, which is a mixture of various alkanes including hexane.
Alternative Organic Solvent Extraction
Requirements of Organic Solvent Extraction
In response to the limitations of hexane extraction discussed earlier, there has been an active search for alternative organic solvents for the defatting of soybeans. The general requirements for a replacement solvent include (1) plentiful supply, (2) safety in terms of human and environmental toxicity, (3) non-flammability, (4) low cost, (5) oil extraction efficiency, and (6) industrial practicality, i.e., easily separated from extraction material and chemically stable (Lusas et al. 1990).
Johnson and Lusas (1983) listed over 70 solvents which have been used to extract oil from oilseeds. The list reported included pure solvents such as other alkanes (propane, butane, pentane, and heptane) to benzene, chlorinated hydrocarbons, alcohols, ketones, and other organic solvents and organic solvent mixtures. Of these, the bulk of recent work has focused primarily on ethyl and isopropyl alcohols (IPA) and mixtures thereof (Lusas et al. 1990; Seth et al. 2007, 2010). Discussions presented here will focus on recent developments in the use of (1) pure solvents, (2) sequential pure solvent extraction, and (3) solvent mixtures, including (a) mixtures of organic solvents, (b) aqueous–organic solvent mixtures, and (c) azeotropic mixtures (Table 1).
Pure Organic Solvent Extraction
In comparison to hexane, pure organic solvents showed comparable levels of soy oil extraction efficiency, although slightly altered extraction conditions were required in some cases. Oil extractability of pure n-heptane, n-propanol, IPA, and ethanol was evaluated by Gandhi et al. (2003). All extractions were carried out in the same manner, i.e., full fat soy flakes were refluxed with pure solvents after which the extracted oils were recovered by evaporating the solvent present in the extracted miscella. The IPA extraction was the only exception in that oil was recovered by chilling the IPA miscella, as IPA requires considerably more energy to vaporize in comparison to the other solvents tested. In these trials, after 10 h of refluxing extraction, all of the pure solvents yielded complete oil recovery. The comparative recovery, after 2 h refluxing, was however lower in all cases, compared to hexane (Table 1).
Sequential Pure Solvent Extraction
The application of the two-phase consecutive solvent extraction of soy material with organic solvents was investigated by Nieh and Snyder (1991). In this experiment, full fat soy flour was first batch-extracted with pure hexane, followed by a wash with an aqueous ethanol solvent mix ranging from 50% to 90% ethanol. The aim of this study was to determine the ability of ethanol to displace residual hexane trapped in the defatted meal, with the goal of increasing the overall oil yield (Table 1). It was found that full fat soy flour submitted to a 1-min hexane extraction followed by a 30-s 50% ethanol extraction led to the highest oil yield, which was comparable to a pure hexane extraction. Upon further analysis, it was observed that the concentration of the ethanol used in the wash step affected the quality of the soy oil extracted and consequently the quality of the residual soy meal (i.e., the lower the concentration of ethanol used, the greater the amount of phospholipids found in the extracted oil due to their affinity for the more polar extraction solvent). In all cases, pure hexane extraction was found to be more effective at removing fat from full fat soy flour than the two-phase consecutive solvent extraction (Nieh and Snyder 1991).
Mixtures of Organic Solvents
The advantage of using mixed solvent systems is that the polarity of the extraction medium can be controlled by varying the ratio of the selected solvents in order to facilitate the removal of specific soybean components. Wiese and Snyder (1987) compared pure hexane extraction of full fat soy flour and full fat soy flakes to a mixed solvent solution extraction using hexane spiked with 1% IPA (Table 1). In the case of full fat soy flour, the increase in total oil yield was significantly higher using 1% IPA spiked hexane, than with pure hexane. Thus, more total oil was extracted by increasing the polarity of the extraction medium. Li et al. (2004) also reported a higher oil yield from ground soy flakes using mixed solvents (hexane/isopropanol 60:40% v/v) for extraction than hexane or isopropanol alone. Unfortunately, neither the quality of the oil nor meal was evaluated as the increase in total oil yield may have been a result of increased extraction of phospholipids. The use of the 1% IPA-spiked hexane did not have a significant effect on the oil yield from full fat soy flakes, which may be the result of limited solvent diffusion into the larger soy flakes (Wiese and Snyder 1987).
Aqueous–Organic Mixed Extraction Solvents
Organic solvents most amenable to mixing with water are those with higher polarities, i.e., alcohols. The extraction of expanded soy collets using 95% IPA (5% water) was carried out at 74 °C, due to increased miscibility of IPA with soy oil at elevated temperatures; the residual oil level in the extracted collets (0.5%) was comparable to a similar sample extracted with pure hexane at 57 °C (Lusas 1997). Gandhi et al. (2003) investigated three alcohols namely, n-propanol, IPA, and ethanol at both 10% and 20% concentrations in water for the extraction of oil from full fat soy flakes (Table 1). The 10% IPA mixture had the greatest oil recovery after 2 h, comparable to the extraction rate of pure hexane (Gandhi et al. 2003). The mixtures all yielded lower recoveries even after 10 h of refluxing. In general, the higher the concentration of alcohol, the larger the quantity of soy oil extracted after 2 h of refluxing. However, Seth et al. (2007) reported higher oil recovery (98.7%) from soy flakes extracted with a mixture of IPA and water (90.5 ± 9.5% w/w at solvent flow rate of 7.75 mL/min for 16 h) than n-hexane extraction (94.2%). The higher extraction rate with IPA was probably due to the higher extraction temperature used for IPA (i.e., actual temperature used for the study was 100–110 °C; however, boiling point of pure IPA for 95% evaporation is 81–82.5 °C; additionally, specific gravity of IPA at 20 °C is 0.784–0.786, latent heat for vaporization is 80 cal g−1, and flash point is 18 °C) compared to n-hexane extraction (actual temperature used for the study was 65–70 °C; boiling point of pure n-hexane for 95% evaporation is 65–70 °C; specific gravity at 20 °C is 0.656–0.656; latent heat of vaporization is 206 cal g–1 and flash point is −14 °C) (Gandhi et al. 2003; Seth et al. 2007). Using aqueous solvent mixtures has the advantage of decreasing the quantity of organic solvent used; however, the extraction temperature often has to be elevated to ensure adequate solubilization of soy oil which, when coupled with the higher heat treatments required to remove the solvent and moisture from the meal, negatively impacts on the functional properties of the residual soy meal (Gandhi et al. 2003).
Azeotropic Extraction Solvents
By mixing predetermined ratios of certain solvents together, one can create azeotropic mixtures with unique physical properties for solvent extraction. Table 1 provides experimental results obtained for seven different organic and aqueous solvent azeotropes used to defat full fat soy flakes. In all cases, the azeotropic extractions had lower oil recovery levels after 2 h of continuous refluxing compared to pure hexane. Recovery levels were only comparable to pure hexane after 10 h of refluxing (Table 1). As with the aqueous–organic extraction solvent mixtures described above, the elevated temperatures used to remove residual solvent from the meal again had a negative impact on the soy meal and further translated into higher processing costs (Gandhi et al. 2003). However, the use of aqueous alcohol azeotropes further poses practical problems in that during extraction the concentrated alcohol solutions can extract moisture from the soy material being extracted, thereby decreasing the extraction medium’s solvency in oil and consequently decreasing the extraction yield (Lusas and Gregory 1998).
Advantages and Disadvantages of Alternative Organic Solvent Extraction of Oil from Soybeans
The advantages and disadvantages of the use of organic solvents are summarized in Table 2. For the most part, the use of pure solvents, sequential pure solvent extraction, and solvent mixtures has been shown to be possible, with total oil extraction yields comparable to that of pure hexane (Gandhi et al. 2003). In particular, the use of both ethanol and IPA has particular advantages in that ethanol is a commonly used solvent, while IPA is lower in cost and is not subject to the regulation and taxation of ethanol (Lusas et al. 1990; Lusas et al. 1995). Both ethanol and IPA are also desirable given that the cost of retrofitting plants currently using hexane as an extraction solvent is minimal when compared to other alternative approaches (Lusas 1997). Moreover, crude soybean oil extracted with ethanol and IPA contains higher neutral oil content (99%), is low in phosphorus (4–5 ppm), and requires less refining (Gandhi et al. 2003). The residual meal from alcoholic extraction (IPA and ethanol) showed better sensory characteristics such as improved color and flavor and was blander in taste than meal obtained by hexane extraction (Beckel et al. 1948). In contrast, Seth et al. (2010) reported that oil and residual meal extracted from soy flakes using azeotropic IPA mixture (91% IPA + 9% water w/w) was darker in color than those extracted by n-hexane. The inconsistent results may be due to differences in moisture content of soy flakes which has a strong impact on the quality of oil and meal. Beckel et al. (1948) used low moisture soy flakes for oil extraction (6–8%), whereas Seth et al. (2010) used high moisture soy flakes (13.4%). Depending on the requirements for the soy oil extracted, or the remaining defatted meal, alternative solvent approaches can also lead to phospholipid-free meals, improving their oxidative stability and functional properties (Wiese and Snyder 1987; Nieh and Snyder 1991). Finally, IPA is a safer solvent than hexane, given its higher flashpoint (18 °C) and auto-ignition temperatures (425 °C) (Lusas 1997).
Hexane has not been replaced with an alternative organic or organic–aqueous solvent mixture due to some of its inherent drawbacks. Alternative pure solvents cost more than hexane and require more energy to vaporize, especially IPA (Gandhi et al. 2003). While the use of aqueous azeotropes of alcohols, in particular IPA, may have the advantage of decreasing the total amount of organic solvent used, the main limitation to their use is that they have limited solubility for oil which is further complicated by the absorbance of moisture from the soybean meal being extracted (Lusas et al. 1995; Lusas and Gregory 1998). Another drawback specific to IPA is its greater density, which increases the energy required for pumping (Lusas 1997). Overall, it has been shown that alternative solvents, such as IPA, require considerably increased energy requirements, on the scale of 30–40% in comparison to hexane (Lusas 1997); this is perhaps the strongest deterrent to their implementation as alternatives for the removal of oil from soybeans. One noticeable exception is the use of compressed petroleum gases (CPG) for oil extraction developed in China in the 1990s, with over 20 production plants being started between 1993 and 2003 (Xuede and Liu 2005). The CPG extraction process uses a pressurized mixture of liquefied propane and butane. The main advantage is low temperature extraction and desolventizing which preserves the quality of the final products. Unfortunately, this process cannot be retrofitted to actual plants.
Aqueous Extraction
History and Principles of Aqueous Extraction
Aqueous separation of oil and protein from oilseeds was reported as early as the 1950s (Sugarman 1956). Initially, the technique was developed for processing high fat oilseeds such as peanuts. It was eventually suggested that the technique could be applied to soybeans (Cater et al. 1974) which led to a focused effort to develop aqueous extraction techniques for the simultaneous extraction of oil and protein from soybeans. The bulk of this work was carried out at the Food Protein Research and Development Center at Texas A & M University in the late 1970s and early 1980s (Cater et al. 1974; Lawhon et al. 1981a; Lusas et al. 1982).
In the aqueous approach, water is used as the extracting solvent and defatting is based on the insolubility of the oil in water (Lawhon et al. 1981a; Rosenthal et al. 1996). By coupling aqueous extraction with the separation of different phases using physical techniques, it is possible to extract and separate protein and lipid fractions from full fat soy materials.
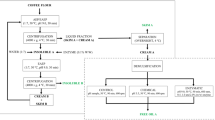
The unit operations for aqueous extraction of oil and protein from soy have remained largely unchanged since they were initially described (Sugarman 1956; Cater et al. 1974; Lusas et al. 1982; Rosenthal et al. 1996). The process involves various steps which are highlighted in Fig. 2 (Lawhon et al. 1981a).
General flow diagram illustrating the unit operations for aqueous extraction of full fat soy material (modified from Lawhon et al. 1981b)
The first step involves comminuting the full fat soybeans in order to facilitate an increase in surface area for the efficient extraction of oil and protein (Fig. 2). This comminution may be either cracking the seeds into smaller pieces, grinding the seeds into full fat soy flour, tempering and flaking the seeds into full fat soy flakes, or passing full fat soy material through an expander/expeller to produce full fat expanded soy collets.
After comminution, the full fat soy material is solubilized to perform a solid–liquid extraction/separation. During this step, insoluble compounds are removed, leaving a liquid solution containing both proteins and lipids. This solution is further separated in the third unit operation which is three-phase centrifugation. The solubilized components are separated into solid, aqueous, and oil/emulsion phases. Finally, each separate phase is further processed through a series of unit operations. The aqueous fraction is submitted to an acid/alkali precipitation after which it is centrifuged and the precipitated protein is dried to produce a soy protein concentrate/isolate. The oil/emulsion fraction is demulsified and separated into its constituents, and the insoluble residue is dried forming a high fiber animal feed (Fig. 2).
The bulk of the experimental work carried out regarding aqueous techniques for the simultaneous removal of oil and protein from soybeans was aimed at the development of a technique to extract a minimally denatured low fat soy protein isolate (Rosenthal et al. 1996). The extraction of pure soybean oil is, thus, a secondary concern in comparison to the production of an oxidatively stable soy protein isolate. For this reason, the experimental results summarized in Table 3 include only the proximate analysis data for soy protein isolates (i.e., they only present the fat and protein contents of the soy protein isolate after its separation and drying).
In practically all cases, the smaller the particle size, the more efficient the extraction of protein and oil (Lusas et al. 1982), with the main limiting factor being the equipment to handle and process the starting material. Rosenthal et al. (1998) found that oil recovery increased from 22% to 65% when particle size of full fat soy flour was reduced from 1,200 to 100 μm during aqueous extraction. In all instances presented in Table 3, the alkaline solubilization progressed under very similar conditions, i.e., a solids/liquid ratio of 1:12, a pH range of 8–9, a temperature between 40 and 60 °C, and an extraction time of 30–40 min. Experimental differences occurred mostly in the protein precipitation steps during the aqueous extraction process.
As presented in Table 3, washing the acid-precipitated soy protein isolate can have an effect on the levels of residual fat and protein content of soy protein isolate. By washing the precipitated protein with pH 4.5 wash water, then resolubilizing at pH 9.0 culminating with a final pH 4.5 precipitation, one can decrease the fat content from 7–9% to 3–6%, while increasing the protein content to such a degree as to extract a soy protein isolate (90–92%) from what was, without the added washing step, a soy protein concentrate (81–85%) (Mattil et al. 1979). These results were confirmed by Lawhon et al. (1981b) who also showed that solubilizing the full fat soy flour in water under controlled conditions (1:12 w/w solid to water ratio, pH 9.0 at 60 °C for 30 min), separating the aqueous phase from the slurry by centrifugation and washing the residue with water (1:5 w/w solid to water ratio) at pH 9.0, precipitating the combined aqueous phase at pH 4.5, and then washing the curd with acid water (pH 4.5) decreased the fat content to 3.6%, while ensuring a protein content approaching 90%. The addition of extra washing steps ultimately increases the efficiency of oil extraction. Unfortunately, these studies did not comment on protein yield.
Lawhon et al. (1981a) also reported that the extractability of protein in aqueous medium (water) from full fat soy flour was higher when using higher flour to water ratio (i.e., 1:30 or 1:25 soy flour to water ratio by weight), than when using lower flour to water ratio (i.e., 1:12 or using a double extraction of 1:10 followed by 1:6). In the former case, extraction at 60 °C for 30 min at pH 6.6, 8.0, and 9.0 gave nitrogen recoveries of 91.4%, 94.2%, and 89.3%, respectively. In the latter case (1:12 flour to water ratio), the extraction was done at pH 9.0 and a recovery of 80.4% nitrogen was obtained, whereas for the double extraction (1:10 followed by 1:6) a pH of 2.5 was used and a recovery of 81.8% nitrogen was obtained. The authors indicated that flour: water ratios influenced nitrogen extractability more significantly than pH. The data presented in the paper are however limited, making a generalization of the statement difficult to validate.
Aqueous Extraction and Membrane Isolation Process
Based on the principles of the aqueous extraction protocol described above, a technique was developed to couple the defatting potential of aqueous extraction with the separation capabilities of membrane isolation. Membrane filtration is increasingly used today as an alternative to isoelectric precipitation in various food applications. The technique has been effectively used for the preparation of protein concentrates and isolates from whey, peas, chickpeas, and lentils (Sachdeva and Buchheim 1997; Fredrikson et al. 2001; Rombaut et al. 2007; Mondor et al. 2009; Boye et al. 2010). Other workers have used microfiltration and ultrafiltration effectively to remove fat from dairy milk and also to fractionate or enrich the phospholipid content in buttermilk (Sachdeva and Buchheim 1997; Goudedranche et al. 2000; Morin et al. 2006). Depending on the order of unit operations, it was possible to create a full fat, intermediate fat, and low fat soy protein isolate (Lawhon et al. 1981a, b); the low fat extraction protocol is outlined in Fig. 3. Full fat soy protein isolate was prepared by directly passing the full fat aqueous extract of the soy flour slurry obtained after the two-phase centrifugation through the ultrafiltration membrane. An intermediate fat containing soy protein isolate was produced by removing residue and oil emulsion from the membrane-filtered concentrate of the full fat aqueous extract by centrifugation. To prepare a low fat protein isolate (Fig. 3), a three-phase centrifugation of the soy flour slurry was done to separate residue as well as protein from oil and water prior to membrane filtration. Experimentally, these soy protein isolates had fat contents ranging from 1.9% (low fat SPI) through 9.8% (intermediate fat SPI) to 32.3% (full fat SPI) as shown in Table 4. Clearly the low-fat extraction protocol is an effective technique to remove oil from soy. Unfortunately, the soy protein isolates extracted using a combination of all of the processes described above did not have a protein content above 80%, which renders this technique less effective than aqueous extraction alone.
General flow diagram illustrating the unit operations for aqueous extraction combined with membrane isolation (modified from Lawhon et al. 1981a)
Aqueous Extraction Process and Ultrasonication
As a novel alternative approach, the effect of ultrasonication on the aqueous extraction of oil and protein from full fat soy flour was evaluated (Yoon et al. 1991), the results of which are summarized in Table 5. In this study, full fat soy flour was ground to a particle size ranging from 120 to 150 mesh, solubilized in a 1:6 flour/water ratio (w/v), and extracted at pH 8.0 at a temperature of 40 °C. During the alkaline extraction period, the solution was subjected to a constant level of sonication, for varying amounts of time (0 to 15 min). After sonication, the solution was centrifuged and the three resultant phases were separated and dried. It was anticipated that subjecting the aqueous solution to sonication would induce cell rupture, increasing the release of oil and protein during extraction (Yoon et al. 1991). The response variables for this study were both the oil and protein recovery. The results of this study indicated that ultrasonication can substantially increase the simultaneous release of oil (87% oil recovery) and protein (85% protein recovery) from full fat soybean flour, in as little as 5 min of sonication time, compared to a control sample which was not sonicated (62% and 68% oil and protein recovery, respectively); however, 90% recovery of both oil and protein was achieved when sonication was done for 15 min (Table 5). This is a novel approach to the removal of fat and protein from full fat soybean flour, which requires further experimentation to determine its viability as a complementary technique for aqueous defatting of soy material.
Aqueous Extraction Using the FRIOLEX® Process
One promising technique that has yet to be experimentally applied to the aqueous defatting of full fat soy material is the FRIOLEX® process. This is a commercially available physical oil extraction process which uses water and a water-soluble extraction aid (15–50% short-chain aliphatic alcohols) to physically remove oil from oilseeds, providing oil recoveries of 95–99% (Hruschka and Frische 1998; Anonymous 1998). In general, the oilseed is milled and then solubilized in water containing the extraction aid, making an aqueous sludge which is then separated through a combination of decanters, separators, and dryers. It is claimed that this technique has been applied to defatting soybean (Hruschka and Frische 1998; Anonymous 1998), but unfortunately no experimental data were presented.
Advantages and Disadvantages of Aqueous Extraction
The aqueous techniques described above are generally more environmentally friendly than the traditional hexane extraction (Hruschka and Frische 1998; Anonymous 1998) (Table 2). Using water as the main extraction solvent eliminates the risk of explosion and facilitates the ease of start-up and shut down during processing. It also decreases capital investment and the day-to-day costs associated with explosion-proofing and implementing the necessary safety measures required with hexane extraction (Lawhon et al. 1981a, b). Another major advantage is that because only water is used, there is minimal protein denaturation during fat removal; thus, the functional properties of the residual soy proteins are more likely to be maintained throughout extraction (Rosenthal et al. 1996). Aqueous solubilization of the soy material further allows for the removal of water-soluble undesirable constituents found in raw soy material (Lusas et al. 1982).
In spite of these advantages, aqueous extraction techniques generally leave higher residual fat contents than are found in products extracted with solvent. The higher the content of oil in the final soy protein isolate, the greater its susceptibility to oxidation and the development of off-flavors and odors (Lawhon et al. 1981b). Some preliminary experiments have, however, shown that protein isolates with higher oil contents can be fairly stable to oxidative degradation (Mattil et al. 1979; Lawhon et al. 1981b); a more detailed and systematic study is required to validate these findings. Another disadvantage of aqueous extraction is that the oil removed from soy flour is often in the form of an emulsion and requires further processing to break the emulsion to release the oil (Lusas et al. 1982; Lamsal et al. 2006; Chabrand et al. 2008). Finally, the use of water as the extraction solvent brings with it the need to maintain hygienic processing conditions to decrease the potential of microbial deterioration and spoilage (Lawhon et al. 1981a, b).
Supercritical Carbon Dioxide Extraction
History and Principles of Supercritical Carbon Dioxide Extraction
The application of SC-CO2 extraction to the removal of oil from soybeans was first demonstrated in the early 1980s (Stahl et al. 1980; Friedrich et al. 1982) and has garnered considerable interest as an alternative to the less environmentally friendly hexane extraction process. In contrast to the aqueous extraction techniques described above, which focused on protein extraction, the development of this technology has focused mainly on the use of SC-CO2 as a lipid extraction medium and its oil extraction efficiency; the quality of the residual meal has been of secondary concern. In spite of this, the results can be interpreted in a manner that sheds light on the utility of this technique as a defatting technique for the down-stream processing of soy protein isolates.
The definition of a supercritical fluid is a gas existing in a physical state above its critical temperature and pressure, which upon compression increases in its density (i.e., it is a gas having the density of a liquid, while maintaining the diffusivity of a gas) (Friedrich et al. 1982). Under these conditions, the use of a supercritical fluid leads to rapid oil extraction in comparison to traditional solvents, due to its higher diffusivity and lower density which improves mass transfer from both solid and liquid matrices (Kim and Yoon 1991; Lancas et al. 1994; Ueno et al. 2008). Given these improved extraction characteristics, the application of SC-CO2 to soybean oil extraction has been studied by many workers and the results are summarized in Table 6.
Experimental Results Using Supercritical Carbon Dioxide Extraction
A number of parameters affect oil extraction and consequently the amount of residual oil in SC-CO2-defatted soy material. Firstly, the physical state of the starting material has a considerable effect on oil removal (Table 6), the most poorly extracted being cracked full fat soybeans, having residual oil levels of ∼20% (Snyder et al. 1984). Snyder et al. (1984) illustrated that more efficient oil extraction is facilitated by further comminution of the starting material, to either full fat soy flour or full fat soy flakes (both of which yielded final residual oil contents of ∼2%). It was further found that the thinner the soy flake, the greater the oil yield, approaching virtually complete soy oil extraction at a soy flake thickness of 0.10 mm (Table 6). For practical application, full fat soy flakes were found to be easier to manipulate, as full fat soy flour had a tendency to pack which led to a reduction in fluid flow (Snyder et al. 1984).
Different combinations of extraction pressure, temperature, and time provide differing oil yields and recoveries and consequently different amounts of residual oil in the meal (Table 6). It is difficult to compare between the treatments presented in Table 6, as all of the experimental parameters listed, i.e., starting material, particle size, particle moisture content, extraction pressure, temperature, and time are all integrally related and combinations of these parameters each have their own effect on the oil yield (Friedrich and Eldridge 1985). In some experimental combinations, the amount of residual oil was comparable to that found with traditional hexane extraction, i.e., <1% (Friedrich and List 1982; Friedrich and Pryde 1984; Friedrich and Eldridge 1985). Unfortunately, in other experimental studies, it was found that the residual oil levels were elevated well above those levels found in traditional hexane extraction (Stahl et al. 1980; Friedrich et al. 1982; Dobarganes Nodar et al. 2002). The results are at best inconsistent, leaving the comparison of the use of SC-CO2 to traditional hexane extraction inconclusive. In spite of this uncertain conclusion, it has been found that in the case where there is an elevated oil content in the residual soy flour after SC-CO2 extraction, the flour has been relatively stable to autoxidative deterioration under mildly accelerated conditions (37 °C) indicating that SC-CO2 extraction either removed some of the lipid fractions most susceptible to autoxidation or was integral in decreasing the activity of lipoxygenase. Differences in flavor scores for defatted soy flour held at 37 °C for 2 months did not differ statistically from the same sample stored at 2 °C (Eldridge et al. 1986).
Evaluation of Supercritical Carbon Dioxide Extraction
In summary, the main strength of SC-CO2 extraction is that it is easily removed from food samples and processing environments and is neither nutritionally nor environmentally toxic (Stahl et al. 1980; Ueno et al. 2008) (Table 2). Much like the aqueous extraction, this extraction medium is non-flammable, lending to a safe working environment (Dobarganes Nodar et al. 2002). SC-CO2 is also readily available and inexpensive (Friedrich and Pryde 1984; Ueno et al. 2008). Friedrich et al. (1982) compared the physicochemical quality of crude soy oil extracted by SC-CO2 and hexane and reported that oil extracted using SC-CO2 was light in color, had lower free fatty acid content (FFA −0.3%), and contained less phosphorus and iron (0.3 and 45 ppm, respectively) than oil extracted using hexane (0.6% FFA, 1.45 ppm iron and 505 ppm phosphorus). Moreover, the refined soy oils from both hexane and SC-CO2 extractions had equivalent odor and flavor scores after 5 days of storage at 60 °C (Friedrich et al. 1982). Dobarganes Nodar et al. (2002) also noticed that the physicochemical quality of crude soy oil obtained by SC-CO2 extraction was comparable to the Food and Agriculture Organization (FAO) standard values of soy oil for human consumption. Finally, the relatively mild conditions of extraction lead to defatted meals with a relatively high content (49–50%) of minimally denatured soy protein with excellent nutritional, functional, and organoleptic properties, as well as high oxidative stability (Stahl et al. 1980; Friedrich and Pryde 1984; Friedrich and Eldridge 1985; Eldridge et al. 1986). Hexane is completely soluble in SC-CO2 when pressurized at >8 MPa and 40 °C; thus, it could be used as a solvent to remove hexane from miscella in conventional oil extraction processes (Wagner and Wichterle 1987). Currently, heated evaporators and reduced pressure strippers are used to separate hexane from soy oil miscella which requires a lot of energy (Woerfel 1995). Reverchon et al. (2000) attempted to eliminate hexane from soy oil/hexane mixture (10% w/w) based on the solubility of hexane in SC-CO2 using continuous counter-current packed tower operating system. Their results indicated that supercritical CO2 treatment effectively removed hexane from soy oil, leaving residual hexane levels as low as 20 ppm under controlled operating conditions (12 MPa pressure, 40 °C, 180 min). Supercritical CO2 has also been used for post-processing of vegetable oils such as deacidification of oils and removal of impurities, separation of free fatty acids from triglycerides, deoiling of raw lecithin, and isolation of polyunsaturated fatty acids and phosphatidylcholine from oil and lecithin, respectively, as well as extraction of bioactive compounds (e.g., essential oils, phenolic compounds, carotenoids, tocopherols, and tocotrienols) from plant materials (Peter 1996; Ueno et al. 2008; Pereira and Meireles 2010).
The practical application of SC-CO2 extraction is limited as the technology is prohibitively expensive for bulk extraction of oil from oilseeds (Hruschka and Frische 1998). The efficiency of this technology is also not consistently comparable to hexane oil extraction. The use of small particle size full fat flour has proven problematic, in spite of the fact that smaller particle sizes lead to greater extraction efficiency (Snyder et al. 1984). Furthermore, percolation extraction of small particle size soy flour leads to a non-uniform extraction of oil (i.e., soy flour closest to the inlet of neat SC-CO2 has lower residual oil levels than soy flour close to the outlet being extracted by oil-saturated SC-CO2) (Stahl et al. 1980; Friedrich and List 1982).
Enzyme-Assisted Extraction
History and Principles of Enzyme-Assisted Extraction of Protein and Oil from Soybeans
The application of enzymes during or prior to the extraction of oil from soybeans was first demonstrated in the early 1980s (Fullbrook 1983; Fullbrook 1984). Since then, the investigation of this approach using single and multiple enzyme systems for the liberation of oil and recovery of protein from soybeans has continued. The main principle of enzymatic assisted extraction is the use of enzymes which damage and/or degrade plant cell walls increasing the permeability of the oil in the oilseed (Dominguez et al. 1994).
The two main approaches used include the use of single and mixed enzymatic systems. The latter has increased utility given that the mixed systems allow for various enzymes to simultaneously act on the cellular structures, leading to a more effective release of oil (Fullbrook 1984; Dominguez et al. 1993). There are four main ways in which this technology is used: (1) enzyme-assisted aqueous soybean oil extraction, (2) simultaneous oil extraction using enzymes and hexane, (3) low moisture enzymatic hydrolysis followed by solvent extraction, and (4) low moisture enzymatic hydrolysis followed by mechanical extraction.
Enzyme-Assisted Aqueous Soybean Oil Extraction
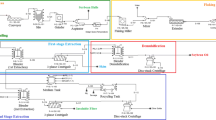
The use of enzymes during aqueous extraction is a variation of the aqueous extraction technique described earlier. Generally, it involves the addition of an enzyme incubation and an enzyme inhibition step into the standard aqueous extraction protocol (Fig. 4). In some cases, the enzyme incubation step is carried out prior to alkaline extraction (i) (Fullbrook 1983; Rosenthal et al. 2001), whereas in other cases enzyme incubation is carried out during aqueous extraction (ii) (Fullbrook 1984; Marek et al. 1990; Yoon et al. 1991). Subsequent enzyme inactivation is carried out by raising the temperature of the solution (Fullbrook 1983, 1984; Marek et al. 1990).
A summary of the experimental conditions and results for the aqueous enzymatic extraction of oil and protein from soybeans is presented in Table 7. In most of the cases, a finely ground full fat soy flour was used as the starting material. In comparison to a non-enzyme-treated control, the use of a mixed enzyme system obtained by using Bacillus subtilis showed an increase in oil yield from 2.7% to as much as 9.2% depending on the enzyme concentration used; although this is an improvement, it is well below the maximum yield of ∼20% (Fullbrook 1983). In another study, Yoon et al. (1991) obtained 62% oil extraction when flour particle size was reduced to <150 mesh (compared to 15% at 32–48 mesh). The recovery further increased to 85% when the flour was treated with an alkaline protease (Alcalase®) obtained from Bacillus lichenthinous, and 86% when treated with Neutrase® protease enzymes from Aspergillus oryzae before aqueous extraction. Flaking and extrusion prior to enzyme treatment (0.5% w/w Multifect Neutral® bacterial protease) also enhanced oil recovery from 68% to 88% in enzyme-assisted aqueous extraction (Lamsal et al. 2006). Additionally, de Moura et al. (2008) recorded higher oil recoveries (93% and 96–97%) from extruded full fat soy flakes when treated with proteases (0.5% w/w Protex 7L–Bacillus amyloliquefaciens and 0.5–1.0% Protex 6L–Bacillus licheniformis, respectively). They, however, observed that very fine grinding of soybean produced a thicker, more viscous emulsion cream phase compared to the emulsion obtained from extruded full fat flakes which was difficult to separate by centrifugation due to the high emulsion stability of the soy proteins and lecithin (Lamsal and Johnson 2007). In contrast to these studies, other workers have reported no improvements in oil yield by using single and mixed enzymatic systems coupled with aqueous extraction (Marek et al. 1990; Rosenthal et al. 2001). These conflicting results indicate that the application of enzymes during the aqueous extraction of oil and protein from soybeans might be limited. The putative benefits of this experimental approach must be weighed with the additional costs, along with the traditional limitations of the aqueous extraction protocol previously mentioned.
Simultaneous Oil Extraction Using Enzymes and Hexane
The integration of enzymatic techniques and solvent extraction aims to combine the strength of hexane extraction with the increased oil permeability caused by enzymatic degradation of the cellular constituents of soybeans. There are two main forms of this complementary approach including (1) enzymatic treatment during simultaneous aqueous and hexane extraction (Fig. 5) and (2) simultaneous enzymatic and hexane extraction (Fig. 6).
General flow diagram illustrating enzymatically assisted extraction of soy oil from soy material using simultaneous aqueous and hexane extraction (modified from Fullbrook 1983)
As shown in Table 8, for full fat flour, the introduction of hexane coupled with increasing concentrations of all of the enzymes evaluated led to a significant increase in oil yield. As an example, the oil yield for full fat soy flour extracted using 3% B. subtilis under aqueous conditions (9.2%) (Table 7) is 3% less than that obtained for full fat flour extracted using 3% B. subtilis and an aqueous system that included hexane (12.5%) (Table 8). The use of Aspergillus niger, in the study conducted by Fullbrook (1983), instead of B. subtilis increased the yield further. A. niger 1 and 2 were used as a source of β-glucanase and hemicellulase, respectively. The highest concentration of A. niger 2 used (3%) led to an oil yield of 17.7% which is much closer to the average 20% oil content of soybeans; this is, however, still lower than the results that are typically obtained with hexane extraction alone.
Table 9 presents the experimental parameters and the results for soybeans treated with enzyme mixtures while being concurrently extracted with hexane alone (Fig. 6). The starting materials for these experimental extractions were crushed full fat soybeans (Bhatnagar and Johari 1987) and cracked full fat soybeans (Dominguez et al. 1995). Bhatnagar and Johari (1987) found that for all the conditions studied, the oil yield increased up to 24.1% which is quite considerable when compared to the 20.3% oil yield of a control (non-enzymatically treated) sample. An increase in oil yield of 5–9.0% was similarly reported by Dominguez et al. (1995), Kashyap et al. (2006), and Kashyap et al. (2007). The high oil yields (>20%) are to be expected given that the extraction medium was pure hexane. The use of enzymes would also be expected to facilitate the extraction of oil by disrupting cell wall structure which under control conditions would not have been extracted, thus leading to an increase in oil yield. Free fatty acid and phosphorus levels in the extracted oils were increased in comparison to an untreated control indicating the removal of undesirable lipid fractions from the meal (Dominguez et al. 1995); this can be advantageous for the down-stream processing of the defatted meal into soy protein isolates, but problematic in terms of extracted oil quality.
Low Moisture Enzymatic Hydrolysis Followed by Solvent Extraction
The use of enzymes as a pre-treatment to solvent extraction has also been investigated. This approach differs from the two presented above in that enzymes are added to pre-tempered soybeans and incubated at a defined temperature and for a specific time period. After this incubation, the soybeans are dried at an elevated temperature to inactivate the enzyme and decrease the moisture content to allow for effective solvent extraction (Fig. 7).
An increase in oil yield due to the increased permeability of the extraction solvent was obtained using this process. Table 10 shows the experimental parameters used and the results obtained for these studies. An increase in total oil yield ranging from 6.3% for full fat soy brokens (Smith et al. 1993), to 8.5% (Kashyap et al. 1997) and 8.8% (Shankar et al. 1997) for full fat soy flakes, and to 8–10% for cracked full fat soy grits (Dominguez et al. 1995) was obtained. In the cases listed above, the increase in oil yield resulted in the removal of more than the standard 20% extractable total oil levels reached using hexane extraction. Bargale et al. (2000) reported up to 50.0% oil yield increase for enzyme-treated full fat soy flakes. It must be noted that, in addition to the effect of enzymatic treatment, this large increase in oil yield was also partially due to the fact that the control treatment had a very low oil yield, i.e., 11–12.0%, due to the fact that intact soy flakes were extracted. It is clear from these results that low moisture enzymatic pre-treatment increases the total amount of extractable oil.
For low moisture enzymatic pre-treatment prior to solvent extraction, the starting material used affects oil yield, as illustrated by the higher change in oil yield for full fat soy flakes relative to full fat soy grits (Bargale et al. 2000). Bargale et al. (2000) reported that an ideal extraction combination is the use of full fat soy flakes and the Driselase® multi-enzymes from Basidiomycetes sp. (containing cellulase, xylanase, and laminarinase). Using this combination led to a maximum oil recovery of 84.3% of the total oil available, corresponding to a 50.0% increase in oil yield in comparison to a non-enzymatically treated control (Table 10). As shown in Table 10, some enzymatic treatments decreased oil yield in comparison to non-enzymatically treated controls regardless of the starting material, reinforcing the need for a judicious choice of enzymatic pre-treatment prior to solvent extraction.
Low Moisture Enzymatic Hydrolysis Followed by Mechanical Extraction
The use of enzymes as a type of pre-treatment for full fat soy material prior to mechanical pressing has also been investigated. Mechanical pressing is generally not as efficient as solvent defatting; hence, the use of enzymes is intended to facilitate oil extraction and increase yield. As with enzyme-assisted solvent extraction, the starting full fat materials are adjusted to the desired moisture content with an aqueous enzyme solution and incubated under varying conditions of time and temperature. The incubation temperature is subsequently increased to inactivate the enzymes, and the moisture content is adjusted for maximum efficiency during mechanical pressing (Fig. 7).
Overall, some treatments showed a considerable increase in oil yield. Smith et al. (1993) observed an 11.7% increase in oil yield, which corresponded to an optimized maximum total oil yield of ∼15% based on the initial sample weight, while Shankar et al. (1997) reported a 16.7% total oil yield based on initial moisture-free sample weight. Other workers (Bargale et al. 2000) have, however, indicated that enzymatic treatment prior to mechanical pressing led to decreased oil yield in some of their experimental trials (Table 11). Any increments that were noted were not practically interesting, given the limitations inherent to mechanical pressing. The increase in oil yield presented in Table 11 corresponds to percent increases above and beyond initial mechanical pressing oil recovery levels of 44.3% (soy grits), 48.4% (soy flakes), and 48.2% (expanded soy collets at 37 MPa) (Bargale et al. 2000), which are considerably lower than other alternative methods. On the other hand, full fat expanded soy collets treated under 65 MPa pressing conditions had an initial oil recovery level of 78.0%. In this case, the influence of enzymatic pre-treatment, particularly that obtained from the use of Driselase® from Basidiomycetes sp. at 0.5%, led to the highest total overall oil recovery of all the starting soy materials tested, i.e., total oil recovery was raised from 78.0% to 87.6%, which corresponds to a 12.3% increase in oil yield (Table 11) (Bargale et al. 2000). Enzyme hydrolysis along with conventional pre-treatments like heating/steaming, high pressure, and extrusion also increased oil yield from soy flakes by mechanical pressing (Shankar et al. 1997; Smith et al. 1993; Bargale et al. 2000).
Advantages and Disadvantages of Enzyme-Assisted Extraction of Protein and Oil from Soybeans
Enzymatic treatment as a complementary technique has been applied to aqueous, aqueous/organic solvent, organic solvent, and mechanical extraction of oil from soybeans. Overall extraction efficiency is directly related to the efficiency of the complimentary extraction process (i.e., enzyme-assisted mechanical extraction gives a lower oil yield compared to enzyme-assisted hexane extraction based primarily on the original methods’ strengths and weaknesses) (Table 2).
In addition to increasing oil yield, a high quality residual meal is also obtained due to the avoidance of drastic processing conditions and the minimization of undesirable by-products (Dominguez et al. 1994). Kashyap et al. (2007) found that enzymatic hydrolysis reduced the time required for 99% oil extraction from 28 to 14 h for soy flakes and 32 to 21 h for soy brokens using hexane. The technique is, thus, efficient and may be environmentally safer (Fullbrook 1984).
The fact that enzymatic pre-treatments have been applied to the various extraction processes described above illustrates its adaptability and its ease of integration into existing extraction techniques (Rosenthal et al. 1996). A potential drawback, however, is that proteolytic enzymes can sometimes negatively affect the functional properties of the residual meal proteins (Rosenthal et al. 1996). Jung et al. (2006) evaluated the functional properties of SPI produced by enzyme-assisted aqueous extraction. No significant changes in the solubility and foaming properties of the enzyme-treated SPI compared to untreated SPI were observed; however, a decrease in emulsifying properties was reported. Clearly, the effect on protein functionality will depend on the type of enzymes used (i.e., proteases or carbohydrases) and processing parameters. In addition to this, a major drawback to the use of enzymatic pre-treatment is its cost (Rosenthal et al. 1996; Dominguez et al. 1994). Smaller particle size starting materials have been shown to lead to increased oil extraction efficiency when using enzymatic pre-treatments (Dominguez et al. 1995); however, these materials are difficult to handle in an industrial scale. The development of industrial equipment to handle such material may lead to greater interest in enzymatic pre-treatments for the extraction processes reviewed. The increase in oil extractability has the potential to off-set the cost of acquiring new industrial equipment, as well as the costs of the required enzymes and processing aids.
Conclusions
The alternative defatting techniques described here were all successful in removing oil from soybeans. Unfortunately, not all were equivalent in terms of the overall amount of oil extracted. To facilitate process comparison, representative optimal oil yields for each of the alternative extraction technique are presented, in descending efficiency, in Table 12. There are five alternative approaches that have oil recoveries close to or higher than that seen with pure hexane (i.e., oil recoveries >97%) and seven extraction processes which have oil recoveries less than 97%.
The majority of the alternative extraction techniques that had oil yields higher than pure hexane were slightly altered forms of the traditional hexane extraction process (Table 12). Unfortunately, the top three techniques do not eliminate the problems associated with hexane extraction discussed earlier and as such cannot be considered to be alternative environmentally friendly and safer defatting techniques. A process using lower boiling point alkanes (a mixture of propane and butane) is used in China to replace hexane. The process uses a non-polar solvent liquefied using high pressure equipment which permits lower desolventizing temperatures. Unfortunately, no data were available for comparison.
The other alternative extraction technique with very high oil recovery (99.5%) similar to pure hexane was SC-CO2 extraction (Eldridge et al. 1986). Unfortunately, SC-CO2 extraction has inherent limits on an industrial scale, as there is a paucity of extraction equipment that will allow for continuous SC-CO2 extraction at the scale required in the soy oil processing industry; hence, its utility is limited to batch-type extractions of high value products (Lusas et al. 1990).
Of the seven extraction techniques classified as having lower soy oil yields, some have oil extraction levels comparable to pure hexane, but unfortunately have significant limitations in terms of other practical factors (Table 12). The use of 95% IPA had a virtually identical theoretical oil yield (23.9%) as pure hexane-extracted full fat soy flour, yet the increase in energy required for removal of the IPA, including evaporation of solvent from meal and the removal of solvent from oil through chilling, limits the practicality of this approach (Lusas 1997). The combination of aqueous extraction and membrane isolation also yielded a very low oil content protein isolate, indicating a moderate theoretical oil yield (18.7%). Unfortunately, the technique was intended for the extraction of protein isolates; thus, with the very low purity of the protein isolates obtained, the technique pales in comparison to the protein extraction and purification efficiency of other aqueous techniques (Lawhon et al. 1981b).
The remaining techniques with lower oil yields are primarily those based on aqueous extraction approaches. In all cases, aqueous techniques gave lower oil yields (15.5-17.7%) except for the ultrasonic and enzyme assisted aqueous extraction which gave 22.5 and 19.3% oil yield, respectively. The lowest oil yields are seen in the most environmentally safe extraction procedures, including aqueous extraction and low moisture enzymatically pre-treated soy mechanical expression. Both of these approaches have oil yields approximately 10% below that which is achievable using pure hexane.
In summary, the best way to maximize oil yield is to use a traditional or modified hexane extraction, or alternative solvent combinations to defat soy material. Unfortunately, the increased costs of these alternative solvents do not justify the replacement of hexane, and as such, there is yet to be found a defatting technique that is more efficient and as cost effective as pure hexane oil extraction. Future possibilities for oil removal are contingent on the development of new and improved technologies (examples of which include the design and practical implementation of large-scale high throughput continuous SC-CO2 extraction equipment) and the further application of the FRIOLEX® aqueous defatting process to full fat soy material.
For defatting of full fat material with the intention of producing a low fat protein isolate, the aqueous and mechanical extraction techniques are again at a disadvantage due to the high residual oil levels found in the defatted meals. Further investigation is required to determine the degree of stability of the residual oil found in the protein concentrates and isolates. By ensuring the oxidative stability of the aqueous and mechanically defatted meals, it may be possible to use environmentally friendly processing techniques to produce relatively low fat soy protein isolates while concomitantly extracting soy oil.
In addition to oil, soy is also highly valued for its protein due to the well-known nutritional, functional, and biological qualities of soy protein (Liu 1997; Friedman and Brandon 2001; Anderson 2008). Moreover, soy proteins are widely used as ingredients in many food applications (Kinsella 1979; Singh et al. 2008). It is therefore important to preserve the qualities of soy proteins during defatting, and as such, the influence of the conditions used for defatting must be studied in detail and critically evaluated in order to identify suitable extraction techniques/conditions for soy oil separation which are economically viable and which remain respectful of the environment.
Abbreviations
- AE:
-
Aqueous extraction
- CFFSB:
-
Cracked full fat soybean
- CPG:
-
Compressed petroleum gas
- EFFSF:
-
Extruded full fat soy flakes
- EtOH:
-
Ethanol
- FAO:
-
Food and Agriculture Organisation (United Nations)
- FDA:
-
Food and Drug Administration (United States)
- FFA:
-
Free fatty acid
- FFEC:
-
Expanded full fat soy collets
- FFF:
-
Full fat soy flour
- FFSB:
-
Full fat soy brokens
- FFSF:
-
Full fat soy flakes
- Hex:
-
Hexane
- HPLC:
-
High performance liquid chromatography
- Incub. temp:
-
Incubation temperature
- Incub. time:
-
Incubation time
- IPA:
-
Isopropyl alcohol
- MIP:
-
Membrane isolation process
- n/a:
-
Not available
- Press temp:
-
Pressing temperature
- SC-CO2 :
-
Supercritical carbon dioxide
- SFFSF:
-
Steam-conditioned full fat soy flakes
- SPI:
-
Soy protein isolate
- Temp:
-
Temperature
- w/v:
-
Weight by volume
- w/w:
-
Weight by weight
References
Anderson, J. W. (2008). Beneficial effects of soy protein consumption for renal function. Asia Pacific Journal of Clinical Nutrition, 17(S1), 324–328.
Anonymous. (1998). Friolex process from Westfalia—A new concept in oil recovery. Oils and Fats International, 14(4), 32–34.
Bargale, P. C., Sosulski, K., & Sosulski, F. W. (2000). Enzymatic hydrolysis of soybean for solvent and mechanical oil extraction. Journal of Food Process Engineering, 23(4), 321–327.
Beckel, A. C., Belter, A., & Smith, A. K. (1948). Solvent effects on the products of soybean oil extraction. Journal of the American Oil Chemists’ Society, 25(1), 7–9.
Bhatnagar, S., & Johari, B. N. (1987). Microbial enzymes in the processing of oilseeds. Current Science, 56(15), 775–776.
Boye, J. I., Aksay, S., Roufik, S., Ribérea, S., Mondor, M., Farnworth, E., et al. (2010). Comparison of the functional properties of pea, chickpea and lentil protein concentrates processed using ultrafiltration and isoelectric precipitation techniques. Food Research International, 43(2), 537–546.
Cater, C. M., Rhee, K. C., Hagenmaier, R. D., & Mattil, K. F. (1974). Aqueous extraction—An alternative oilseed milling process. Journal of the American Oil Chemists’ Society, 51(4), 137–141.
Chabrand, R. M., Kim, H. J., Zhang, C., Glatz, C. E., & Jung, S. (2008). Destabilization of the emulsion formed during aqueous extraction of soybean oil. Journal of the American Oil Chemists’ Society, 85(4), 383–390.
de Moura, J. M. L. N., & Johnson, L. A. (2009). Two stage countercurrent enzyme assisted aqueous extraction processing of oil and protein form soybeans. Journal of the American Oil Chemists’ Society, 86(3), 283–289.
de Moura, J. M. L. N., Campbell, K., Mahfuz, A., Jung, S., Glatz, C. E., & Johnson, L. (2008). Enzyme assisted aqueous extraction of oil and protein from soybeans and cream de-emulsification. Journal of the American Oil Chemists’ Society, 85(10), 985–995.
Dobarganes Nodar, M., Molero Gomez, A., & Martinez de la Ossa, E. (2002). Characterisation and process development of supercritical fluid extraction of soybean oil. Science and Technology International, 8(6), 337–342.
Dominguez, H., Nunez, M. J., & Lema, J. M. (1993). Oil extractability from enzymatically treated soybean and sunflower: Range of operational variables. Food Chemistry, 46(3), 277–284.
Dominguez, H., Nunez, M. J., & Lema, J. M. (1994). Enzymatic pretreatment to enhance oil extraction from fruits and oilseeds: A review. Food Chemistry, 49(3), 271–286.
Dominguez, H., Nunez, M. J., & Lema, J. M. (1995). Enzyme-assisted hexane extraction of soya bean oil. Food Chemistry, 54(2), 223–231.
Eldridge, A. C., Friedrich, J. P., Warner, K., & Kwolek, W. F. (1986). Preparation and evaluation of supercritical carbon dioxide defatted soybean flakes. Journal of Food Science, 51(3), 584–587.
Fredrikson, M., Biot, P., Alminger, M. L., Carlsson, N. G., & Sandberg, A. S. (2001). Production process for high quality pea protein isolate with low content of oligosaccharides and phytate. Journal of Agricultural and Food Chemistry, 49(3), 1208–1212.
Friedman, M., & Brandon, D. L. (2001). Nutritional and health benefits of soy proteins. Journal of Agricultural and Food Chemistry, 49(3), 1069–1086.
Friedrich, J. P., & Eldridge, A. C. (1985). Production of defatted soybean products by supercritical fluid extraction. US Patent No 4493854.
Friedrich, J. P., & List, G. R. (1982). Characterization of soybean oil extracted by supercritical carbon dioxide and hexane. Journal of Agricultural and Food Chemistry, 30(1), 192–193.
Friedrich, J. P., & Pryde, E. H. (1984). Supercritical CO2 extraction of lipid-bearing materials and characterization of the products. Journal of the American Oil Chemists’ Society, 61(2), 223–228.
Friedrich, J. P., List, G. R., & Heakin, A. J. (1982). Petroleum-free extraction of oil from soybeans with supercritical CO2. Journal of the American Oil Chemists’ Society, 59(7), 288–292.
Fullbrook, P. D. (1983). The use of enzymes in the processing of oilseeds. Journal of the American Oil Chemists’ Society, 60(2), 476–478.
Fullbrook, P. D. (1984). Extraction of vegetable oils. UK Patent No 2127425.
Gandhi, A. P., Joshi, K. C., Jha, K., Parihar, V. S., Srivastav, D. C., Raghunadh, P., et al. (2003). Studies on alternative solvents for the extraction of oil-I soybean. International Journal of Food Science and Technology, 38(3), 369–375.
Goudedranche, H., Fauquant, J., & Maubois, J. L. (2000). Fractionation of globular milk fat by membrane microfiltration. Lait, 80(1), 93–98.
Hruschka, S., & Frische, R. (1998). A new oil extraction process: Friolex. Oleagineux Corps Gras Lipides, 5(5), 356–360.
Johnson, L. A., & Lusas, E. W. (1983). Comparison of alternative solvents for oils extraction. Journal of the American Oil Chemists’ Society, 60(2), 181A–194A.
Jung, S., Lamsal, B. P., Stepien, V., Johnson, L. A., & Murphy, P. A. (2006). Functionality of soy protein produced by enzyme-assisted extraction. Journal of the American Oil Chemists’ Society, 83(1), 71–78.
Kashyap, M. C., Agrawal, Y. C., Sarkar, B. C., & Singh, B. P. N. (1997). Response surface analysis of enzyme aided extraction of soybean. Journal of Food Science and Technology, 34(5), 386–390.
Kashyap, M. C., Agrawal, Y. C., Ghosh, P. K., Jayas, D. S., Sarker, B. C., & Singh, B. P. N. (2006). Enzymatic hydrolysis pretreatment to solvent extraction of soybrokens for enhanced oil availability and extractability. Journal of Food Process Engineering, 29(6), 664–674.
Kashyap, M. C., Agrawal, Y. C., Ghosh, P. K., Jayas, D. S., Sarker, B. C., & Singh, B. P. N. (2007). Oil extraction rates of enzymatically hydrolyzed soybeans. Journal of Food Engineering, 81(3), 611–617.
Kim, I. H., & Yoon, S. H. (1991). Extraction of soybean oil using supercritical carbon dioxide and its characteristics. Korean Journal of Food Science and Technology, 23(6), 677–682.
Kinsella, J. E. (1979). Functional properties of soy proteins. The Journal of American Oil Chemists’ Society, 56(3), 242–256.
Lamsal, B. P., & Johnson, L. A. (2007). Separating oil from aqueous extraction fractions of soybean. Journal of the American Oil Chemists’ Society, 84(8), 785–792.
Lamsal, B. P., Murphy, P. A., & Johnson, L. A. (2006). Flaking and extrusion as mechanical treatments for enzyme-assisted aqueous extraction of oil from soybeans. Journal of the American Oil Chemists’ Society, 83(11), 973–979.
Lancas, F. M., Queiroz, M. E. C., & da Silva, I. C. E. (1994). Seed oil extraction with supercritical carbon dioxide modified with pentane. Chromatographia, 39(11/12), 687–692.
Lawhon, J. T., Manak, L. J., Rhee, K. C., Rhee, K. S., & Lusas, E. W. (1981a). Combining aqueous extraction and membrane isolation techniques to recover protein and oil from soybeans. Journal of Food Science, 46(3), 912–916. 919.
Lawhon, J. T., Rhee, K. C., & Lusas, E. W. (1981b). Soya protein ingredients prepared by new processes—Aqueous processing and industrial membrane isolation. Journal of the American Oil Chemists’ Society, 58(3), 377–384.
Li, H., Pordesimo, L., & Weiss, J. (2004). High intensity ultrasound-assisted extraction of oil from soybeans. Food Research International, 37(7), 731–738.
Liu, K. (1997). Soybeans: Chemistry, technology and utilization (pp. 25–95). New York: Chapman and Hall.
Lusas, E. W. (1997). Final report: IPA as an extraction solvent. INFORM, 8(3), 290–292. 294-306.
Lusas, E. W., & Gregory, S. R. (1998). New solvents and extractors. In S. S. Koseoglu, K. C. Rhee, & R. F. Wilson (Eds.), Proceedings of the World Conference on oilseed and edible oils processing, volume 1, emerging technologies, current practices, quality control, technology transfer and environmental issues (pp. 204–219). Champaign: AOCS.
Lusas, E. W., Lawhon, J. T., & Rhee, K. C. (1982). Producing edible oil and protein from oilseeds by aqueous processing. Oil Mill Gazetteer, 86(10), 28–30. 32–34.
Lusas, E. W., Watkins, L. R., & Rhee, K. C. (1990). Separation of fats and oils by solvent extraction: Non-traditional methods. In D. R. Erickson (Ed.), Edible fats and oils processing: Basic principles and modern practices (pp. 56–78). Champaign: AOCS.
Lusas, E. W., Watkins, L. R., & Koseoglu, S. S. (1991). Isopropyl alcohol to be tested as solvent. INFORM, 2(11), 970–976.
Lusas, E. W., Koseoglu, S. S., Rhee, K. C., Watkins, L. R., Hernandez, E., Doty, S. C., et al. (1995). Progress in IPA extraction. Oil Mill Gazetteer, 101(4), 24–27. 29–34.
Marek, E., Schalinatus, E., Weigelt, E., Mieth, G., Kerns, G., & Kude, J. (1990). On the application of enzymes in the production of vegetable oil. Progress in Biotechnology, 6, 471–474.
Mattil, K. F., Rhee, K. C., & Cater, C. M. (1979). Soybean protein extract. US Patent No 4151310.
Messina, M. J. (1997). Soyfoods: Their role in disease prevention and treatment. In K. Liu (Ed.), Soybeans: Chemistry, technology and utilization (pp. 442–477). New York: Chapman and Hall.
Mondor, M., Aksay, S., Drolet, H., Roufik, S., Farnworth, E., & Boye, J. I. (2009). Influence of processing on composition and antinutritional factors of chick pea protein concentrates produced by isoelectric precipitation and ultrafiltration. Innovative Food Science and Emerging Technologies, 10(3), 342–347.
Morin, P., Pouliot, Y., & Jiménez-Flores, R. (2006). A comparative study of the fractionation of regular buttermilk and whey buttermilk by microfiltration. Journal of Food Engineering, 77(3), 521–528.
Mustakas, G. C. (1980). Recovery of oil from soybeans. In D. R. Erickson, E. H. Pryde, O. L. Brekke, T. L. Mounts, & R. A. Falb (Eds.), Handbook of soy oil processing and utilization (pp. 49–65). Champaign: American Soybean Association and AOCS.
Nelson, A. I., Wijeratne, W. B., Yeh, S. W., Wei, T. M., & Wei, L. S. (1987). Dry extrusion as an aid to mechanical expelling of oil from soybeans. Journal of the American Oil Chemists’ Society, 64(9), 1341–1347.
Nieh, C. D., & Snyder, H. E. (1991). Solvent extraction of oil from soybean flour. II. Pilot plant and two-solvent extractions. Journal of the American Oil Chemists’ Society, 68(4), 250–253.
NIOSH/OSHA/DOE Health Guidelines (1996). Occupational safety and health guideline for n-hexane. Occupational Safety and Health Administration (OSHA), United States Department of Labour, Washington DC, USA. Available at: http://www.osha.gov/SLTC/healthguidelines/n-hexane/recognition.html). Accessed 12 July 2009.
Pereira, C. M., & Meireles, A. A. (2010). Supercritical fluid extraction of bioactive compounds: Fundamentals, applications and economic perspectives. Food and Bioprocess Technology, 3(3), 340–372. doi:10.1007/s11947-009-0263-2.
Peter, S. (1996). Supercritical fractionation of lipids. In J. W. King & G. R. List (Eds.), Supercritical fluid technology in oil and lipid chemistry (pp. 74–75). Champaign: AOCS Press.
Reverchon, E., Poletto, M., Osseo, L. S., & Somma, M. (2000). Hexane elimination from soybean oil by continuous packed tower processing with supercritical CO2. Journal of the American Oil Chemists’ Society, 77(1), 9–14.
Rombaut, R., Dejonckheere, V., & Dewettinck, K. (2007). Filtration of milk fat globule membrane fragments from acid buttermilk cheese whey. Journal of Dairy Science, 90(4), 1662–1673.
Rosenthal, A., Pyle, D. L., & Niranjan, K. (1996). Aqueous and enzymatic processes for edible oil extraction. Enzyme and Microbial Technology, 19(6), 402–420.
Rosenthal, A., Pyle, D. L., & Niranjan, K. (1998). Simultaneous aqueous extraction of oil and protein from soybean: Mechanisms for process design. Food and Bioproducts Processing, 76(4), 224–230.
Rosenthal, A., Pyle, D. L., Niranjan, K., Gilmour, S., & Trinca, L. (2001). Combined effect of operational variables and enzyme activity on aqueous enzymatic extraction of oil and protein from soybean. Enzyme and Microbial Technology, 28(6), 499–509.
Sachdeva, S., & Buchheim, W. (1997). Recovery of phospholipids from buttermilk using membrane processing. Kieler Milchwritschaftliche Forschungsberichte, 49(1), 47–68.
Serrato, A. G. (1981). Extraction of oil from soybeans. Journal of the American Oil Chemists’ Society, 58(3), 157–159.
Seth, S., Agrawal, Y. C., Ghosh, P. K., Jayas, D. S., & Singh, B. P. N. (2007). Oil extraction rates of soya bean using isopropyl alcohol as solvent. Biosystems Engineering, 97(2), 209–217.
Seth, S., Agrawal, Y. C., Ghosh, P. K., & Jayas, D. S. (2010). Effect of moisture content on the quality of soybean oil and meal extracted by isopropyl alcohol and hexane. Food Bioprocess Technology, 3(1), 121–127.
Shankar, D., Agrawal, Y. C., Sarkar, B. C., & Singh, B. P. N. (1997). Enzymatic hydrolysis in conjunction with conventional pretreatments to soybean for enhanced oil availability and recovery. Journal of the American Oil Chemists’ Society, 74(12), 1543–1547.
Singh, P., Kumar, R., Sabapathy, S. N., & Bawa, A. S. (2008). Functional and edible uses of soy protein products. Comprehensive Reviews in Food Science and Food Safety, 7(1), 14–28.
Smith, D. D., Agrawal, Y. C., Sarkar, B. C., & Singh, B. P. N. (1993). Enzymatic hydrolysis pretreatment for mechanical expelling of soybeans. Journal of the American Oil Chemists’ Society, 70(9), 885–890.
Snyder, J. M., Friedrich, J. P., & Christianson, D. D. (1984). Effect of moisture and particle size on the extractability of oils from seeds with supercritical CO2. Journal of the American Oil Chemists’ Society, 61(12), 1851–1856.
Stahl, E., Schutz, E., & Mangold, H. K. (1980). Extraction of seed oils with liquid and supercritical carbon dioxide. Journal of Agricultural and Food Chemistry, 28(6), 1153–1157.
Sugarman, N. (1956). Process for simultaneously extracting oil and protein from oleaginous materials. US Patent No 2762820.
Ueno, H., Tanaka, M., Machmudah, S., Sasaki, M., & Gotom, M. (2008). Supercritical carbon dioxide extraction of valuable compounds from Citrus junos seed. Food and Bioprocess Technology, 1(4), 357–363.
USDA-FAS. (2009). Oilseeds: World markets and trade. Circular Series FOP 1-09. Available at: http://www.fas.usda.gov/oilseeds/circular/2009/January/Oilseedsfull0109.pdf. Accessed 26 July 2009.
Wagner, Z., & Wichterle, I. (1987). High-pressure, vapour–liquid equilibrium in systems containing carbon dioxide, 1-hexene and hexane. Fluid Phase Equilibria, 33(1–2), 109–123.
Wiese, K. L., & Snyder, H. E. (1987). Analysis of the oil extraction process in soybeans: A new continuous procedure. Journal of the American Oil Chemists’ Society, 64(3), 402–406.
Williams, M. A. (1995). Extrusion preparation for oil extraction. INFORM, 6(3), 289–293.
Woerfel, J. (1995). Extraction. In D. R. Erikson (Ed.), Practical handbook of soybean processing and utilization (pp. 65–92). Champaign: AOCS.
Xuede, W., & Liu, K. (2005). Extraction with compressed petroleum gases for speciality oil and meal products. INFORM, 16(4), 264–362.
Yoon, S. H., Kim, I. H., Kim, S. H., & Kwon, T. W. (1991). Effects of enzyme treatments and ultrasonification on extraction yields of lipids and protein from soybean in aqueous process. Korean Journal of Food Science and Technology, 23(6), 673–676.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Russin, T.A., Boye, J.I., Arcand, Y. et al. Alternative Techniques for Defatting Soy: A Practical Review. Food Bioprocess Technol 4, 200–223 (2011). https://doi.org/10.1007/s11947-010-0367-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-010-0367-8