Abstract
The effect of thermosonication (TS) and pulsed electric fields (PEF) on inactivation of Staphylococcus aureus (SST 2.4) and selected quality aspects in orange juice was investigated. Conventional pasteurization (HTST, 94 °C for 26 s) was used as a control. TS (10 min at 55 °C) applied in combination with PEF (40 kV/cm for 150 μs) resulted in a comparable inactivation of S. aureus to that achieved by conventional HTST. TS/PEF did not affect the pH, conductivity, or °Brix and had a milder impact on the juice color than thermal treatment. Furthermore, the non-enzymatic browning index was significantly affected by HTST (P < 0.05) but not by TS and PEF. Ascorbic acid retention was almost complete after TS and PEF (96.0%), but it was substantially lower (P < 0.05) after HTST (80.5%). Residual activity of pectin methyl esterase (PME) decreased as PEF field strength and treatment time increased; however, applying TS and PEF in combination left a greater residual PME activity than HTST (12.9 vs 5.0%, respectively).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
As consumers continue to increase their demand for safe foods with a fresh-like quality and longer shelf-life, a growing interest in food preservation technologies capable of producing high-quality minimally processed products is emerging. Pulsed electric fields (PEF) and ultrasound are two of the emerging technologies that are often classified as “non-thermal” food preservation processes (Hoover 1997; Señorans et al. 2003). These technologies have been reported to reduce the amount of microorganisms present in liquid foods while their “non-thermal” nature minimizes adverse effects of heat on quality attributes (e.g., nutrients, flavor, and color).
While the underlying modes of action are different, it is well established that both PEF (Doevenspeck 1961) and ultrasound (Harvey and Loomis 1929) may contribute to a reduction in the microbial population. When PEF treatment is applied, a transmembrane potential is established, which can lead to pore formation (i.e., electroporation) and subsequent leakage of intracellular components (Zimmermann et al. 1974). Electroporation can be either reversible or irreversible, depending on the intensity and the duration of the electric field (Schoenbach et al. 2002). The inactivation mechanism of ultrasound is thought to involve the process of cavitation (i.e., rapid creation, growth, and abrupt breakdown of liquid bubbles; Sala et al. 1995). This results in an antimicrobial effect caused by either temperature–pressure peaks or formation of free radicals (Fellows 2000; Butz and Tauscher 2002). Recent research suggests that the bactericidal effect of ultrasound can be enhanced by combination with heat (thermosonication, TS), pressure (manosonication), or both (manothermosonication; Piyasena et al. 2003).
The use of hurdle strategies (i.e., preserving foods by a combination of processes) for food preservation is an area of importance. Hurdles can be classified as physical (e.g., temperature, packaging), physicochemical (e.g., pH, a w), or microbial (e.g., bacteriocins; Leistner and Gorris 1995). The combination of these techniques is aimed at ensuring the necessary microbial safety of the product while maintaining a higher overall quality than is achievable by traditional methods.
Although extensive research on orange juice treated with PEF (Raso et al. 1998; McDonald et al. 2000; Yeom et al. 2000; Ayhan et al. 2002; Roodenburg et al. 2005; Cserhalmi et al. 2006) has been carried out in the past, so far, no information is available on the combination of PEF and ultrasound for orange juice processing.
When standard thermal pasteurization is applied to reconstituted fruit juice, it is usually heated in a high-temperature short-time (HTST) approach for approximately 10–30 s at 95 °C (Hicks 1990), achieving sufficient microbial safety and enzymatic stability.
Therefore, the aims of this study were to compare the bactericidal effect of conventional pasteurization with TS and PEF, applied individually and in combination, on Staphylococcus aureus in reconstituted orange juice. S. aureus was chosen because it has been one of the most frequently studied bacteria for investigations of effects of non-thermal processing (Patterson et al. 1995; Lamb et al. 2002). In addition, it is considered to be one of the most resistant bacteria to PEF treatment, and apparently, longer treatment times are necessary to achieve an acceptable degree of inactivation (Qin et al. 1998). A further objective was to determine the effect of the alternative and conventional processing on selected quality attributes of orange juice.
Materials and Methods
Product Preparation
Orange juice used in this experiment was reconstituted from concentrate in fresh drinking water 1 to 6.15 (v/v), yielding 11.5°Brix. When the juice was destined for microbial inoculation (as described below), it was autoclaved for 15 min at 121 °C before inoculation. For quality-related measurements, plain reconstituted orange juice was used without any sterilization or inoculation steps.
Microbiological Preparation and Counts
The study was conducted using S. aureus (SST 2.4), which was obtained from the microbial culture collection of the University College Cork, Ireland. A concentrated pure strain suspension of the bacteria was maintained at −16 °C. Broth subcultures were prepared by inoculating 5 ml of sterile tryptone soya broth (TSB; CM129, Oxoid Ltd., Basingstoke, Hampshire, UK) with 20 μl of the strain. These subcultures were incubated at 37 °C for 24 h in a heated rotary bath at 70 rpm (OLS 200, Grant Instruments Ltd., Cambridge, UK).
After the incubation period, the subcultures were further diluted (1:100) in sterile TSB and reincubated as above. The resulting cultures were used to inoculate sterile orange juice so that, before processing, the initial population of S. aureus was approximately 1011 CFU/ml for TS, PEF, TS/PEF, and HTST pasteurization. The initial population was estimated by collecting samples of inoculated juice immediately before processing. Bacterial numbers were estimated on tryptone soya agar (TSA; CM0131, Oxoid Ltd.) by surface inoculating with 0.1 ml of the neat solution or successive tenfold dilutions in Ringers solution (BR0052G, Oxoid Ltd.). Triplicate samples were spread on the agar surface using a sterile glass rod. Plates were incubated at 37 °C for 24 h. After incubation, the number of colony forming units (CFU) on each plate was counted, and the log cycle reductions of the bacteria (log N/N o) were plotted.
Possible recovery of the bacterial cells after treatment was investigated by spreading the samples on modified TSA (addition of 3% sodium chloride that is broadly in agreement with the method used by García et al. 2005) in the same manner as stated before and extending the incubation period to 48 h. The amount of sublethally injured cells was estimated by comparing the bacterial counts after 24 h growth on non-selective TSA to the counts after 48 h on selective, modified TSA.
Thermosonication
It is well established that TS is a more effective antimicrobial treatment than ultrasonication at ambient temperature (Piyasena et al. 2003). A temperature of 55 °C was chosen to match the maximum temperature within the PEF treatment cell (see “Microbial Inactivation with Pulsed Electric Fields”). For the TS hurdle, a temperature-controlled, 30-kHz ultrasound bath (U500, Ultrawave Limited, Cardiff, UK) was used, employing treatment times of 5, 10, and 20 min. Orange juice (200 ml) was inoculated with a subculture of S. aureus (200 μl) and incubated for 30 min in a shaking water bath (70 rpm and 37 °C) before TS for the adaptation of the microbial inoculum. The juice was then placed in the ultrasonic bath for the selected treatment time. In a similar manner, inoculated orange juice was placed in a heated water bath at 55 °C without ultrasound as a control. In both cases, the final temperature of the inoculated orange juice was in equilibrium with the water bath at 55 °C. Juice samples before and after the batch TS or the control treatment were plated and counted as described in “Microbiological Preparation and Counts.”
Pulsed Electric Fields
Treatment with PEF was carried out using a continuous treatment chamber (Fig. 1), which was connected to a laboratory-scale PEF unit (C-Tech Innovation Ltd., Capenhurst, UK). The treatment chamber consisted of two stainless steel electrodes housed in Teflon blocks and separated by a 2.5-mm-thick Perspex slotted spacer, which provided the flow channel for the juice. The total electrode area exposed was 7 × 10−4 m2.
PEF was applied using monopolar square-wave pulses (1-μs pulse width, frequency of 15 Hz), and the pulses were monitored using a high-voltage probe (P6015A, Tektronix, Beaverton, OR, USA) connected to a digital oscilloscope (TDS 2012, Tektronix). A peristaltic pump (SR25 S300, ESSKA Maschinen GmbH, Hamburg, Germany) was used to pump orange juice through the system at controlled flow rates of 63.0, 31.5, 15.8, and 11.8 ml/min, corresponding to a total pulse times of 25, 50, 100, and 150 μs, respectively. Electric field strengths were applied for each of the four treatment times, producing specific energy inputs of 25.5, 51.0, 102.0, and 153.1 kJ/l for 20 kV/cm; 73.7, 147.5, 295.0, and 442.4 kJ/l for 30 kV/cm; and 102.0, 204.1, 408.2, and 612.3 kJ/l for 40 kV/cm. Temperatures at all critical points along the system were monitored by T-type thermocouples (Industrial Temperature Sensors, Naas, Ireland) and recorded in 1-s intervals using a data logger (Squirrel SQ1600, Grant Instruments Ltd.). Immediately pre- and post-PEF treatment, the juice was cooled to <10 °C using cooling coils in a refrigerated water bath (Viscotherm VT100, Physica, Stuttgart, Germany). Under the most severe treatment conditions, the juice entered the PEF treatment chamber at 9.7 °C and left the chamber at 56 °C. Samples of orange juice pre- and post-PEF processing were plated and counted following the methods outlined in “Microbiological Preparation and Counts.”
TS/PEF Hurdle Treatment
Combined treatments consisted of batch TS for 10 min as described in “Thermosonication” followed by PEF at 30 and 40 kV/cm for different treatment times (as mentioned before in “Pulsed Electric Fields”). The temperature profile of the orange juice during the combined treatment was the same as when describing the hurdles individually. Orange juice samples before and after combined processing were plated and counted according to the descriptions outlined in “Microbiological Preparation and Counts.”
Heat Pasteurisation
Orange juice was pasteurized at 94 °C in a tubular heat exchanger unit (FT74 UHT/HTST processing system, Armfield Technical Education Co. Ltd., Ringwood, UK). The flow rate was adjusted to allow a residence time of 26 s in the holding tube. The juice was entering and exiting the heat exchanger at ambient temperature. The come-up time of the heat exchanger was 3.5 min, and the come-down time was 2.5 min for a total power consumption of 0.43 kJ/l. The juice samples before and after thermal processing were plated and counted as described in “Microbiological Preparation and Counts.”
Conductivity, pH, and °Brix Measurement
The pH, °Brix, and conductivity were measured in triplicate with a pH meter (Unicam 9450, Pye-Unicam, Cambridge, UK), a hand-held refractometer (0–50%, Bellingham and Stanley Ltd., Tunbridge Wells, UK), and a conductivity meter (Cyberscan CON 400 Series, Eutech Instruments, Singapore), respectively.
Color and Non-Enzymatic Browning Index Measurement
Colorimetric measurements were taken with a tristimulus colorimeter (CR 300, Minolta Co. Ltd., Osaka, Japan) in Hunter L* a* b* color space values and total color difference (ΔE) was calculated as follows:
ΔE were then classified as not noticeable (0–0.5), slightly noticeable (0.5–1.5), noticeable (1.5–3.0), well visible (3.0–6.0), and greatly different (6.0–12.0; Cserhalmi et al. 2006). The non-enzymatic browning index (NEBI) was determined using the method described by Meydav et al. (1977) measuring the UV absorbance of clarified juice at 420 nm.
Measurement of Pectin Methyl Esterase Activity and Ascorbic Acid Retention
The pectin methyl esterase (PME) activity was assayed by the method of Kimball (1991). Citrus fruit pectin, sodium chloride, and sodium hydroxide were purchased from Sigma-Aldrich (St. Louis, USA).
A 10-ml aliquot of sample juice was mixed with 40 ml of 1% pectin sodium chloride substrate and equilibrated to 30 °C in a water bath. The solution was adjusted to pH 7.0 with 2.0 M NaOH. The pH of the mixture was then quickly adjusted to pH 7.75 with 0.05 M NaOH, after which, exactly 100 µl of additional 0.05 M NaOH was added. The time (expressed in minutes) for the pH of the solution to return to a value of 7.75 was then measured. The assay was performed in duplicate for all samples. Pectin esterase units (PEU) were calculated according to Eq. 2, and relative PME activity was calculated using Eq. 3 (Kimball 1991):
Ascorbic acid (AA) content was measured using the redox titration between AA and dichloroindophenol as described by Tillmans et al. (1932). AA retention of treated samples was calculated using the following formula:
Statistical Analysis
Data from triplicate batches (n = 3) were analyzed statistically using GenStat version 8.1 (GenStat, VSN International Ltd., Hemel Hempstead, UK). Analysis of variance and, where appropriate, Tukey’s test for comparison of treatment means were used to determine statistical significance at a 5% level. The type of response (linear, quadratic) of microbial inactivation to increasing TS time and increasing PEF treatment time was further investigated using orthogonal polynomials in Genstat.
Results and Discussion
Microbial Inactivation with TS
The bactericidal effect of TS in reconstituted orange juice as a function of treatment time is shown in Fig. 2. A linear reduction (P < 0.05) in S. aureus numbers was detected when the juice was exposed to TS for 5, 10, and 20 min, achieving 0.8, 1.8, and 3.3 log cycle reductions, respectively. The magnitude of microbial inactivation in the present study is in line with the findings of other researchers who also investigated the effect of ultrasound treatment on liquid media, including low-pH products similar to orange juice (Utsunomiya and Kosaka 1979). Moreover, the impact of product pH on resistance to TS (i.e., increased lethality at lower pH) was shown using another microorganism (Lactobacillus acidophilus) in a study by Zenker et al. (2003) carried out in orange juice.
In another study, comparable inactivation of Zygosaccharomyces bailii in orange juice of up to 3.0 log cycles was observed by Earnshaw et al. (1995) under similar treatment conditions (55 °C at 20 kHz), although with a shorter treatment time of 6 min. Morphological and physiological differences could account for the different susceptibilities of S. aureus and Z. bailii to heat and ultrasound. Larger yeast cells are reported to be more prone to ultrasonic waves than smaller bacterial cells (Ahmed and Russell 1975) because of increased surface area, which is intensively stressed by pressure from ultrasonic cavitation.
Microbial Inactivation with PEF
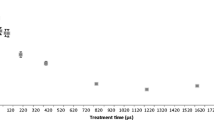
The effect of electric field strength and treatment time on S. aureus inactivation is presented in Fig. 3. Electric field strengths of 20 and 30 kV/cm were moderately effective in terms of microbial inactivation with, for example, a 3.0-log cycle reduction being obtained at 30 kV/cm and treatment time of 150 μs; however, an increase in field strength to 40 kV/cm led to a more significant microbial kill amounting to 4.5 and 5.5 log cycle reductions at 100 and 150 μs, respectively. The latter PEF conditions caused a moderate temperature increase of 36 °C, whereby juice entering the treatment chamber at 9.7 °C had increased to a temperature of 56 °C at the chamber outlet. Overall, there was a linear response to increasing treatment time in the microbial inactivation (P < 0.05).
Pothakamury et al. (1995) and Evrendilek et al. (2004) evaluated the impact of PEF on S. aureus in simulated milk ultrafiltrate (SMUF) or skim milk, respectively. While these workers reported a 3.0–4.0 log cycle reduction, it is difficult to compare their findings to those of the present study because the medium composition (SMUF/skim milk instead of orange juice), wave form (exponential vs square), electrical field strength (16 kV/cm vs 20–40 kV/cm), and pulse duration (200–300 μs instead of 25–150 μs) all differ from those used in the current work. All of these factors have been found to significantly affect the efficiency of microbial inactivation (Rüegg et al. 1977; Zhang et al. 1995; Barsotti et al. 1999). While McDonald et al. (2000) have shown the effectiveness of PEF in orange juice against other bacteria, such as Escherichia coli, Listeria innocua and Leuconostoc mesenteroides, the PEF experimental conditions were sufficiently dissimilar to those used in the present study so as not to allow direct comparisons to be made.
Impact of Hurdle Treatment with TS and PEF
The target of the hurdle approach used in this study was to obtain a comparable level of microbial inactivation to that obtained by a conventional thermal method. In choosing a hurdle approach, it was assumed that a combination of TS and PEF at their maximum level of effectiveness (i.e., 3.3 and 5.5 log cycles, respectively) theoretically would have produced a microbial inactivation in excess of the heat pasteurization treatment (i.e., 6.8 log cycles) used in this study. As treatment exposure times of TS and PEF were vastly different (minute vs microsecond, respectively), a shorter TS treatment time of 10 min was chosen to match the microbial inactivation of conventional pasteurization while minimizing the thermal exposure of the juice.
The combined effect of batch TS and continuous PEF on the inactivation of S. aureus is shown in Fig. 4. Because of the low inactivation shown when PEF was applied at a field strength of 20 kV/cm, the combined treatment consisted of TS and PEF with electric field strengths of 30 and 40 kV/cm. Maximum bacterial inactivation was observed under the most severe conditions (TS for 10 min with PEF at 40 kV/cm for 150 μs), resulting in an overall bacterial reduction of 6.8 log cycles, similar to the 6.8 log cycle reduction obtained by conventional thermal pasteurization (P ≥ 0.05). An additive effect on microbial inactivation between TS (1.8 log) and PEF (5.5 log) was detected when operating in combination at their respective maximum level (6.8 log cycles in total), which was not significantly lower than the reduction theoretically obtainable (7.3 log cycles) by adding the two individual effects. A possible explanation for these results could be the processing sequence where TS acted on the most sensitive cells of S. aureus, leaving the remaining and more resistant cells for inactivation by PEF treatment. It is well documented that TS has an ‘all or nothing’ inactivation effect on microorganisms (Barbosa-Cánovas et al. 2005; Mañas and Pagán 2005), which is in line with the findings of this study. Therefore, the total inactivation of a combined TS/PEF treatment can only be determined by the severity of the PEF processing conditions applied. In regard to sublethal injury, no difference in bacterial counts was observed between the samples grown non-selectively for 24 h and incubated for 48 h on selective, modified agar. Shortening the treatment time from 150 to 100 μs at 40 kV/cm led to a reduced microbial inactivation of 5.1 log cycles for S. aureus, which fulfils the microbiological recommendations of the Food and Drug Administration (FDA 2001) for citrus juices. The application of square-wave PEF (15–28 kV/cm) achieved a higher inactivation of S. aureus strains in phosphate–citrate buffer (pH 7) compared to both ultrasound treatment under pressure (117 μm, 20 kHz, 200 kPa) and thermal treatment (58 °C), as reported by Rodríguez-Calleja et al. (2006).
When PEF was applied on its own at 40 kV/cm for 150 μs, it resulted in a 5.5-log cycle reduction of S. aureus, which is in line with the above-mentioned FDA recommendations; however, when combined with TS, the same PEF conditions brought about a 6.8-log cycle reduction, which was equivalent to the reduction achieved by a standard heat pasteurization (P ≥ 0.05). Therefore, a beneficial effect was achieved by combining the two technologies in a hurdle approach.
A comparable reduction of 4.2 log cycles in total aerobic plate counts in orange juice before and after continuous stand-alone PEF treatment at 30 kV/cm and 19.8 pulses over 60 μs was reported by Qiu et al. (1998). Rodríguez-Calleja et al. (2006) proposed that PEF could be a potent alternative instead of the typical thermal treatment because of the distinct sensitivity of most S. aureus strains to electropermeabilization.
Gram-positive bacteria such as S. aureus have been reported to be generally more resistant to PEF than yeasts (Qin et al. 1998) or Gram-negative bacteria (Hülsheger et al. 1983) because of their different cell size or cell structure, respectively. However, the distinct efficacy of PEF toward S. aureus may not be related entirely to the fact that electroporation showed a greater bactericidal effect on S. aureus than ultrasonic cavitations; it is possible that the morphological and physiological properties of the cell could have affected its disruption which is, for instance, indicated by higher resistance of rod-shaped, Gram-positive Listeria monocytogenes to PEF (Rodríguez-Calleja et al. 2006). Jeyamkondan et al. (1999) suggested that an increase in local perturbations in the membrane surface, changes in membrane conformation by dipolar reorientation in phosphor-lipid monolayers, or denaturation of voltage-sensitive protein channels in the cell membrane could be among the factors which contribute to membrane breakdown and, thus, cell disruption. As cell disruption has not been reported unambiguously and organism-specific in literature, more research on explicit changes in morphology and physiology induced by PEF in microorganisms is recommended.
Effect of TS and PEF on Juice Quality Attributes
No significant changes in conductivity, pH, and °Brix were detected for any of the treatments conducted in this study (P ≥ 0.05), with average values of 4.2 mS/cm, 3.7, and 11.0, respectively. The overall color attributes for untreated orange juice were 42.84, −6.31, and 18.99 for L*, a*, and b*, respectively. Colorimetric data of reconstituted orange juice processed by hurdle approach (Table 1) showed significant differences from that obtained by thermal treatment (P < 0.05).
Thermal treatment of orange juice caused an overall darkening of the sample when increasing the redness and decreasing the yellowness. By contrast, TS showed a significant increase in L*, whereas a minor decrease in a* and increase in b* were detected; however, when TS was followed by PEF, a gradual compensating effect was observed with increasing electric field strength and PEF duration. As a consequence, the TS/PEF-treated product that was most similar to untreated juice was that obtained using PEF at 40 kV/cm for 150 µs after TS. When considering the total color difference (ΔE), the heat pasteurization caused a ΔE of 2.7, which is a “noticeable” change according to the criteria described in “Conductivity, pH, and °Brix Measurement.” This is in agreement with Lee and Coates (2003), who showed a significant ΔE of 2.9 between fresh and heat-treated orange juice samples. This confirms the adverse effects of thermal processing on orange juice color as also reported by other scientists (Sizer and Waugh 1988; Yeom et al. 2000). The combination of TS with shorter PEF treatment (i.e., 25 and 50 µs) produced “noticeable” changes in ΔE values, which were in the range of 1.5–3.0. In contrast, the hurdle approach (TS and PEF at 40 kV/cm for 150 µs) led to a “not noticeable” ΔE of 0.3.
Ayhan et al. (2002) observed that PEF-treated orange juice differed significantly in all color attributes from fresh, untreated orange juice, showing an increase in L* and b* and a decrease in a* after continuous PEF treatment with 35 kV/cm for 59 μs. However, these changes in color were not regarded as detrimental but could be considered as contrasting with the results of the current study.
Different findings, such as no change of L* between untreated, PEF-treated, and heat-pasteurized orange juice; a decrease in a* after either treatment; and a decrease of b* by PEF, were reported by Sánchez-Moreno et al. (2005), using PEF at 35 kV/cm for 750 μs and HTST pasteurization at 90 °C for 60 s. These differences could be caused by the longer treatment exposures employed by these authors and their use of fresh rather than reconstituted orange juice.
The effect of TS, alone and in combination with PEF, and thermal treatment on NEBI, AA retention, and PME activity is shown in Table 2. Processing-reconstituted orange juice by conventional heat treatment resulted in the lowest residual activity of PME compared to the untreated sample (5.0%). While TS alone proved unsuccessful in causing any appreciable inactivation of PME, when the orange juice was subject to TS followed by PEF, the PME residual activity decreased significantly (P < 0.05) in response to the PEF treatment time. Increasing the electric field strength also had a significant effect in decreasing the residual activity of PME (P < 0.05). A change in the conformation of the enzyme is generally claimed to be the explanation of the mechanism of inactivation of enzymes by PEF. In particular, in a study by Yeom et al. (2002), the total treatment time seemed to contribute to a decrease in the relative PME activity once the intensity of the field applied was sufficiently high (35 kV/cm). In this study, when 40 kV/cm were applied after TS, a more pronounced effect of treatment time was noted than when PEF was applied at 30 kV/cm: at 40 kV/cm, PME residual activity decreased from 82.7 and 76.3% when the product was treated for 25 and 50 μs, and to 19.2 and 12.8% when the treatment time was 100 and 150 μs, respectively. The same sequence of treatment times but at 30 kV/cm of field intensity caused a decrease from 86.5 and 79.6% to only 56.6 and 43.2%, respectively. However, even when the most severe combination treatment was applied (TS for 10 min at 55 °C, PEF at 40 kV/cm for 150 μs), the residual activity was still higher than that caused by thermal pasteurization (12.8 vs 5.0%, P < 0.05).
In a study also carried out on orange juice by Yeom et al. (2002), a significant effect of PEF strength was noted, and a field of 35 kV/cm applied for 184 μs led to a PME residual activity of 16.8%, which is broadly in agreement with the data presented in the current study. Other studies reported an overall PME residual activity of 21% (Rodrigo et al. 2003) in mixed orange and carrot juice after PEF treatment. These findings contrast with those of Van Loey et al. (2002) who found that PEF applied at 35 kV/cm for up to 1,000 μs caused a 10% maximum decrease in PME in enzyme-enriched orange juice. This low inactivation may have been caused by post-treatment release of intracellular PME as a consequence of electroporation. However, the difference in applied pulse frequency and, hence, the moderate temperature rise because of PEF treatment could partly contribute to the discrepancy in the results. The PME activity measured after pasteurization by heat exchanger was similar to that reported by Yeom et al. (2000) and was in line with the 0–10% figure reported for commercial-heat-pasteurized orange juice by Irwe and Olsson (1994).
Compared to the untreated samples, juice processed by either TS or TS in combination with PEF did not show any significant difference in the AA retention. By contrast, a significant decrease of 19.5% (P < 0.05) was noted in samples of orange juice processed by conventional thermal pasteurization. This observation is in agreement with the results reported by Min et al. (2003), who noted a loss of 19% in AA caused by heat treatment, whereas a PEF treatment of 40 kV/cm for a total treatment time of 97 μs did not change significantly the original AA concentration in freshly squeezed orange juice. Similar results (a 2.5% decrease in AA concentration of orange juice) were found by Hodgins et al. (2002) who employed PEF at 80 kV/cm for between 40 and 60 μs. The results obtained in the present study may indicate that the relatively low temperature induced following the treatment with TS or TS/PEF does not affect the retention of AA.
The difference in NEBI showed agreement with the colorimetric data, with the largest difference in NEBI between the unprocessed and processed samples noted when orange juice was pasteurized by conventional methods. There was less difference comparing the samples processed by TS or TS followed by PEF, regardless of the total PEF treatment time. Significant correlations (P < 0.001) were obtained between the differences in color attributes and the NEBI values caused by the process treatments (r = 0.87, 0.86, and 0.84 for ΔL, Δa, and Δb, respectively).
Conclusions
When considered as a stand-alone preservation technology, TS, as used in this study, required a long processing time to achieve significant reduction of S. aureus. By contrast, PEF reduced the S. aureus population by a maximum of 5.5-log cycles, which was less than the conventional pasteurization treatment examined but was in line with FDA recommendations for juice processing. However, a semi-batch hurdle approach consisting of batch TS followed by continuous PEF treatment at electric field strength of 40 kV/cm for 150 μs was found to significantly reduce S. aureus growth by up to 6.8 log cycles in reconstituted orange juice. This approach produced comparable results to those obtained with conventional pasteurization while reducing the heat input. When considering the comparable microbial inactivation achieved and the quality aspects observed, a hurdle approach consisting of TS and PEF (40 kV/cm and 150 μs) shows great potential for improving the quality and the safety of orange juice. As semi-batch TS/PEF is limited in terms of product throughput by the batch TS and further quality improvement could be expected by shorter treatment exposure, further research on process optimization toward a fully continuous setup is suggested. Investigation of additional quality aspects will also be necessary to explore the full potential of combining these two technologies.
References
Ahmed, F. I. K., & Russell, C. (1975). Synergism between ultrasonic waves and hydrogen peroxide in the killing of microorganisms. Journal of Applied Bacteriology, 39(1), 31–40.
Ayhan, Z., Zhang, Q. H., & Min, D. B. (2002). Effects of pulsed electric field processing and storage on the quality and stability of single strength orange juice. Journal of Food Protection, 65(10), 1623–1627.
Barbosa-Cánovas, G. V., Tapia, M. S., & Cano, M. P. (Eds.) (2005). Novel food processing technologies. Boca Raton, USA: CRC Press.
Barsotti, L., Merle, P., & Cheftel, J. C. (1999). Food processing by pulsed electric fields, I. Physical aspects. Food Review International, 15(2), 163–180.
Butz, P., & Tauscher, B. (2002). Emerging technologies: Chemical aspects. Food Research International, 35(2–3), 279–284.
Cserhalmi, Zs., Sass-Kiss, Á., Tóth-Markus, M., & Lechner, N. (2006). Study of pulsed electric field treated citrus juices. Innovative Food Science and Emerging Technologies, 7(1–2), 49–54.
Doevenspeck, H. (1961). Influencing cells and cell walls by electrostatic impulses. Fleischwirtschaft, 13(12), 986–987.
Earnshaw, R. G., Appleyard, J., & Hurst, R. M. (1995). Understanding physical inactivation processes: Combined preservation opportunities using heat, ultrasound and pressure. Journal of Food Microbiology, 28(2), 197–219.
Evrendilek, G. A., Zhang, Q. H., & Richter, E. R. (2004). Application of pulsed electric fields to skim milk inoculated with Staphylococcus aureus. Biosystems Engineering, 87(2), 137–144.
FDA (2001). Hazard analysis and critical control point (HACCP): Procedures for the safe and sanitary processing and importing of juices. Final rule. Federal Register, 66(13), 6137–6202.
Fellows, P. J. (2000). Food processing technology: Principles and practice. New York, USA: CRC Press.
García, D., Hassani, M., Mañas, P., Condón, S., & Pagán, R. (2005). Inactivation of Escherichia coli O157:H7 during the storage under refrigeration of apple juice treated by pulsed electric fields. Journal of Food Safety, 25(1), 30–42.
Harvey, F., & Loomis, A. (1929). The destruction of luminous bacteria by high frequency sound waves. Journal of Bacteriology, 17(5), 373–376.
Hicks, D. (1990). Production and packaging of non-carbonated fruit juices and fruit beverages. Glasgow, UK: Blackie and Son.
Hodgins, A. M., Mittal, G. S., & Griffiths, M. W. (2002). Pasteurization of fresh orange juice using low-energy pulsed electrical field. Journal of Food Science, 67(6), 2294–2299.
Hoover, D. G. (1997). Minimally processed fruits and vegetables: Reducing microbial load by nonthermal physical treatments. Food Technology, 51(6), 66–71.
Hülsheger, H., Potel, J., & Niemann, E. G. (1983). Electric field effects on bacteria and yeast cells. Radiation and Environmental Biophysics, 22(2), 149–162.
Irwe, S., & Olsson, I. (1994). Reduction of pectinesterase activity in orange juice by high-pressure treatment. In R. P. Singh, & F. A. R. Oliveira (Eds.) Minimal processing of foods and process optimization (pp. 35–42). Ann Arbor, MI, USA: CRC Press.
Jeyamkondan, S., Jayas, D. S., & Holley, R. A. (1999). Pulsed electric field processing of foods: A review. Journal of Food Protection, 62(9), 1088–1096.
Kimball, D. (1991). Citrus processing: Quality control and technology. New York, USA: Springer.
Lamb, J. L., Gogley, J. M., Thompson, M. J., Solis, D. R., & Sen, S. (2002). Effect of low-dose gamma irradiation on Staphylococcus aureus and product packaging in ready-to-eat ham and cheese sandwiches. Journal of Food Protection, 65(11), 1800–1805.
Lee, S. H., & Coates, G. A. (2003). Effect of thermal pasteurization on Valencia orange juice color and pigments. Lebensmittel-Wissenschaft und -Technologie, 36(1), 153–156.
Leistner, L., & Gorris, L. G. M. (1995). Food preservation by hurdle technology. Trends in Food Science and Technology, 6(2), 41–46.
Mañas, P., & Pagán, R. (2005). Microbial inactivation by new technologies of food preservation. Journal of Applied Microbiology, 98(6), 1387–1399.
McDonald, C. J., Lloyd, S. W., Vitale, M. A., Petersson, K., & Innings, F. (2000). Effects of pulsed electric fields on microorganisms in orange juice using electric field strengths of 30 and 50 kV/cm. Food Engineering and Physical Properties, 65(6), 984–989.
Meydav, S., Saguy, I., & Kopelman, I. J. (1977). Browning determination in citrus products. Journal of Agriculture and Food Chemistry, 25(3), 602–604.
Min, S., Jin, Z. T., Min, S. K., Yeom, H., & Zhang, Q. H. (2003). Commercial-scale pulsed electric field processing of orange juice. Journal of Food Science, 68(4), 1265–1271.
Patterson, M. F., Quinn, M., Simpson, R., & Gilmour, A. (1995). Sensitivity of vegetative pathogens to high hydrostatic pressure treatment in phosphate-buffered saline and foods. Journal of Food Protection, 58(5), 524–529.
Piyasena, P., Mohareb, E., & McKellar, R. C. (2003). Inactivation of microbes using ultrasound: A review. International Journal of Food Microbiology, 87(3), 207–216.
Pothakamury, U. R., Monsalve-González, A., Barbosa-Cánovas, G. V., & Swanson, B. G. (1995). Inactivation of Escherichia coli and Staphylococcus aureus in model foods by pulsed electric field technology. Food Research International, 28(2), 167–171.
Qin, B. L., Barbosa-Cánovas, G. V., Swanson, B. G., Pedrow, B. G., & Olsen, R. G. (1998). Inactivation of microorganisms using pulsed electric field continuous treatment system. Institute of Electrical and Electronics Engineers Transaction on Industry Application, 34(1), 55–60.
Qiu, X., Sharma, S., Tuhela, L., Jia, M., & Zhang, Q. H. (1998). An integrated PEF pilot plant for continuous non-thermal pasteurization of fresh orange juice. Transactions of the American Society of Agricultural Engineers, 41(4), 1069–1074.
Raso, J., Calderón, M. L., Góngora, M., Barbosa-Cánovas, G. V., & Swanson, B. G. (1998). Inactivation of mold ascospores and conidiospores suspended in fruit juices by pulsed electric fields. Lebensmittel-Wissenschaft und -Technologie, 31(7–8), 668–672.
Rodrigo, D., Barbosa-Canovas, G. V., Martinez, A., & Rodrigo, M. (2003). Pectin methyl esterase and natural microbial flora of fresh mixed orange and carrot juice treated with pulsed electric fields. Journal of Food Protection, 66(12), 2336–2342.
Rodríguez-Calleja, J. M., Cebrián, G., Condón, S., & Mañas, P. (2006). Variation in resistance of natural isolates of Staphylococcus aureus to heat, pulsed electric fields and ultrasound under pressure. Journal of Applied Microbiology, 100(5), 1054–1062.
Roodenburg, B., Morren, J., Berg, H. E., & De Haan, S. W. H. (2005). Metal release in a stainless steel pulsed electric field (PEF) system Part II. The treatment of orange juice; related to legislation and treatment chamber lifetime. Innovative Food Science and Emerging Technologies, 6(3), 337–345.
Rüegg, M., Moor, U., & Blanc, B. (1977). A calorimetric study of the thermal denaturation of whey proteins in simulated milk ultrafiltrate. Journal of Dairy Research, 44(3), 509–520.
Sala, F. J., Burgos, J., Condón, S., López, P., & Raso, J. (1995). Effect of heat and ultrasound on microorganisms and enzymes. In GWGould (Ed.) New methods of food preservation (pp. 176–204). London, UK: Blackie Academic & Professional.
Sánchez-Moreno, C., Plaza, L., Elez-Martínez, P., De Ancos, B., Martín-Belloso, O., & Cano, M. P. (2005). Impact of high pressure and pulsed electric fields on bioactive compounds and antioxidant activity of orange juice in comparison with traditional thermal processing. Journal of Agricultural and Food Chemistry, 53(11), 4403–4409.
Schoenbach, K. H., Katsuki, S., Stark, R. H., Buescher, E. S., & Beebe, S. J. (2002). Bioelectrics—New applications for pulsed power technology. Institute of Electrical and Electronics Engineers Transactions on Plasma Science, 30(1), 293–300.
Señorans, F. J., Ibáñez, E., & Cifuentes, A. (2003). New trends in food processing. Critical Reviews in Food Science and Nutrition, 43(5), 507–526.
Sizer, C. E., & Waugh, P. L. (1988). Maintaining flavor and nutrient quality of aseptic orange juice. Food Technology, 42(6), 152–159.
Tillmans, J., Hirsch, P., & Siebert, F. (1932). Das Reduktionsvermögen pflanzlicher Lebensmittel und seine Beziehung zum Vitamin C. Zeitschrift für Lebensmitteluntersuchung und -Forschung A, 63(1), 21–30.
Utsunomiya, Y., & Kosaka, Y. (1979). Application of supersonic waves to food. Journal of the Faculty of Applied Biological Sciences Hiroshima University, 18(2), 225–231.
Van Loey, A., Verachtert, B., & Hendrickx, M. (2002). Effects of high electric field pulses on enzymes. Trends in Food Science and Technology, 12(3–4), 94–102.
Yeom, H. W., Streaker, C. B., Zhang, Q. H., & Min, D. B. (2000). Effects of pulsed electric fields on the activity of microorganisms and pectin methyl esterase in orange juice. Journal of Food Science, 65(8), 1359–1363.
Yeom, H. W., Zhang, Q. H., & Chism, G. W. (2002). Inactivation of pectin methyl esterase in orange juice by pulsed electric fields. Journal of Food Science, 67(6), 2154–2159.
Zenker, M., Heinz, V., & Knorr, D. (2003). Application of ultrasound-assisted thermal processing for preservation and quality retention of liquid foods. Journal of Food Protection, 66(9), 1642–1649.
Zhang, Q. H., Barbosa-Cánovas, G. V., & Swanson, B. G. (1995). Engineering aspects of pulsed electric field pasteurisation. Journal of Food Engineering, 25(2), 261–281.
Zimmermann, U., Pilwat, G., & Riemann, F. (1974). Dielectric breakdown in cell membranes. Biophysical Journal, 14(11), 881–899.
Acknowledgment
The authors acknowledge the financial support of the Non-Commissioned Food Institutional Research Measure, funded by the Department of Agriculture, Fisheries and Food, Ireland.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Walkling-Ribeiro, M., Noci, F., Riener, J. et al. The Impact of Thermosonication and Pulsed Electric Fields on Staphylococcus aureus Inactivation and Selected Quality Parameters in Orange Juice. Food Bioprocess Technol 2, 422–430 (2009). https://doi.org/10.1007/s11947-007-0045-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11947-007-0045-7