Abstract
The effect of blanching by ohmic heating (OH) on the kinetics of osmotic dehydration of strawberries was studied. Ohmic heating parameters obtained at two temperatures (65 and 85 °C). The osmotic dehydration (OD) parameters are the temperature (26 and 37 °C) and the sucrose in osmotic solution (30–70 °B). An approximate solution of Fick’s law for unsteady state mass transfer in spherical configuration has been used to calculate the effective diffusion coefficients of water and sucrose. Results show that ohmic heating increases drastically the mass transfer and the effective diffusion rates. After 4 h of OD (without OH) in a sucrose solution (at 37 °C and 70 °B), the dry matter of the untreated strawberry halves was 20.3%; while it reached 68% when OD was combined with blanching by OH at 85 °C for 3-min. Ohmic blanching permits the effective damage of cells by combination of electrical and thermal effects. That result has an important enhancement of water and sugar transfers during osmotic dehydration of strawberries.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Osmotic dehydration (OD), which is better defined as Dewatering Impregnation–Soaking in concentrated solution (Ponting et al. 1966), is a technique that removes the water and increases solid concentration in food. The process is carried out by immersing whole fruits or pieces in concentrated solutions of substances compatible with the material to be treated. It is governed by difference between osmotic pressure of the food material (hypotonic medium) and the concentrated osmotic solution (hypertonic medium). Usually, when the product is put into contact with a low-water activity solution, a two-way mass transfer appears: (1) water is transferred from the product to the solution and in the opposite direction (2) the solute is transferred from the solution to the fruit. As a consequence of those exchanges, the product loses a part of its weight.
As an alternative step or pretreatment technology for freezing (Ponting 1973) or air drying of foods (Nanjundaswamy et al. 1978), OD improves product quality of food without affecting its integrity (Le Maguer 1988; Ramaswamy and Nsonzi 1998; Krokida et al. 2000).
The efficiency of the OD process is dependent on many factors, such as the solution concentration and the temperature, the immersion time, the speed of stirring, the size and shape of food, as well as the solution-to-food ratio (Raoult-Wack et al. 1992; Torreggiani 1993; Fito 1994; Raoult-Wack 1994; Rastogi and Raghavarao 1995, 1996).
It was reported that the increase in concentration of the osmotic solution increased water loss (WL) and sugar gain (SG); (Lenart 1992). However, the observed increase in WL is much higher than the increase in SG. As a result, increased concentration of solution lead to increased weight reduction (Hawkes and Flink 1978; Conway et al. 1983; Lenart 1992). In the case of OD, maximum possible water loss along the minimum possible sugar gain is often desired (Bonaui et al. 1996). However, in some applications, the increased sugar gain is searched. For instance, jam production by OD was proposed to increase the quality of product (Garcia-Martiez et al. 2002; Allali 2008). In this case, the increased sugar gain by fruit is needed in order to reach a soluble dry matter of 45 °B minimum.
It is well recognised that diffusion is a temperature-dependent phenomenon. Higher process temperature seems to promote faster water loss through swelling and plasticizing of cell membranes, faster water diffusion within the product, and better water transfer characteristics on the product surface due to lower viscosity of the osmotic solution. In the case of fruits and vegetables, there is an upper temperature limit beyond which there is a damage of the cellular tissue with negative impact on product quality (softening, browning and flavour losses). This temperature limit is conventionally assumed to be around 50 °C (Ponting et al. 1966).
The cellular membrane exerts high resistance to mass transfer and slows down the OD rate. Even with the optimisation of parameters previously mentioned, the OD rate is not rapid enough and should be improved with the permeabilisation of cell membranes (i.e., pore formation and/or membrane breakage).
The damage of membranes can be achieved by blanching or non-thermal pretreatment, such as the application of pulsed electric field (Angersbach and Knorr 1997; Rastogi et al. 1999; Bazhal 2001; Lebovka et al. 2001, 2002; Amami et al. 2005), high pressure (Taiwo et al. 2001) or ultra-sound (Simal et al. 1998). These various pretreatments all have an important effect on the cellular permeability and, thus, on the mass transfer within the product, but the methods of blanching are of additional interest to attack the integrity of the tissue (solubilisation of the pectic substances constituting the cellular wall), to cause a degasification of the product and to reduce the microbial load and to inactivate the enzymes (Cano 1996). In the case of the conventional blanching, generation of heat within the solid particles depends of the thermal conductivity of the liquid, thus, the overheating of the liquid is possible. It should be noted that a long blanching time has adverse effects on the quality of fruits and yield of the final product (Icier et al. 2006).
The ohmic heating process can be used as an alternative blanching method for vegetables (Mizrahi 1996). The ohmic heating concept is not new and was already used in the beginning of the twentieth century for milk pasteurisation by application of an electrical current through the product placed between two electrodes connected to a generator. Thus, the product behaves as an electrical resistance (Reznick 1996). The heat generated inside the food is directly related to the current induced by the voltage gradient in the field, and the electrical conductivity of the product (Sastry and Li 1996).
The application of OH in the food industry is very wide and includes cooking, pasteurisation, blanching, evaporation and dehydration (Sastry et al. 2001). Mizrahi (1996) reported that blanching by ohmic heating considerably reduced the extent of solid leaching as compared to a hot water process and had short blanching time regardless of the shape and size of the product. Ohmic heating yields better products, clearly superior in quality than those processed by conventional heating. This is mainly due to its ability to heat materials rapidly and uniformly leading to a less aggressive thermal treatment.
Ohmic heating appears to be an effective method for enhancement of processes controlled by mass transfer since it affects the integrity of biological tissue by solubilizing the pectic substances constituting the cellular wall and by providing electroporation of cell membranes (Lebovka et al. 2004; Praporscic et al. 2006).
This paper is an investigation on ohmic heating as a method for fruits blanching before osmotic dehydration. The quality of blanching by ohmic heating is related to the kinetic changes in mass transfer during the subsequent osmotic dehydration. For osmotic dehydration, the studied parameters are temperature (26 and 37 °C) and sucrose concentration (30–70 °Brix). The mass transfer kinetics is evaluated by means of the effective diffusion coefficients.
Materials and Methods
Raw Material
Fresh strawberries were purchased from a local market and processed on the same day. Before the study, the fruits were selected by visual observation for colour, size and physical damage. After washing under tap water and removing of surface water using absorbent paper, strawberries were cut in halves along the fruit axis (Castello et al. 2006) to optimise the contact between the fruit and the osmotic solution and thus to improve the mass transfer during osmotic dehydration.
Blanching by Ohmic Heating
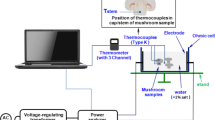
The blanching of the strawberries by ohmic heating is carried out in a parallelepipedic cell (14.5 × 6.6 × 8 cm) with two planar electrodes (14.5 × 8 cm) connected to a generator of alternative current (50 Hz, voltage and intensity up to 230 V and 18 A conceived by Electronic Service UTC, Compiegne, France). Strawberry halves (°Brix ≈ 8°B, radius ≈ 0.8 cm and mass ≈ 7 g) were immersed in a sucrose syrup (fruit/syrup ratio = 1/2). Syrup concentration was 8% w/w in order to limit the mass transfer between the fruit and the syrup. The mixture (syrup + fruits) was then directly placed in the parallelepipedic cell, between the electrodes. Temperature was measured with type-K thermocouples ((NiCr/NiAl) wrapped in Teflon to avoid the interferences with the electric field); one was placed in the syrup at the center of the ohmic cell and the second one was inserted in the geometrical center of the fruit halves. The power delivery of the generator was regulated on the temperature of the fruit particles (on/off regulation in order to reach and maintain the requested blanching temperature value). A data-logger was employed to record simultaneously the current intensity, voltage and temperature (Electronic Service UTC, Compiegne, France; Fig. 1).
The selected range of temperature was 60 to 85 ± 2 °C and heating time from 0 to 3 min. During heating, the temperature difference between fruit and syrup ranged from 2 to 4 °C.
When blanching was over, the pretreated strawberry halves were gently rinsed under tap water and put into the osmotic solution.
Osmotic Treatment
Commercial sucrose was dissolved in distilled water to obtain the osmotic solution. The initial studied concentrations considered for this study were 30, 50 and 70 °B and the solution temperatures were 26 and 37 ± 2 °C. In typical experiments, the ratio of the mass of osmotic solution to the mass of solid sample was 10:1.
The process was carried out in four glass beakers containing the osmotic solution. Temperature regulation and magnetic mixing are provided by heating agitator (Fischer Bioblock) with a Pt100 probe for temperature control (Velp Scientifica, Milan, Italy). Each experiment (with the replications) was carried out the same day with 20 samples (allowing an analysis of variance of the obtained results). The four untreated samples (blank) were used directly to characterise the fresh product by measuring its moisture content. The 16 remained samples were treated by ohmic heating. At the end of the treatment, the fruits were separated from the syrup used for the blanching and were placed in a perforated basket then immersed into the osmotic solution. During the osmotic dehydration, the cutting section of strawberry halves remained non-immersed in the osmotic solution and did not participate in the mass transfer. When the immersion time was reached (respectively 60, 120, 180 and 240 min), samples were taken out from the glass beakers, gently rinsed under tap water (in order to eliminate the possible layer of sugar formed at the fruit surface) and slightly blotted with absorbent paper and analysed.
The sample mass and moisture content were determined by placing the sample in a ventilated oven (AOAC, 1996) at 105 °C for 24 h (Amami 2006). Soluble solids (°B) in the solution were measured with a digital refractometer (Reichert Leica AR200).
Mass Transfer Model
Rates of water loss and solids gain during osmotic dehydration were modelled by an approximate analytical solution of Fick’s second law for unsteady state diffusion (Crank 1975).
The following assumptions were used:
-
–The strawberry halves are semi-spherical.
-
–The diffusion is only radial.
-
–The initial water and solute concentrations throughout the strawberries are uniform.
-
–The counter-current diffusions are simultaneous; only the diffusion of water from the strawberries and of sugar into the strawberries are considered. Other mass transfers are neglected.
-
–The external mass transfer resistance is neglected (agitation level of osmotic solution is sufficient).
-
–The concentration of the osmotic solution is constant.
In the case of a spherical geometry, the second Fick’s law applied for the mass transfer by diffusion in transient state is described by the following equation (Crank 1975):
The initial and boundary conditions are:
At t = 0, C = C 0 for 0 < r < R, and for t > 0, C = C e at r = R.where C is the sugar concentration in the fruit. Mass fraction (g/g) can be used in place of molar concentration until volume changes of fruit particles are negligible; D is the effective diffusion coefficient (m2/s); R is the radius of fruit (m); r is radius in fruit (m) at t time immersion (s). C 0 and C e are, respectively, the initial concentration and the concentration at equilibrium stage.
In order to analyse the data and indicate the overall exchange of sugar and water between the fruit and the osmotic solution, the following equations (Giangiacomo et al. 1987) are considered to calculate the water loss (WL) and the solids gain (SG);
where W t is the fruit mass at time t; WSt is the solids mass in the fruit at time t; WS0 is the initial mass of solids; WW0 is the initial mass of water.
The solutions of the Fick’s equation in spherical coordinates for the water loss (WL) and sugar gain (SG) are given as (Crank 1975):
where D w and D s are, respectively, the effective diffusion coefficients of water and sugar (m2/s). WL 0 = 0 is the water loss at time 0 (g/g); WL t is water loss at time t; WL∞ is water loss at equilibrium; SG0 = 0 is the sugar gain at time 0 (g/g); SG t is sugar gain at time t; SG∞ is sugar gain at equilibrium; π ≈ 3.14.
After some initial period of diffusion, Eqs. (4) and (5) can be reduced to the first term (n = 1; Crank 1975):
The strawberry halves are semi-spherical. Eqs. (6) and (7) should be modified consequently supposing that the water and solute rates would be the same for the semi-spherical and spherical fruit having the same volume (Veinik 1958). In this case, the equivalent diffusion coefficient for the spherical fruit D eq can be expressed on the base of the experimentally obtained diffusion coefficient for the semi-spherical fruit, D 1/2 as: \(D_{{\text{eq}}} = \frac{{S_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} }}{{S_{{\text{eq}}} }}D_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} \), where S 1/2 is the surface of semi-spherical fruit without its cutting section, which was not involved into the mass transfer, \(S_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} = 2\pi R_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}^2 \), S eq is the surface area of the equivalent sphere, \(S_{{\text{eq}}} = 4\pi R_{{\text{eq}}}^2 \), R 1/2 and R eq are the radius of the semi-spherical fruit and the equivalent sphere, \(R_{{\text{eq}}} = \frac{{R_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} }}{{\sqrt[3]{2}}}\). Finally, Eqs. (6) and (7) may be presented as the following:
Where D w1/2 and D s1/2 are respectively the effective diffusion coefficients of water and sugar for the semi-spherical fruit (m2/s). A linear regression of experimental data was used to determine \({{ - D_{w_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} } \pi ^2 } \mathord{\left/ {\vphantom {{ - D_{w_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} } \pi ^2 } {R_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}^2 }}} \right. \kern-\nulldelimiterspace} {R_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}^2 }}\) for water diffusion and \({{ - D_{s_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} } \pi ^2 } \mathord{\left/ {\vphantom {{ - D_{s_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}} } \pi ^2 } {R_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}^2 }}} \right. \kern-\nulldelimiterspace} {R_{{1 \mathord{\left/ {\vphantom {1 2}} \right. \kern-\nulldelimiterspace} 2}}^2 }}\) for sucrose diffusion.
Statistical Analysis
Each experiment was replicated thrice to ensure the repeatability and the reproducibility of the results. All data were subjected to statistical analysis using the analysis of variance (ANOVA); in all cases, the standard error (SE) was less than 6%.
Results and Discussion
Osmotic Dehydration of Fresh Strawberries
A first set of tests was carried out with four strawberry halves of the same size dehydrated in osmotic solutions of different concentrations (30, 50 and 70 °B) maintained at 26 and 37 °C.
Figure 2 shows the influence of temperature (26 and 37 °C) on the water loss and sugar gain during OD of fresh strawberry halves (in an osmotic solution with initial sucrose concentration of 70 °B). We observe that the WL increases with an increasing of temperature from 26 to 37 °C, while SG did not change significantly. The changes due to the heating were particularly visible during the first 2 h of immersion. During this time, the impregnation was mainly done near the particle surface and into the extra cellular volumes. After a 2-h period of osmotic dehydration, the mass transfer kinetics were slower and were not significantly affected by temperature changes. This result is in agreement with literature data (Lenart and Flink 1984). It was reported that for temperatures lower than 50 °C, cell membranes do not lose their selectivity and mass transfer enhancements should be mainly due to the thermal effects.
The effect of initial sucrose concentration (30, 50 and 70 °B) was also studied. Figure 3 shows that an increase in initial sucrose concentration leads to a higher water loss and to a higher sugar gain. It can be observed that after 4 h of osmotic dehydration at 37 °C, the fruit water loss obtained in the solution of 70 °B was two times higher than that in the solution with initial sucrose concentration of 50 °B. Similar results were observed at 26 °C. This can be explained by a strong reduction in water activity of the sucrose syrup between 50 and 70 °B (Galmarini et al. 2008). For such concentrated osmotic solutions, dehydration kinetics depends mainly on water activity and less on water mass fraction.
Figure 4 shows the evolution of dry matter content (DM) and selectivity of transfer (WL/SG) during OD of untreated strawberry halves.
The evolution of mass transfer selectivity (WL/SG) indicates that water loss is about eight times faster than the sucrose gain by the fruit. The ratio WL/SG slightly decreased with time. This phenomenon has already been mentioned and allotted to the death of cells consecutive to the increase of sugar concentration in the tissue (Ferrando and Spiess 2003; Mavroudis et al. 2004, Marchal et al. 2005).
The values of calculated D w1/2 and D s1/2 according to the temperature and to the concentration of osmotic solutions are given in Table 1. As can be seen from Table 1, the increase of temperature and initial sucrose concentration leads to the enhancement of water and sugar diffusivities. The values of calculated diffusion coefficients were found to be in the range of 10−10 m2/s. They are in agreement with literature values (Hough et al. 1993; Kaymak-Ertekin and Cakaloz 1996). The regression coefficient R 2 (R 2 ranged between 0.96 and 0.99, whereas standard error (SE) were less than 6% in all cases) displays good correlations between the experiments and a model described in Eqs. (8) and (9). They confirmed that the mass transfer mechanism involved during osmotic dehydration of strawberry halves was close to a diffusive one.
Effect of Blanching by Ohmic Heating on the Kinetics of Osmotic Dehydration
In this part of the study, the effects of the ohmic heating (OH) on the kinetics of osmotic dehydration and on the coefficients of effective diffusion were investigated. Osmotic dehydration was carried out in the solution of 70 °B at 37 °C supporting faster process kinetics.
Figure 5 presents the effect of blanching pretreatment by OH on the kinetics of water loss during osmotic dehydration of strawberries. Results are compared with those obtained for osmotic dehydration of untreated strawberries. Dehydration rates are much more important when osmotic dehydration is coupled with ohmic heating, even with a short-time (1 min) blanching by OH at both the blanching temperature of 65 °C and 85 °C.
Effect of blanching by ohmic heating on the kinetics of water loss during osmotic dehydration of strawberries in the solution with initial sucrose concentration of 70 °B and temperature of 37 °C: dash OD of untreated strawberries, empty square, filled upright triangle, filled diamond OD after blanching (at 85 °C during 1, 2 and 3 min, respectively)
The effect of blanching temperature on the mass transfer in a subsequent osmotic dehydration was also studied. The dry matter (DM) and the mass transfer selectivity (WL/SG ratio) were evaluated and presented in Fig. 6. There was a noticeable influence of the OH pretreatment on WL/SG ratio. For the untreated strawberries, the WL/SG ratio amounted to near 8 (Fig. 4). After the ohmic heating at 65 °C for 3 min, the ratio WL/SG was initially 3.5 and then decreased to about 2.1 probably due to the death of cells remaining intact after the OH blanching. When the temperature of OH was increased to 85 °C, all cells were probably destroyed. The ratio WL/SG was decreased to about 1.5 and remained the same during osmotic dehydration.
Evolution of DM and selectivity of mass transfer (WL/SG) of strawberries during the osmotic dehydration of strawberries blanched by OH. filled upright triangle % DM, during OD after blanching by OH at 85 °C for 3 min; filled square, filled diamond WL/SG ratio, during OD after blanching by OH for 3 min at 65 °C and 85 °C, respectively
In any case, the loss of selectivity indicates noticeable changes in the fruit structure due to the blanching by OH.
After 4 h of OD (without OH) in a sucrose solution (at 37 °C and 70 °B), the dry matter of the untreated strawberry halves was 20.3%, while it reached 68% when OD was combined by a blanching by OH at 85 °C for 3 min. That result can be explained by the capability of ohmic heating to permeabilise efficiently the cellular membrane and to enhance the mass transfer during the OD.
The D w1/2 and D s1/2 values for strawberries osmotically dehydrated after the OH pretreatment are presented in Table 2. Blanching temperature seems to have more pronounced effect than blanching duration on the water and sugar diffusivity during OD. As it was already put in evidence through selectivity results (Fig. 6), the diffusion of sugar is much more sensitive to the ohmic pretreatment than the diffusion of water. This reflects the loss of selectivity of the cellular structure (cell membranes and walls). As the pretreatment is effective even for short heating at moderate temperature, a non-thermal effect of ohmic heating on the fruit structure is suspected. For the studied temperatures of ohmic heating (65 and 85 °C), electroporation of cell membranes seems to be possible even when the electrical field is moderate (60 V cm−1). For longer treatments (3 min), there is an additional increase of the diffusion coefficients that is possibly due to the thermal effect of blanching upon the cell wall structure.
The qualitative analysis of strawberries blanched by ohmic heating should be provided in future studies to compare with fruit blanched by traditional way.
Conclusion
The data shows that WL and SG increased with the increase of both temperature and concentration of the osmotic solution. The values of calculated diffusion coefficients were found to be in the range of 10−10 m2/s and were in agreement with literature values.
Ohmic heating enhances mass transfer during the osmotic dehydration of strawberry halves. Water loss and sugar gain rose with an increase of temperature and duration of blanching. The coupling of ohmic heating with osmotic dehydration makes it possible to obtain higher water and sugar diffusion rates at a moderate temperature of 37 °C. Those results can be explained by the ability of ohmic heating to permeabilise efficiently the cellular membranes.
As a perspective work, the quality evaluation of the OD product is lacking to indicate adverse or beneficial effects of the increased diffusivity of sugar. The tissue modifications involved during the electrical and thermal treatments should be clarified. This could be carried out by an electronic microscope analysis and/or measuring the impedance and the texture as shown in recent works (Angersbach et al. 1999; Lebovka et al. 2004).
Abbreviations
- OD:
-
osmotic dehydration.
- OH:
-
ohmic heating.
- °B :
-
Brix
- C e :
-
concentration at equilibrium, g/g.
- C :
-
sugar concentration in the fruit, g/g.
- C 0 :
-
initial sugar concentration, g/g.
- D eq :
-
equivalent diffusion coefficient for the spherical fruit, m2/s.
- D 1/2 :
-
effective diffusion coefficient for the semi-spherical fruit, m2/s.
- D w :
-
effective diffusion coefficient of water for the spherical fruit, m2/s.
- D s :
-
effective diffusion coefficient of sugar for the spherical fruit, m2/s.
- D w1/2 :
-
effective diffusion coefficient of water for the semi-spherical fruit, m2/s.
- D s1/2 :
-
effective diffusion coefficient of sugar for the semi-spherical fruit, m2/s.
- R 1/2 :
-
radius of semi-sphere, m.
- R eq :
-
radius of sphere, m.
- S eq :
-
surface of spherical fruit, m2.
- S 1/2 :
-
surface of semi-spherical fruit, m2.
- SG:
-
sugar gain, g/g.
- SG t :
-
sugar gain at time t, g/g.
- SG0 :
-
sugar gain at time 0, g/g.
- SG∞ :
-
sugar gain at equilibrium, g/g.
- t :
-
immersion time, s.
- W t :
-
fruit weight at the end of treatment, g.
- WL:
-
water loss, g/g.
- WL t :
-
water loss at time t, g/g.
- WL0 :
-
water loss at time 0, g/g.
- WL∞ :
-
water loss at equilibrium, g/g.
- WL/SG:
-
selectivity.
- WS t :
-
solids weight in the fruit at the end of treatment, g.
- WS0 :
-
initial weight of solids, g.
- WW0 :
-
initial weight of water, g.
References
Allali, H. (2008). Production of fruits jams by osmotic dehydration coupled with ohmic heating. PhD thesis, Génie des Procédés Industriels: Université de Technologie de Compiègne, Compiègne, France.
Amami, E. (2006). Amélioration de la déshydratation osmotique des produits végétaux par champ électrique pulsé. Thèse de doctorat, Génie des Procédés Industriels: Université de Technologie de Compiègne, Compiègne, France.
Amami, E., Vorobiev, E., & Kechaou, N. (2005). Effect of pulsed electric field on the osmotic dehydration and mass transfer kinetics of apple tissue. Drying Technology, 23(3), 581–595. doi:10.1081/DRT-200054144.
Angersbach, A., & Knorr, D. (1997). High intensity electric field pulses as pretreatment for affecting dehydration characteristics and rehydration properties of potato cubes. Nahrung, 41, 194–200. doi:10.1002/food.19970410403.
Angersbach, A., Heinz, V., & Knorr, D. (1999). Electrophysiological model of intact and processed plant tissues: cell disintegration criteria. Biotechnology Progress, 15, 753–762.
Bazhal, M. (2001) Etude du mécanisme d’électroperméabilisation des tissus végétaux. Application à l’extraction du jus de pommes. Thèse de doctorat. Génie des Procédés Industriels, Université de Technologie de Compiègne, Compiègne, France.
Bonaui, C., Dumoulin, E., Raoult-Wack, A. L., Berk, Z., Bimbenet, J. J., Courtois, F., et al. (1996). Food drying and dewatering. Drying Technology, 14(9), 2135–2170. doi:10.1080/07373939608917199.
Cano, M. P. (1996). Vegetables. In L. E. Jeremiah (Ed.), Freezing effects on food 604 quality (p. 520). New York: Marcel Dekker.
Castello, M. L., Fito, P. J., & Chiralt, A. (2006). Effect of osmotic dehydration and vacuum impregnation on respiration rate of cut strawberries. LWT-Food Science and Technology, 39, 1171–1179.
Conway, J., Castaigne, F., Picard, G., & Vovan, X. (1983). Mass transfer considerations in the osmotic dehydration of apples. Canadian Institute of Food Science and Technology Journal, 16, 25–29.
Crank, J. (1975). The mathematics of diffusion, 2nd Ed. New York: Oxford University Press.
Ferrando, M., & Spiess, W. (2003). Mass transfer in strawberry tissue during osmotic treatment I: microstructural changes. Journal of Food Science, 68, 1347–1355. doi:10.1111/j.1365-2621.2003.tb09649.x.
Fito, P. (1994). Modelling of vacuum osmotic dehydration of food. Journal of Food Engineering, 22, 313–228. doi:10.1016/0260-8774(94)90037-X.
Galmarini, M. V., Chirife, J., Zamora, M. C., & Pérez, A. (2008). Determination and correlation of the water activity of unsaturated, supersaturated and saturated trehalose solutions. LWT-Food Science and Technology, 41(4), 628–631.
Garcia-Martiez, E., Ruiz, G., Martinez-Monzo, J., Camatcho, M. M., Martinez-Navarette, N., & Chiralt, A. (2002). Jam manufacture with osmodehydrated fruit. Food Research International, 35, 301–306. doi:10.1016/S0963-9969(01)00200-9.
Giangiacomo, R., Torreggiani, D., & Abbo, E. (1987). Osmotic dehydration of fruit, Part I: sugar exchange between fruit and extracting syrup. Journal of Food Processing and Preservation, 11, 183–195.
Hawkes, J., & Flink, J. M. (1978). Osmotic concentration of fruit slices prior to freeze dehydration. Journal of Food Processing and Preservation, 2, 265–284. doi:10.1111/j.1745-4549.1978.tb00562.x.
Hough, G., Chirife, J., & Marini, C. (1993). A simple model for osmotic dehydration of apples. Lebensmittel-Wissenschaft und-Technologie, 26, 151–156.
Icier, F., Yildiz, H., & Baysal, T. (2006). Peroxidase inactivation and colour changes during ohmic blanching of pea puree. Journal of Food Engineering, 74(3), 424–429. doi:10.1016/j.jfoodeng.2005.03.032.
Kaymak-Ertekin, F., & Cakaloz, T. (1996). Osmotic dehydration of peas: I. Influence of process variables on mass transfer. Journal of Food Processing and Preservation, 20, 87–104. doi:10.1111/j.1745-4549.1996.tb00848.x.
Krokida, M. K., Karathanos, V. T., & Maroulis, Z. B. (2000). Effect of osmotic dehydration on colour and sorption characteristics of apple and banana. Drying Technology, 18, 937–950. doi:10.1080/07373930008917745.
Le Maguer, M. (1988). Osmotic dehydration: review and future directions. In Proceedings of the International Symposium on Progress in Food Preservation Processes 1: 283–309 Brussels, CERIA.
Lebovka, N. I., Bazhal, M. I., & Vorobiev, E. (2001). Pulsed electric field breakage of cellular tissues: visualization of percolative properties. Innovative Food Science & Emerging Technologies, 2(2), 113–125. doi:10.1016/S1466-8564(01)00024-8.
Lebovka, N. I., Bazhal, M. I., & Vorobiev, E. (2002). Estimation of characteristic damage time of food materials in pulsed-electric fields. Journal of Food Engineering, 54, 337–346. doi:10.1016/S0260-8774(01)00220-5.
Lebovka, N. I., Praporscic, I., & Vorobiev, E. (2004). Combined treatment of apples by pulsed electrical fields and by heating at mo derate temperature. Journal of Food Engineering, 65, 211–217. doi:10.1016/j.jfoodeng.2004.01.017.
Lenart, A. (1992). Mathematical modelling of osmotic dehydration of apple and carrot. Journal of Food and Nutrition Sciences, 1(42), 33–44.
Lenart, A., & Flink, J. M. (1984). Osmotic concentration of potato: II. Spatial distribution of osmotic effect. Journal of Food Technologyogy, 19, 65–89.
Marchal, L., Allali, H., & Vorobiev, E. (2005). Blanchiment de fraises par chauffage ohmique: incidence sur la cinétique de déshydratation impregnation par immersion, In: Récents Progrès en Génie des Procédés, Numéro 92 ISBN 2-910239-66-7, Ed. Lavoisier, Paris, France.
Mavroudis, N. E., Dejmek, P., & Sjöholm, I. (2004). Osmotic treatment induced cell death and osmotic processing kinetics of apples with characterised raw material properties. Journal of Food Engineering, 63, 47–56. doi:10.1016/S0260-8774(03)00281-4.
Mizrahi, S. (1996). Leaching of soluble solids during blanching of vegetables by ohmic heating. Journal of Food Engineering, 29, 153–166. doi:10.1016/0260-8774(95)00074-7.
Nanjundaswamy, A. M., Radhakrishnaiah Setty, G., Balachandran, C., Saroja, S., & Murthy Reddy, K. B. S. (1978). Studies on development of new categories of dehydrated product from indigenious fruits. Indian Food Packer, 22, 91–93.
Ponting, J. D. (1973). Osmotic dehydration of fruits: recent modifications and applications. Process Biochemistry, 8, 18–20.
Ponting, J. D., Watters, G. G., Forrey, R. R., Jackson, R., & Stanley, W. L. (1966). Osmotic dehydration of fruits. Journal of Food Technology, 29(10), 125–129.
Praporscic, I., Lebovka, N. I., Ghnimi, S., & Vorobiev, E. (2006). Ohmically heated, enhanced expression of juice from soft vegetable tissues. Biosystems Enginering, 93, 199–204. doi:10.1016/j.biosystemseng.2005.11.002.
Ramaswamy, H. S., & Nsonzi, F. (1998). Convective-air drying kinetics of osmotically pre-treated blueberries. Drying Technology, 16, 743–59. doi:10.1080/07373939808917433.
Raoult-Wack, A. L. (1994). Advances in osmotic dehydration. Trends in Food Science & Technology, 5, 255–260. doi:10.1016/0924-2244(94)90018-3.
Raoult-Wack, A. L., Lenart, A., & Guilbert, S. (1992). Recent advances in dewatering through immersion in concentrated solution. In A. S. Majumdar (Ed.), Drying solids (pp. 21–51). New York: International Science.
Rastogi, N. K., Eshtisghi, M. N., & Knorr, D. (1999). Accelerated mass transfer during osmotic dehydration of high intensity electrical field pulse pretreated carrots. Journal of Food Science, 64, 1020–1023. doi:10.1111/j.1365-2621.1999.tb12272.x.
Rastogi, N. K., & Raghavarao, K. S. M. S. (1995). Kinetics of osmotic dehydration of coconut. Journal of Food Process Enginering, 18, 187–97. doi:10.1111/j.1745-4530.1995.tb00362.x.
Rastogi, N. K., & Raghavarao, K. S. M. S. (1996). Kinetics of osmotic dehydration under vacuum. Lebensmittel-Wissenschaft Technologie, 29, 669–672. doi:10.1006/fstl.1996.0103.
Reznick, D. (1996). Ohmic heating of fluid foods: ohmic heating for thermal processing of foods: Government, industry, and academic perspectives. Food Technologyogy, 50, 250–251.
Sastry, S. K., & Li, Q. (1996). Modelling the ohmic heating of foods. Food Technology, 50, 246–248.
Sastry, S. K., Yousef, A., Cho, H. Y., Unal, R., Salengke, S., Wang, W. C. et al. (2001). Ohmic heating and moderate electric field (MEF) processing. In: Engineering and Food for the 21st Century.
Simal, S., Benedito, J., Sanchez, E. S., & Rossello, C. (1998). Use of ultrasound to increase mass transport rates during osmotic dehydration. Journal of Food Engineering, 36, 323–336. doi:10.1016/S0260-8774(98)00053-3.
Taiwo, K. A., Angersbach, A., Ade-Omowaye, B. I. O., & Knorr, D. (2001). Effect of pretreatments on the diffusion kinetics and some quality parameters of osmotically dehydrated apple slices. Journal of Agricultural and Food Chemistry, 49, 2804–2811. doi:10.1021/jf0009798.
Torreggiani, D. (1993). Osmotic dehydration in fruit and vegetable processing. Food Research International, 26, 59–68. doi:10.1016/0963-9969(93)90106-S.
Veinik, A. (1958). Approximative calculations of the thermo-conductivity processes, Moscow, Gosenegoizdat. p. 169–175.
Acknowledgment
The authors would like to thank the “Pole Regional Genie des Procédés” (Picardie, France) for providing financial support.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Allali, H., Marchal, L. & Vorobiev, E. Blanching of Strawberries by Ohmic Heating: Effects on the Kinetics of Mass Transfer during Osmotic Dehydration. Food Bioprocess Technol 3, 406–414 (2010). https://doi.org/10.1007/s11947-008-0115-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-008-0115-5