Abstract
The effect of heating duration and applied voltage were investigated on heat and mass transfer of the ohmic blanching mushroom. The drying kinetics and energy consumption of blanched/control samples were studied in a microwave dryer. Results showed that the mass loss during ohmic blanching depended on heating duration and applied voltage. Variation of electrical conductivity of mushroom was calculated using basic model and validated by the first law of thermodynamics (energy balance). The electrical conductivity of mushroom, water, and mixture increased with increasing temperature. At high voltage and longer heating duration, the blanched mushrooms dried faster than un-blanched mushroom while the drying of both samples occurred in a falling rate period. Moisture diffusion for blanched samples varied in the range of 4.23–6.08 × 10−7 m2/s while it was 4.22 × 10−7 m2/s for the control sample. Blanched sample at low voltage and heating duration consumed the minimum total energy during the drying process.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
1 Introduction
The current industrial process for blanching of vegetables, which is still based on the conventional hot water, involves the immersion of the fresh product for a prescribed time into hot water kept at a constant temperature [14, 27, 35]. This technique is limited due to the low heat penetration rate into the center of the large vegetables that may provide undesired softening of the products [14].
Ohmic treatment is one of the electron heating methods based on the passage of electrical current through a food product having electrical resistance [16, 36]. The electrical energy is converted to heat while the amount of heat generated through the food product is directly related to the voltage gradient and the electrical conductivity [6, 34]. Ohmic heating as an alternate processing method has shown to yield foods with higher quality compared to the conventional heating. This difference is mainly due to its ability to heat materials rapidly and uniformly leading to a less aggressive thermal treatment [1, 4, 13]. Xin et al. [43] reported that novel thermal blanching methods such as ohmic heating provide rapid and uniform heating, reduce the loss of soluble nutrients, and minimize thermal damage compared to conventional hot water blanching.
Sensoy and Sastry [35] investigated the effect of waveform and frequency on ohmic blanching of mushroom (mushroom/water ratio: 1:30) and found that the frequency (1–100 Hz) and waveform (sinusoidal, square, and triangular) did not affect the weight loss during ohmic blanching. Allali et al. [2] studied the effect of blanching by ohmic heating on the kinetics of osmotic dehydration of strawberries and found that ohmic heating increases drastically the mass transfer and the effective diffusion rates during osmotic dehydration of strawberries. In another study, Allali et al. [1] investigated the effect of blanching duration by ohmic heating on the osmotic dehydration behavior of apple cubes and reported that ohmic heating enhances mass transfer. Sarang et al. [31] developed the ohmic blanching method as a pretreatment method to improve uniformity the heating of solid-liquid food mixtures. They reported that particles and liquid in the mixture heat at rates depending on relative conductivities of the phases and the volume fractions of the respective phases.
Farahnaky et al. [13] investigated the effects of ohmic blanching on the kinetics of textural softening of cylindrical pieces of carrot roots, red beet, and golden carrots compared with that of hot water and microwave blanching. They reported that ohmic heating resulted in greater softening rates and weight losses and considerably less firm products than those blanched with either hot water or microwave blanching methods.
Icier [15] studied the effect of ohmic blanching as a pretreatment method of fluidized bed drying of artichoke and found that dried ohmic-blanched artichoke byproducts had higher total phenolic contents and fresh-like green color values than the conventional blanching. Guida et al. [14] reported that the losses in both protein and polyphenolic content of artichoke heads in conventional hot water blanching samples are greater than those measured in samples pre-treated by ohmic blanching. Icier et al. [17] showed that the ohmic blanching increased the quality parameters of pea puree than the water blanching. Ohmic blanching of root vegetables of carrot, red beet, and golden carrot resulted in greater softening rates. Moreover, the final hardness of the samples treated by ohmic heating was significantly lower than those of other samples treated by either conventional or microwave blanching method [13]. The literature review showed that the hot-air drying rate of vegetable tissue was accelerated with ohmic heating pretreatment [23, 41, 45]. Because ohmic heating accelerates moisture loss, it could have important commercial uses. Thus, further research is needed to elucidate mass transfer mechanisms during ohmic heating is important.
The electrical conductivity behavior depends to a large extent on whether a cellular structure exists within the material, the fiber orientation, and the size and shape of the solid food [24, 33]. The electrical conductivity of mushroom was measured by sandwiching samples between two electrodes in a closed compartment. The mushroom was compressed to fill the fuel cell and fully cover the electrodes of the ohmic cell. By this means, the porous structure of the mushroom has changed and the electrical conductivity of the material may be affected. On the other hand, several studies have been conducted to evaluate the pretreatment such as hot water and osmotic on drying of mushroom. However, there is no information regarding the effect of ohmic pre-treatment on the drying kinetics of mushroom in the literature.
This article shows an investigation on ohmic heating as a method for mushroom blanching and ohmic blanching as a pre-treatment technique for mushroom microwave drying. Also, the variation of heat and mass transfer, electrical conductivity, drying kinetics, and energy consumption during ohmic blanching and blanching-microwave drying of mushroom were determined under different conditions of duration blanching and applied voltage.
2 Materials and methods
2.1 Experimental material
Fresh white button mushrooms were obtained from Vartash mushroom farm, Gorgan, North of Iran. The samples were washed with tap water to remove dirt. The moisture content of mushroom was determined by heating in a drying oven at 103 °C for 24 h [10] and obtained 13.71 kg water/kg dry matter (or 93.2 ± 1.5% wet basis).
2.2 Ohmic blanching experiments
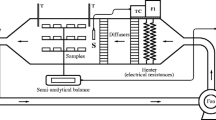
The ohmic heating system (Fig. 1) used in this study consists of a rectangular glass cell (length: 11 cm, width: 8 cm, height: 15 cm), two 30 6 L stainless steel electrodes (2 mm thickness) with a 11 cm gap between them, a power analyzer (Lutron DW-6090, Thailand), a voltage-regulating transformer (1 kW, 0–320 V, 50 Hz, MST - 3, Toyo, Japan), three K-type thermocouples (Teflon-coated), and a microcomputer. Power analyzer had a ± 1% accuracy 0.1 V resolution for voltage measurement and ± 1% accuracy and 1 mA resolution for electrical current measurement. One of the thermocouples was placed in the center of a mushroom stem (Fig. 1), the second thermocouple was placed in the center of the mushroom cap, and the other thermocouple was placed in the geometric center of the ohmic cell to measure of mixture temperature. Thermocouples were connected to the microcomputer by a multi-channel thermometer (TESTO 735–1, Germany) with a 0.1 °C resolution and ± 0.3 °C accuracy. The ohmic heating process was performed at voltages 90, 120, and 150 V [16, 29, 30, 38] under different duration heating 5, 10, and 15 min. In each experiment, about 54.77 ± 6.23 g of mushroom and 600 g water was used. The tap water with little removable ionic concentration has a very low electrical conductivity even under high voltage gradient. Due to this effect, the temperature of the water was raised slowly. In order to increase the electrical conductivity of tap water, about 1% salt (NaCl) was added to the mixture. Current, voltage, and temperature data were recorded at 1 s intervals during heating and monitored in the microcomputer. After the ohmic heating process, mushroom samples were moved from the water for measuring the mass changes in using a digital balance (A&G, GF-600, Japan) with a ± 0.01 g accuracy.
2.3 Microwave drying process
After cooling the ohmic blanched samples, they were transferred to the microwave dryer. Microwave drying experiments were carried out in a domestic digital microwave oven (M945, Samsung Electronics Ins) with the technical feature of 230 V, 50 Hz, and 1000 W. The moisture from oven chamber was removed with magnetron fan by passing it through the openings on the right side of the oven wall to the outer atmosphere. To evaluate the effect of ohmic pretreatment on the drying kinetics, experiments were performed for both non-blanched (as control group) and blanched mushrooms at only 900 W microwave power levels. In order to cover the entire in of the microwave tray by mushroom, about 164.77 ± 9.67 g of fresh mushroom was selected to blanch process in each experiment of drying. Samples were divided into three parts of 54.77 (±3.22) g and the blanching process was performed. After blanching of fresh mushroom, the samples were allowed to cool and then the surface water was taken by absorbent paper. Samples were spread uniformly in trays and trays were put in the dryer. Mass losses of samples were recorded at 2-min intervals by removing the samples from dryer using a digital balance of ±0.01 g accuracy. Drying process was stopped when the moisture content of the samples was about 10% ± 1.5 (w.b.). The moisture content at any time in drying experiment was calculated by (Jafari et al. 2015; [38]):
where mt and Xt are the mass (kg) and moisture content (kg water/kg dry matter) of sample at any time of drying, respectively, and m0 and X0 are the initial mass (kg), and moisture content (kg water/kg dry matter) of sample (kg water/kg dry matter), respectively.
2.4 Temperature homogeneity
Temperature homogeneity cap and stem of mushroom during ohmic heating were determined by the ratio of the center temperature to the surface temperature at each time interval as follows [3]:
where TH is the temperature homogeneity (dimensionless) and TC and Ts are the center and surface temperature (°C) of the cap and stem mushroom, respectively. It should be noted that the surface temperature is identical with the water temperature in the mixture of water/mushroom.
2.5 Mass loss
The mass loss of mushroom samples is defined by the following equation:
where ML is the percentage of mass loss (%), mb is the final mass of blanched mushroom (kg), and m0 is the mass of fresh mushroom (kg).
2.6 Modeling of electrical conductivity
The electrical conductivity of the mixture was calculated as follows:
where σmix is the electrical conductivity of the mixture (S/m), I is the current (A), V is the voltage (V), L is the distance between electrodes (m), and A is the surface contact between mixture and electrode (m2).
According to the previous works such as those of Engchuan et al. [11], Jiang et al. [19], Marra et al. [26], and Kanjanapongkul [21], the electrical conductivity-temperature relationship of each part in mixture of solid/liquid can be calculated using the basic model assuming that the electrical field was uniformly distributed inside the mixture (|∇V|l = |∇V|s). In this study, the basic model was used in cases of excluding the convective heat transferred between the mushroom samples and surrounding water as follows [11]:
For mushroom:
The specific heat capacity of mushroom calculated as follows [37]
For surrounding water:
where Cp is the specific heat capacity (J/kg.°C), T is the temperature (°C), ρ is the density (kg/m3), ∇V is the electrical field strength (V/m), σ is the electrical conductivity (S/m), and subscripts of w and m indicated the water and mushroom samples, respectively. The density of fresh mushroom is considered as 1300 kg/m3 [18].
The basic model was validated indirectly from the electrical conductivity of the mixtures. The experimental values of the mixture electrical conductivity were determined using Eq. (4), while the values of the electrical conductivity of the mixture were predicted based on the first law of thermodynamics (energy balance) as follows [11]:

where φ is the volume fraction of mushroom in the mixture (dimensionless), u is the volume (m3), m is the mass (kg), Qloss is the density of power loss (W/m3), h is the convective heat transfer coefficient between ohmic cell wall and ambient temperature (28 W/m2.K) [5], AC is the surface area of convective heat transfer in ohmic cell (0.0264 m2), uc is the volume of ohmic cell (0.00132 m3), um is the volume of mushroom samples (m3), uw is the volume of water (m3), T∞ is the ambient temperature (23 ± 1.5 °C), and Toh is the temperature of ohmic cell (°C). Then, Eq. (11) can be rewritten as follows:
The fitness of the basic model was evaluated by comparing the modeled mixture electrical conductivity (Eq. 13) and the experimental data (Eq. 4). The coefficient of determination (R2) and root mean square error (RSME) of estimate were calculated to determine the basic model performance. The RMSE parameter can be calculated as:
where σmix, exp , i and σmix, pre, i are experimental and predicted electrical conductivities (S/m), respectively, and N is the number of observations. Higher R2 and lower RMSE values indicated a better fit of the experimental data to the model.
2.7 Moisture diffusivity (drying)
The effective diffusivity of moisture was determined based on the change of moisture in mushroom (defined as moisture ratio) during the drying process and the solution of Fick’s law for mushroom as follows [40]:
where MR is the moisture ratio (dimensionless); Xe is the equilibrium moisture content (kg water/kg dry matter); Deff is the effective moisture diffusivity (m2/s); r is the radius and b is the length of a characteristic mushroom (m); t is the drying time (s); and λi is ith root of the Bessel function (2.405, 5.520, 8.654,…) of zero order, i = 1, 2, 3, and
The equilibrium moisture content of mushroom was assumed to be equal to zero during microwave drying (Togrul, 2006). For long drying times (MR < 0.6), when a and r are small and t is large, only the first term of the series solution is used. Then, Eq. (16) can be reduced to:
Thus, λ1 = 2.4048 for Eq. (17).
From Eq. (17), a plot of ln(MR) versus drying time would result in a straight line with slope:
2.8 Energy consumption
The energy consumption during ohmic blanching of mushroom samples was calculated from the following equation [6]:
where EOh is the specific energy consumption during ohmic blanching (J/kg fresh sample), m0 is the mass of fresh sample (kg), and t is the drying time (s).
The total energy consumption is equal to the sum of ohmic heating and microwave energies as follows:
where Et is the total specific energy consumption (J/kg fresh sample), Emic is the energy consumption by microwave dryer (J), P is the microwave power (W), and t is the drying time (s).
2.9 Uncertainty analysis
The uncertainty analysis is necessary to prove the accuracy of the experiments. During measurement and calculation of the parameters, the uncertainties occurring were determined using the following equation and presented in Table 1.
2.10 Statistical analysis
All the obtained data were expressed as mean and standard deviation values from three replicate measurements for different blanching conditions. The comparison of the results was made to analyze the effect of duration ohmic blanching and applied voltage on selected properties by using ANOVA and post hoc (Duncan) tests at the 5% significance level (p ≤ 0.05). The statistical evaluation was performed using SPSS V.18 software.
3 Results and discussion
3.1 Heat and mass transfer during ohmic blanching
Variations of temperature for mushroom (stem and cap) and water are shown in Fig. 2. When samples were heated at higher voltage gradient, increased input energy would increase the activity of water molecules and ionic substances resulting in a higher heating rate [6, 16, 32]. At first heating period, the temperature of the water is higher than that of the mushroom until 60–65 °C than the temperature of mushroom increased with a higher heating rate for all voltages applied. After a warming-up period and reaching the maximum temperature, sample temperature gradually remained constant during evaporation for the high voltages 120 and 150 V. While heating process with 90 V, the warming-up period is long (≥15 min) and thus no evaporation process was observed. Temperature homogeneity of cap/stem of mushroom samples is presented in Fig. 3. As can be seen from Fig. 3, the temperature of samples (cap/stem) not uniformly changed during the warming-up period. Temperature homogeneity varied between 0.62 and 1.27 before starting evaporation of mixture water. This variation is due to different size/texture of cam and stem of mushroom samples, which causes different heating rate in cap/stem of the mushroom.
Changes in the mass of samples are presented in Table 2 as the mass loss percentage. The results indicate that the mass transfer in mushroom samples depended strongly on the voltage applied and duration heating (p < 0.05). During a 5 min heating period, the mass of mushroom increased due to absorption of water. However, with increasing the applied voltage, the value of absorbed water was reduced. After a 10-min heating process, samples heated at low voltage (especially 90 V) were still absorbing water, while the samples heated with high voltage gradient were in water disposal state and reducing mass. Finally, it was observed that water disposal started after 15 min heating period for samples heated with 90 V. At 5 min initial heating process, the mass of sample increased about 0.42–9.58% while the mass loss of varied between 9.82–34.04% after a 15-min heating process.
3.2 Electrical conductivity
Figure 4a presents the experimental electrical conductivity of mixture (Eq. 4) as a function of average temperature. As can be noted, the electrical conductivity of mixture increased with temperature. A similar trend was observed by Assiry et al. (2010), Sakr and Liu (2014); Darvishi et al. [8], Icier et al. (2008), Sarang et al. (2008), and Darvishi et al. [6]. Also, the calculated electrical conductivity of mushroom and water increased with a temperature rise (Fig. 4b). Researchers have explained the increase in the electrical conductivity values with temperature via the reduced drag for the movement of ions [12, 16, 38]. This phenomenon occurs because of structural changes in the tissue-like cell wall protopectin breakdown, the expulsion of non-conductive gas bubbles, and softening and lowering in aqueous phase viscosity.
The electrical conductivity of mushroom, water (+1% NaCl), and mixture (water/mushrooms) varied within a range of 0.29–1.31 S/m, 0.18–0.58 S/m, and 0.24–0.68 S/m, respectively. The electrical conductivity values of the food material were within the general ranges of 0.01 to 10 S/m for biological materials [9, 44]. Sensoy and Sastry (2004) found that the electrical conductivity of mushroom and mixture of mushroom/water during ohmic heating varied with the range of 0.5–1.4 S/m and 0.25–0.70 S/m, respectively. From the electrical values, the electrical conductivity of mushroom is higher than that of the mixture. Also, it was observed that the applied voltage in study range (90-150 V) did not significantly affect the electrical conductivity of mushroom, water, or mixture. The results are generally in agreement with some of the literature on the ohmic heating of various food products [4, 12, 16].
Validation of the basic model was confirmed by comparing the predicted electrical conductivities using Eq. (13) with the measured electrical conductivity (Eq. 4) at different heating conditions. Fig. 5 shows the plot between the predicted and experimental values of the mixture electrical conductivity. A good agreement was observed between experimental and predicted electrical conductivity values, i.e., the data generally banded around the straight line on the plot. These results are confirmed by their high values of R2 (≥0.9799) and low values of RMSE (0.01098, 0.01327, and 0.04860 for 90, 120, and 150 V, respectively). Overall, it can be said that the measured electrical conductivity of mushroom in a mixture using basic model is acceptable. The result of this work was in agreement with the report of Jiang et al. [20] and Marra et al. [26], which reported that basic model has a high accuracy in estimating the temperature-electrical conductivity relationship. The relationship between average values of electrical conductivity and temperature can be represented as:
3.3 Mass transfer during microwave drying
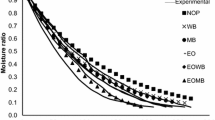
Due to different behavior of the mushroom during ohmic blanching, the mass of samples input to the microwave oven was different, as presented in Table 3. The average moisture content of the blanched and control mushrooms as a function of drying time is presented in Fig. 6. As can be clearly seen, most of the initial moisture was lost within the first few minutes of the drying time because energy absorption in microwave drying process depends on the moisture content of the material. Samples with a higher moisture content absorbed more energy and the heat generated within the sample created a larger vapor pressure difference between the center and the product surface (Wang and Sheng, 2006; [22]) and thus accelerated the interior moisture migration and increased surface water evaporation. Fig. 6 presents that there is no significant difference between drying rates of blanched samples at 5 min and 10 min duration and control sample because the moisture content of mushroom samples is equal at any point of time during drying (after initial drying phase). Results showed that the blanching pretreatment at 5 min (for all applied voltage) and 10 min duration (for 90 and 120 V applied voltage) has no significant effect on drying time.
The values of effective diffusivity obtained by using Eq. (19) and the estimated values are presented in Table 4. It is evident from Table 4 that the moisture diffusion for blanched and control samples varied within the range of 4.23–6.08 × 10−7 m2/s and 4.22 × 10−7 m2/s, respectively. These values fell within the normally expected range of Deff (10−12 to 10−7 m2/s) for food materials [7]. As can be seen, the difference between the moisture diffusivity of the control sample and blanched samples at high applied voltages and duration blanching is high. The obtained values of Deff from this study are comparable with the reported values of 2.99–9.15 × 10−8 m2/s for pre-treated and 1.045–3.68 × 10−8 m2/s for control apple pomace in microwave drying [42]; 0.2–2.9 × 10−8 m2/s and 0.2–2.1 × 10−8 m2/s for blanched and unblanched mushroom, respectively, in convective dryer [28]; and 4.86–11.0 × 10−7 m2/s for Panax notoginseng extract in vacuum belt drying [25].
3.4 Energy consumption
Table 4 shows the energy needed for blanching of mushroom under different conditions. As can be seen, the energy consumption increased with increasing of applied voltage and duration of blanching. At the same duration, energy consumption of low voltage, 90 V, is 3.8–5.3-fold lower than the 120 V applied voltage. One reason may be that the input energy to the system at higher voltage is more than the lower voltage for same duration heating and resulted in the increase in energy consumption [6, 38].
Variations of total specific energy consumption during the drying process of blanched and control mushroom samples are shown in Table 4. Form these data, the specific energy consumption for dying of blanched samples processed at high voltage and longer duration heating is higher than the samples blanched in low voltage and duration time. The reason for this trend can be due to values of disposal and water absorption during ohmic blanching process because the amount of energy absorbed in microwave dryer depends strongly on the moisture content of material [7, 39]. Blanched samples at high voltage and duration heating had low values of moisture content. Thus, absorption of the microwave energy decreased and energy consumption increased although drying time is lower under these conditions. According to these results, it can be stated that the effect of initial moisture content on the specific energy consumption is more than that of the drying time. Compared to controls, samples blanched at low heating voltage and duration consumed less energy while samples blanched at high voltage and duration consumed more energy. Minimum specific energy consumption was found as 3.48 MJ/kg fresh mushrooms for blanched samples at 90 V and 5 min duration heating.
4 Conclusion
Ohmic blanching process and effect of ohmic blanching as a pretreatment on microwave drying kinetics of white button mushroom were studied. The result showed that the blanching duration and voltage had a significant effect on mass loss, heat transfer, variations of electrical conductivity, and energy consumption during blanching process. The electrical conductivity of mushroom was calculated using basic model and validated by the first law of thermodynamics (energy balance). Blanching samples at high applied voltage/duration blanching resulted in an increase in moisture diffusivity and a decrease in drying time while blanching process at low voltage and duration heating did not affect drying kinetics of mushroom. The minimum energy consumption was found as 3.84 MJ/kg fresh mushrooms for blanched samples at a low voltage and duration blanching (i.e., 90 V and 5 min, respectively).
Abbreviations
- A:
-
Surface contact between mixture and electrode (m2)
- AC :
-
Surface area of convective heat transfer (m2)
- b:
-
Length of a characteristic mushroom (m)
- Cp :
-
Specific heat capacity (J/kg.K)
- Cp,m :
-
Specific heat capacity of mushroom (J/kg.K)
- Cp,w :
-
Specific heat capacity of water (J/kg.K)
- Deff :
-
Effective moisture diffusivity (m2/s)
- Emic :
-
Microwave energy consumption (J)
- Eoh :
-
Specific energy consumption for ohmic blanching (J/kg fresh sample)
- Et :
-
Total specific energy consumption (J/kg fresh sample)
- h:
-
Convective heat transfer coefficient (W/m2.K)
- I:
-
Current (A)
- L:
-
Gap between electrodes (m)
- m:
-
Mass (kg)
- M.C:
-
Moisture content (%, wet basis)
- m0 :
-
Mass of fresh mushroom (kg)
- mb :
-
Mass of blanched mushroom (kg)
- ML:
-
Mass loss of sample (%)
- MR:
-
Moisture ratio (−)
- mt :
-
Mass of sample at any time (kg water/kg dry matter)
- mw :
-
Mass of water in ohmic cell (kg)
- N:
-
Number of observations
- P:
-
Microwave power (W)
- Qloss :
-
Density of power loss (W/m3)
- r:
-
Radius (m)
- R2 :
-
Coefficient of determination
- RMSE:
-
Root mean square error (−)
- T:
-
Temperature (°C)
- t:
-
Time (s)
- T∞ :
-
Ambient temperature (°C)
- Tc:
-
Center temperature of the cap or stem mushroom (°C)
- TH:
-
Temperature homogeneity (−)
- Tm :
-
Temperature of mushroom (°C)
- Toh :
-
Temperature of the ohmic cell
- Ts:
-
Surface temperature of the cap or stem mushroom (°C)
- Tw :
-
Water temperature (°C)
- u:
-
Volume (m3)
- uc :
-
Volume of the ohmic cell (m3)
- um :
-
Volume of mushroom samples (m3)
- uw :
-
Volume of water (m3)
- V:
-
Applied voltage (V)
- X0 :
-
Initial moisture content (kg water/kg dry matter)
- Xe :
-
Equilibrium moisture content (kg water/kg dry matter)
- Xt :
-
Moisture content at any time (kg water/kg dry matter)
- Y:
-
Uncertainty
- z:
-
Independent variable
- λi :
-
ith root of the Bessel function
- ρm :
-
Mushroom density (kg/m3)
- ρw :
-
Water density (kg/m3)
- σ:
-
Electrical conductivity (S/m)
- σexp :
-
Experimental electrical conductivity (S/m)
- σm :
-
Electrical conductivity of mushroom (S/m)
- σmix :
-
Electrical conductivity of the mixture (S/m)
- σpre :
-
Predicted electrical conductivity (S/m)
- σw :
-
Electrical conductivity of water (S/m)
- φ:
-
Volume fraction of mushroom in the mixture (−)
- ∇V:
-
Electrical field strength (V/m)
- w:
-
Water
- mix:
-
Mixture
- m:
-
Mushroom
- 0 :
-
Initial
References
Allali H, Marchal L, Vorobiev E (2009) Effect of blanching by ohmic heating on the osmotic dehydration behavior of apple cubes. Dry Technol 27:739–746
Allali H, Marchal L, Vorobiev E (2010) Blanching of strawberries by ohmic heating: effects on the kinetics of mass transfer during osmotic dehydration. Food Bioprocess Technol 3:406–414
Bozkurt H, Icier F (2010) Ohmic cooking of ground beef: effects on quality. J Food Eng 96:481–490
Castro I, Teixeira JA, Salengke S, Sastry SK, Vicente AA (2004) Ohmic heating of strawberry products: electrical conductivity measurements and ascorbic acid degradation kinetics. Innov Food Sci Emerg Technol 5:27–36
Conti R, Gallitto AA, Fiordilino E (2014) Measurement of the convective heat-transfer coefficient. Phys Teach 52. https://doi.org/10.1119/1.4862118
Darvishi H, Hosainpour A, Nargesi F, Fadavi A (2015) Exergy and energy analyses of liquid food in an Ohmic heating process: a case study of tomato production. Innov Food Sci Emerg Technol 31:73–82
Darvishi H, Zarein M, Farhudi Z (2016) Energetic and exergetic performance analysis and modeling of drying kinetics of kiwi slices. J Food Sci Technol 53(5):2317–2333
Darvishi H, Khostaghaza MH, Najafi G (2013) Ohmic heating of pomegranate juice: electrical conductivity and pH change. J Saudi Soc Agric Sci 12(2):101–108
DeAlwis AAP, Halden K, Fryer PJ (1989) Shape and conductivity effects in the ohmic heating of foods. Chem Eng Res Des 67:159–168
Doymaz I (2014) Drying kinetics and rehydration characteristics of convective hot-air dried white button mushroom slices. J Chem 453175:1–8
Engchuan W, Jittanit W, Garnjanagoonchorn W (2014) The ohmic heating of meat ball: modeling and quality determination. Innov Food Sci Emerg Technol 23:121–130
Fadavi A, Yousefi S, Darvishi D, Mirsaeedghazi H (2018) Comparative study of ohmic vacuum, ohmic, and conventional-vacuum heating methods on the quality of tomato concentrate. Innov Food Sci Emerg Technol 47:225–230
Farahnaky A, Azizi R, Gavahian M (2012) Accelerated texture softening of some root vegetables by ohmic heating. J Food Eng 113:275–280
Guida V, Ferrari G, Pataro G, Chambery A, Di Maro A, Parente A (2013) The effects of ohmic and conventional blanching on the nutritional, bioactive compounds and quality parameters of artichoke heads. LWT-Food Sci Technol 53:569–579
Icier F (2010) Ohmic blanching effects on drying of vegetable byproduct. J Food Process Eng 33:661–683
Icier F, Ilicali C (2005) The use of tylose as a food analog in ohmic heating studies. J Food Eng 69(1):67–77
Icier F, Yildiz H, Baysal T (2006) Peroxidase inactivation and colour changes during ohmic blanching of pea puree. J Food Eng 74:424–429
Jadhav HT, Patil ST (2008) Effect of pretreatment, drying temperature and intermittent drying technique on physical properties of oyster mushroom. Int J Agric Eng 1(2):90–92
Jiang X, Li L, Shen W, Zhou J (2010a) Numerical simulation of inhomogeneous food with ohmic heating. Int J Food Eng 6(4):1–17
Jiang X, Li L, Shen W, Zhou J (2010b) Numerical simulation of inhomogeneous food with ohmic heating. Int J Food Eng 6(4):1–17 (Article 6)
Kanjanapongkul K (2017) Rice cooking using ohmic heating: determination of electrical conductivity, water diffusion and cooking energy. J Food Eng 192:1–10
Li Z, Raghavan GSV, Orsat V (2010) Temperature and power control in microwave drying. J Food Eng 97:478–483
Lima M, Sastry SK (1999) The effects of ohmic heating frequency on hot-air drying and juice yield. J Food Eng 41:115–119
Liu L, Llave Y, Jin Y, Zheng DY, Fukuoka M, Sakai N (2017) Electrical conductivity and ohmic thawing of frozen tuna at high frequencies. J Food Eng 197:68–77
Liu X, Qiu Z, Wang L, Cheng Y, Qu H, Chen Y (2009) Mathematical modeling for thin layer vacuum belt drying of Panax notoginseng extract. Energy Convers Manag 50:928–932
Marra F, Zell M, Lyng JG, Morgan DJ, Cronin DA (2009) Analysis of heat transfer during ohmic processing of a solid food. J Food Eng 91:56–63
Rastogi NK, Nayak CA, Raghavarao KSMS (2004) Influence of osmotic pre-treatments on rehydration characteristics of carrots. J Food Eng 65:287–292
Sahbaz F, Uzman D, Palazoğlu TK (2000) Drying kinetics of blanched and unblanched mushrooms. Nahrung 44:283–284
Salengke S, Sastry SK (2007a) Experimental investigation of ohmic heating of solid–liquid mixtures under worst-case heating scenarios. J Food Eng 83:324–336
Salengke S, Sastry SK (2007b) Models for ohmic heating of solid–liquid mixtures under worst-case heating scenarios. J Food Eng 83:337–355
Sarang S, Sastry SK, Gaines J, Yang TC, Dunne P (2007) Product formulation for ohmic heating: blanching as a pretreatment method to improve uniformity in heating of solid-liquid food mixtures. Food Eng Phys Prop 72(5):227–234
Sarkis JR, Jaeschke DP, Tessaro IC, Marczak LDF (2013) Effects of ohmic and conventional heating on anthocyanin degradation during the processing of blueberry pulp. LWT-Food Sci Technol 51:79–85
Sastry SK, Kamonpatana P (2014) Electrical conductivity of foods. In M.A. Rao, S.S.H. Rizvi, A.K. Datta, & J. Ahmed (Eds.), Engineering properties of foods 527–570
Sengun IY, Turp YG, Icier F, Kendirci P, Kor G (2014) Effects of ohmic heating for pre-cooking of meatballs on some quality and safety attributes. LWT-Food Sci Technol 55:232–239
Sensoy I, Sastery KS (2004) Ohmic blanching of mushroom. J Food Process Eng 27:1–15
Shirsat N, Lyng JG, Brunton NP, McKenna B (2004) Ohmic processing: electrical conductivities of pork cuts. Meat Sci 67:507–514
Shrivastava M, Datta AK (1999) Determination of specific heat and thermal conductivity of mushrooms (Pleurotus forida). J Food Eng 39:255–260
Torkian M, Borghei AM, Beheshti B, Hosseini SE (2015) The process of producing tomato paste by ohmic heating method. J Food Sci Technol 52:3598–3606
Torringa E, Esveld E, Scheewe I, Berg R, Bartels P (2001) Osmotic dehydration as a pre-treatment before combined microwave-hot-air drying of mushrroms. J Food Eng 49:185–191
Usub T, Lertsatitthankorn C, Poomsa-ad N, Wiset L, Siriamornpun S, Soponronnarit S (2010) Thin layer solar drying characteristics of silkworm pupae. Food Bioprod Process 88:149–160
Wang W, Sastry S (2000) Effects of thermal and electrotermal pretreatments on hot air drying rate of vegetable tissue. J Food Process Eng 23(4):299–319
Wang Z, Sun J, Chen F, Liao X, Hu X (2007) Mathematical modeling on thin layer microwave drying of apple pomace with and without hot air pre-drying. J Food Eng 80:536–544
Xin Y, Zhang M, Xu B, Adhikari B, Sun J (2015) Research trends in selected blanching pretreatments and quick freezing technologies as applied in fruits and vegetables: a review. Int J Refrig 57:11–25
Zell M, Lyng JG, Morgan DJ, Cronin DA, Morgan DJ (2009) Ohmic heating of meats: electrical conductivities of whole meats and processed meat ingredients. Meat Sci 83:563–570
Zhong T, Lima M (2003) The effect of ohmic heating on vacuum drying rate of sweet potato tissue. Bioresour Technol 87:215–220
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Soghani, B.N., Azadbakht, M. & Darvishi, H. Ohmic blanching of white mushroom and its pretreatment during microwave drying. Heat Mass Transfer 54, 3715–3725 (2018). https://doi.org/10.1007/s00231-018-2393-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-018-2393-4