Opinion statement
Neuromyelitis optica spectrum disorder (NMOSD) is a rare, autoimmune disease of the central nervous system that primarily attacks the optic nerves and spinal cord leading to blindness and paralysis. The spectrum of the disease has expanded based on the specificity of the autoimmune response to the aquaporin-4 water channel on astrocytes. With wider recognition of NMOSD, a standard of care for treatment of this condition has condition based on a growing series of retrospective and prospective studies. This review covers the present state of the field in the treatment of acute relapses, preventive approaches, and therapies for symptoms of NMOSD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neuromyelitis optica spectrum disorder (NMOSD) is a rare, autoimmune disease of the central nervous system (CNS) that primarily attacks the optic nerves and spinal cord leading to blindness and paralysis [1]. NMO was first described and coined in the late 1800s but only recognized to be an entity distinct from multiple sclerosis (MS) over the past 10 years with the discovery of a unique biomarker antibody that identifies the disease in up to 72 % of NMOSD patients with >99 % specificity [2]. NMOSD accounts for approximately 1.5 % of demyelinating diseases in Caucasian populations extrapolating to a prevalence of 0.52 to 4.4 per 100,000 [3]. Although the incidence of demyelinating disease is lower in non-Caucasian countries, the percentage of demyelinating diseases made up by NMOSD is higher [4].
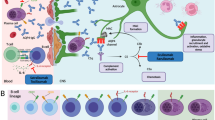
Although rare throughout the world, NMOSD has received widespread attention because of the progress made in understanding the pathogenesis of disease and the identification of druggable targets for therapy. In 2005, the target of the NMO antibody was confirmed to be the aquaporin-4 water channel (AQP4) expressed on the end feet of astrocytes in the central nervous system [5]. The coordinated immunological attack against AQP4 is mediated by B and T cells, innate cells including neutrophils and eosinophils, the complement system, as well as pathogenic antibodies, each of which has been successfully targeted for therapy in NMO. Human treatment studies published to date are mostly retrospective, with a handful of prospective open-label series that provide an insight into the feasibility and potential efficacy of certain treatments. These small studies laid the foundation for investment in three worldwide, blinded, placebo-controlled pivotal trials competing to be the first approved medication for NMOSD. This review will include analysis of the aforementioned retrospective and prospective studies, as well as a discussion about the direction of the field of NMOSD treatment.
Treatment of NMOSD is divided into two goals: suppression of acute inflammatory relapse and prevention of future relapses. For the purposes of this review, we will review the data on these two treatment goals separately.
Acute treatment
NMOSD is a relapsing disease with repeated attacks leading to accumulating neurological damage and disability [6]. At the time of an acute relapse, neurological symptoms and signs localize to the acute NMOSD lesion where dysfunction occurs as a result of direct CNS damage as well as edema and secondary inflammation. The goals of acute treatment are to suppress the acute inflammatory attack, minimize CNS damage, and improve long-term neurological function.
Building on decades of experience using corticosteroids to treat inflammatory attacks in multiple sclerosis and other inflammatory conditions, high-dose intravenous methylprednisolone was widely adopted as a first-line agent to broadly suppress inflammation in acute NMOSD relapses. Data supporting the use of high-dose corticosteroids in MS have recently been challenged by the observation that they do not provide meaningful long-term improvement in neurological function because spontaneous healing and remyelination in MS may be equally effective [7]. This particular concern does not apply to NMOSD where studies have shown that permanent damage from relapses leads to cumulative disability. Therefore, the consensus among experts in NMOSD is that every relapse needs to be treated and high-dose corticosteroids are good starting agents because they are widely available, are simple to administer, and may provide some benefits in suppressing the acute inflammatory response [8].
The typical starting dose for treatment of NMOSD is 1000 mg of methylprednisolone intravenously for 5 days, commonly followed by an oral steroid taper for 2–8 weeks depending on the severity of the attack [8]. Equivalent doses of other corticosteroids are likely equally effective as are other routes of administration given that bioavailability of intravenous versus oral corticosteroids are approximately the same [9]. The initial goal for corticosteroid use in acute NMOSD relapses is to reduce the edema and secondary inflammation in the lesion. This may have the immediate effect of mild to modest improvement in neurological function. For long lesions or severely inflamed attacks, additional steroid doses may be indicated.
If there is minimal or no improvement with high-dose corticosteroids based on the judgment of the treating physician, the use of plasma exchange (PLEX) has been shown to be effective in NMOSD [10]. PLEX involves the use of a centrifuge to separate the cells from a patient’s plasma, whereupon the cells are returned to the patient and the plasma is replaced with saline ± normal serum albumin. PLEX serves to remove plasma components involved in the inflammatory cascade and has the effect of suppressing active CNS inflammatory attack. The first study to demonstrate the benefit of PLEX against a sham control in a small number of subjects with NMOSD in 1999 suggested the procedure was safe and effective [11]. Additional studies since then have suggested that PLEX following a course of high-dose steroid is more effective than corticosteroids alone in achieving pre-attack neurological function [12]. Although the calculated risks of PLEX amount to nearly 36 % chance of line infections, blood clots, and other complications, the benefits of PLEX in acute NMO relapses far outweigh the risks [13].
In both cases of corticosteroids or corticosteroids plus PLEX, the expectation is that after an initial period of improvement due to resolution of secondary inflammation and edema, there is a period of 6–24 months during which the healing process can lead to further improvement in neurological function. After the healing process, there can be additional improvements in function due to recruitment of other neurological circuits optimized by physical and occupational therapy [14].
Beyond high-dose corticosteroids and PLEX, researchers have experimented with other approaches to the treatment of acute NMOSD relapses. Intravenous immunoglobulin (IVIg) has long been used for treatment of a variety of neuroimmunological conditions including Guillain-Barre syndrome and myasthenia gravis [15], but its role in inflammatory disease of the CNS is less clear. In one series of ten subjects with NMOSD unresponsive to corticosteroids ± PLEX, IVIg was helpful in five [16]; this series prompted a larger, multi-center randomized controlled study in NMOSD and acute TM [17]. Small case series also support the use of cyclophosphamide in acute steroid/PLEX-unresponsive NMOSD attacks, especially in the context of systemic lupus erythematosus (SLE) and other systemic autoimmune diseases [18, 19].
More specific acute therapies in NMOSD have been trialed recently. Damage in NMOSD relapses is mediated in part by the classical complement system beginning with antibody fixation and ending with the membrane attack process [20, 21]. An inhibitor of the classical complement cascade, C1-esterase inhibitor (Cinryze®) was recently demonstrated to improve outcomes of nine of ten NMOSD subjects back to their baseline neurological function after an acute relapse of either optic neuritis or transverse myelitis in NMOSD [22]. Another complement inhibitor, eculizumab (Soliris®) is also in trials for benefit in prevention of relapses in NMO (see below).
Preventive treatment
The first preventive therapy study in NMOSD was published in 1998 using azathioprine in seven subjects. Since then, six additional studies of azathioprine have been published worldwide in series as large as 103 subjects. Retrospective and prospective investigations with two other immunosuppressive medications, mycophenolate mofetil and rituximab, have dominated the NMOSD literature since 2006 nearly unanimously declaring a benefit in NMOSD in a majority of subjects [8]. Methotrexate, mitoxantrone, and prednisone have also been examined and used in a fair proportion of NMOSD patients worldwide. These studies are listed in Table 1 and described in more detail below.
Azathioprine
Azathioprine is a purine analog that interferes with DNA synthesis of rapidly proliferating cells, especially B and T lymphocytes. It was first used in heart and kidney transplants in the early 1960s and quickly adopted for treatment of a range of autoimmune diseases from hemolytic anemia to SLE and rheumatoid arthritis. It has been widely considered a first-line immunosuppressant medication for autoimmune diseases in combination with low-dose oral corticosteroids. The first series of seven such subjects with NMOSD was published in 1998 by Dr. Mandler and colleagues who demonstrated that this combination of 2 mg/kg/day of azathioprine plus oral prednisone at 1 mg/kg/day for the first 2 months tapered works to improve neurological function after relapses and to prevent future relapses, at least over 18 months [23]. In this study, patients were enrolled shortly after a relapse so their baseline disability scores were high: the mean Expanded Disability Status Scale score (EDSS) was 8.2, which then improved to a mean of 4.0 at the conclusion of the trial.
This initial success with azathioprine plus prednisone prompted widespread use in NMOSD that continues to this day. Larger series have since supported the initial observations in both children and adults with doses of azathioprine at the higher range of 3 mg/kg/day plus concurrent prednisone showing more benefit than azathioprine monotherapy. In three large NMOSD cohorts of 99 (Mayo), 103 (UK), and 77 (China) subjects treated with azathioprine for at least 1 year, treatment with azathioprine was successful in preventing relapses in 37–57 % of subjects [24–26]. The annualized relapse rate (ARR) in the Mayo cohort dropped from 2.20 to 0.52 in those taking more than 2 mg/kg/day and dropped from 2.09 to 0.82 in those taking less than 2 mg/kg/day, suggesting a dose-response effect [24]. In all three large azathioprine studies, the effect of prednisone was less clear because concurrent prednisone was usually weaned down in subjects who were doing well while purposely maintained or increased among those who relapsed despite azathioprine.
Side effects occurred in up to 60 % of patients taking azathioprine in these studies. The most common side effects were gastrointestinal and hematological, as with other diseases. In the Mayo cohort of 99 NMOSD subjects, three of them (3 %) developed treatment-related lymphoma, a known risk of azathioprine use [27].
The combined experience with azathioprine in NMOSD suggests the medication has an approximately 50/50 chance of preventing additional relapses. Concurrent prednisone use helps to keep patients in remission while azathioprine takes effect, which can take up to 12 months. There are no reliable biomarkers of azathioprine effect used in common clinical practice but consensus opinion recommends increasing the dose of azathioprine closer to 3 mg/kg/day if initial treatment fails. And if the higher dose fails to keep the disease in remission, switching to another medication class, such as rituximab, is suggested [8].
Mycophenolate
Mycophenolate mofetil, like azathioprine, is a purine analog anti-metabolite which interferes with lymphocyte proliferation. But unlike azathioprine, it was developed to be a specific immunosuppressive agent with limited side effects by targeting guanosine more than adenosine. Also, mycophenolate avoids production of thioguanosine which is incorporated into DNA and leads to treatment-related lymphomas with azathioprine. In head-to-head studies in autoimmune disease studies, mycophenolate is safer and slightly more effective than azathioprine [28, 29].
Many autoimmune diseases in which azathioprine have shown a benefit have been tested for improved safety and efficacy with mycophenolate, and NMOSD is no exception. The first series of 24 NMOSD subjects controlled on mycophenolate was published in 2009 and validated by additional groups in the US and Korea cohort sizes between 28 and 59 subjects. Compared to azathioprine, these three studies suggest mycophenolate is more effective at achieving remission in 60–75 % of subjects with fewer side effects and adverse events, most of which were not serious [30–32].
The recommended starting dose for mycophenolate is 500 mg twice daily with up-titration every 6 weeks until the absolute lymphocyte count reaches the stable target of 1000–1500 cells/μl of blood without causing a rise in liver enzymes. Concurrent prednisone of 20–30 mg daily is typically used until the target lymphocyte count is reached, and then the prednisone is weaned off. In a few patients, a small dose of prednisone between 2.5 and 10 mg daily is continued if clinically indicated.
Rituximab
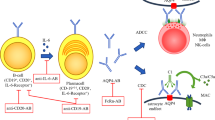
B cell depletion with anti-CD20 monoclonals as a treatment for autoimmune disease was first demonstrated in rheumatoid arthritis using rituximab in 2004 [33], and since then has been used to treat a wide number of autoimmune conditions including myasthenia gravis, lupus, and multiple sclerosis that share immunopathogenic mechanism with NMOSD [34]. In 2005, the first open-label series of rituximab in NMOSD showed promise that has since been supported by 14 additional prospective and retrospective studies around the world with a total patient cohort of well over 300 subjects (Table 1). Although none of these studies were placebo controlled, they all show a sustained and powerful benefit in the treatment of NMOSD. In studies with persistent B cell depletion, remission rates up to 83 % were achieved [32]. In retrospective head-to-head studies comparing azathioprine, mycophenolate, and rituximab in NMO, rituximab was the most effective option, followed by mycophenolate and then azathioprine [32].
The proposed mechanisms of action of rituximab in NMOSD include removal of B cells as antigen-presenting cells and reduction in the CD20+ early plasmablast population generating anti-aquaporin-4 antibodies (ref). Infusion of either 375 mg/m2 or 1000 mg of rituximab leads to rapid depletion of circulating CD20+ mature B cells within a few hours. Additional doses of rituximab (375 mg/m2 ×4 total weekly doses or another 1000 mg 2 weeks after the initial 1000 mg dose) provide long-lasting B cell depletion for 6–8 months. The goal of therapy is to keep B cells depleted at all times, which can be achieved either by scheduled infusions every 6 months or based on CD19/20 B cell counts tested monthly.
B cells in peripheral organs and the CNS cannot be reached by intravenous rituximab and may explain why the risk of opportunistic infections in patients with autoimmune disease on rituximab is not statistically increased compared to placebo [35]. The most common and serious adverse reaction with rituximab is allergy due to lysis of circulating lymphocytes and release of cytokines and is greater in patients with replete B cell counts [36]. True anaphylactic reactions mediated by IgE have been reported [37], but most allergic infusion reactions to rituximab can be prevented by starting with a slow infusion rate and using preventive medications such as diphenhydramine (25–50 mg), methylprednisolone (100 mg), and acetaminophen (650 mg) up to 45 min prior to infusion.
Other preventive immunosuppressants
Corticosteroids have been used extensively in the treatment of autoimmune disease for decades. They are an effective add-on agent to prevent relapses in NMOSD when used with anti-metabolite such as azathioprine or mycophenolate and may be useful as monotherapy as well [38]. However, the use of prednisone in NMOSD is limited by serious complications including hyperglycemia, hypertension, insomnia, mood swings, truncal weight gait, osteoporosis, and glaucoma.
Methotrexate is another anti-metabolite (like azathioprine and mycophenolate) that has been studied in NMOSD. Weekly methotrexate at a dose of 50 mg used as monotherapy or in combination with corticosteroids/cyclophosphamide led to remission in about two thirds of subjects [39, 40]. Methotrexate was generally well tolerated in these cohorts. Mitoxantrone, an anthracenedione antineoplastic medication that intercalates DNA and inhibits topoisomerase II, has been studied in three patient populations (Table 1) and can lead to NMOSD disease remission in up to 70 % of subjects when dosed appropriately [41, 42]. Serious adverse events including heart failure and leukemia observed in these small cohorts have curbed widespread enthusiasm for mitoxantrone in NMOSD.
Ongoing trials in NMOSD
Three promising trials have launched in the NMOSD preventive therapy space, two of which have been tested in pilot studies in NMOSD, eculizumab and tocilizumab.
Eculizumab is a C5 complement inhibitor that blocks the terminal activation of complement and the membrane attack complex. The rationale behind testing a complement inhibitor in NMOSD is based on the pathology of NMO lesions showing extensive complement deposition [20, 43]. In an open-label study of 14 subjects, of which 8 subjects proved unresponsive to other immunosuppressants, eculizumab appeared to suppress nearly all disease activities [44]. Only two relapses occurred to all subjects over the year of treatment, and both of them were mild in severity. One subject became infected with meningococcal bacteremia, a known risk with eculizumab, but was treated and continued in the study. The success of this trial prompted a worldwide, placebo-controlled registrational trial of eculizumab in seropositive NMOSD, which is expected to complete enrollment in 2016.
Tocilizumab is a blocker of the interleukin-6 (IL-6) receptor and has been approved for treatment of rheumatoid arthritis and juvenile idiopathic arthritis. The rationale behind testing an IL-6 receptor antagonist in NMOSD is based on the reportedly high levels of the pro-inflammatory IL-6 measured in the blood and spinal fluid of relapsing, actively inflamed NMOSD patients [45–47]. In a pilot study of seven Japanese NMOSD patients, tocilizumab added to background immunosuppressants such as azathioprione or prednisone provided additional reduction in relapse rates [48]. Plus, tocilizumab reduced pain scores in these subjects due to the role of IL-6 in spinal cord pain pathways. The dual pain/immunosuppressive benefit was confirmed in a German study of eight NMOSD patients on tocilizumab monotherapy [49]. These studies have prompted a worldwide, placebo-controlled registrational trial of SA237, an anti-IL-6 receptor blocking monoclonal antibody applied with recycling antibody technology that extends the dosing frequency to once monthly by intramuscular injection. This study is also expected to complete enrollment in 2016.
A third worldwide, placebo-controlled, registrational trial has launched in NMOSD testing the ability of MEDI-551, a CD19 monoclonal antibody, to prevent relapses [50]. Based on the success of rituximab, a CD19+ B cell-depleting medication would be expected to perform as well or better because earlier B cells and more mature plasmablasts would be depleted compared to rituximab.
Symptomatic treatment
Symptomatic treatment of immobility, neuropathic pain, spasticity, urinary retention/incontinence, depression, fatigue, and cognitive dysfunction in NMOSD patients have not been adequately studied, especially compared to the numerous studies of immunotherapies to prevent relapses. Even after successful induction of remission, NMOSD patients contend with serious complications of the spinal cord and brainstem and optic nerve damage that limit their quality of life. The most common consequence of CNS inflammation in NMOSD is pain, manifesting as a combination of neuropathic pain described as a constant burning, tingling, and electrical discomfort, and spastic pain described as an intermittent muscular tightening pain. A review of the literature in NMOSD symptomatic management yields little in the way of evidence; therefore, the recommendations provided in this section are based on experience and must be tailored to individual circumstances.
Recovery of neurological function
Transverse myelitis attacks in NMOSD tend to be long and severe, causing extensive damage to astrocytes, myelinated tracts, and underlying neural tissue in the gray matter of the spinal cord. With involvement of the corticospinal tracts, anterior corticospinal tracts, or the cerebellar-spinal tracts, patients can present with difficulty walking either due to loss of motor strength in the legs and trunk or due to incoordination. Involvement of sensory tracts, especially the dorsal columns carrying proprioceptive information, can cause walking problems due to sensory ataxia. In the two acute NMOSD studies published to date, the majority of subjects with attacks of transverse myelitis had difficulty walking as part of their presentation [22, 51].
Following acute treatment of NMOSD transverse myelitis with high-dose corticosteroids ± plasma exchange, the objective is to return to baseline neurological function. The initial recovery of function observed in the first few days is due to reduction of inflammation in the spinal cord that directly disrupted signal conduction in the spinal cord. Recovery of the next 6 months (or longer in younger patients) is attributable to biological healing and partial remyelination within the spinal cord. Development of new circuits within the spinal cord and between and the spinal cord and the brain can compensate for pathways that are permanently damaged. These new circuits can be strengthened with continued exercise and guided physical therapy. The purposes of a physical therapy trainer in the recovery process after transverse myelitis are as follows: (1) prevent harm to the patient due to incorrect posture or compensation as a result of the injury, (2) teach and encourage the patient the correct way to practice walking or mobility exercises, and (3) adjust the training program as the patient heals and improves over time.
There is one trial currently underway focused on improving mobility and gait in patients with transverse myelitis including myelitis patients with NMOSD. This trial is testing dalfampridine (Ampyra®), a potassium channel blocker currently approved for multiple sclerosis, at the same dose of 10 mg twice daily in monophasic transverse myelitis patients. While NMOSD subjects are not excluded, subjects can only have one spinal cord lesion because part of the study involves the use of transcranial magnetic stimulation to measure the speed of electrical conduction in the spinal cord across the lesion with and without dalfampridine. The trial is completely enrolled and is expected to report results in spring 2016.
Two remyelination trials may impact on NMOSD mobility in the future. Anti-lingo is an antibody developed to stop the inhibition of remyelination following an inflammatory attack of the CNS. It has been tested in optic neuritis and showed some benefits in objective measures such as visual evoked potential latency. Recombinant human IgM22 (RhIgM22) is an IgM product that binds to myelin and stimulates oligodendrocyte proliferation. It proved safe in a recent phase I study and is now undergoing additional phase I testing. Both of these remyelination approaches may be application following transverse myelitis attacks in NMOSD as well.
Stem cell hold the promise of true regeneration of CNS tissue previously thought to be permanently damaged. At the present, there is one trial of mesenchymal stem cells in NMOSD, two trials of neural stem cells in traumatic spinal cord injury, and several trials of mesenchymal stem cells in spinal cord injury that are all in progress [52]. These important studies will shed light on the potential use of stem cells in CNS regeneration that may hopefully be applied in NMOSD in the future.
Pain
Up to 86 % of NMOSD patients report pain as a consequence of their disease and was the most common initial complaint in clinic [53, 54]. Most patients do not distinguish between neuropathic pain and spastic pain but on careful questioning, neuropathic pain tends to be more common and difficult to treat with conventional therapies.
There are four general classes of pain medications commonly used in NMOSD patients: (1) anti-epileptic medications (e.g., gabapentin, carbamazepine), (2) anti-spasmodics (e.g., baclofen, tizanidine), (3) anti-depressants (e.g., amitriptyline, duloxetine), and (4) analgesics (e.g., tramadol, opiates). First-line agents that have been most effective in treating both neuropathic and spastic pain are anti-epileptic medications. Gabapentin dosing is usually started at 300 mg three times daily and titrated up as needed weekly up to a maximum dose of 2400 mg per day. In the absence of kidney disease, gabapentin monitoring is unnecessary and can be dosed at the minimum effective dose according to patient report of efficacy versus side effects with sedation as the dose-limiting factor. Carbamazepine at 100–200 mg twice daily is also particularly effective at treating both neuropathic and spastic pain in NMO [55] and can be added to gabapentin. When a combination of two anti-epileptic medications is insufficient to manage neuropathic pain, an anti-depressant is usually added to the regimen. Amitriptyline is a particularly effective agent in this regard starting at a dose of 25 mg nightly and titrating up biweekly to 150 mg nightly as tolerated, also limited by sedation. For those who cannot tolerate the sedation from amitriptyline, duloxetine at a dose of 60 mg twice daily is often an effective substitute. For NMOSD patients with persistent tonic spasms, rather than intermittent spasms, an anti-spasmodic can reduce the spasticity. Baclofen at doses of 5–20 mg three times daily is good at reducing spasticity, as are other anti-spasmodics, but they can also make patients feel weaker especially while walking. In a few patients where all efforts have failed to reduce pain to a tolerable level, analgesics can be used sparingly and temporarily while the long-term goal of finding other medications or non-medical interventions continues on a trial-and-error basis.
Urinary retention/incontinence
Micturition is initiated by the brain, which sends tracts to the bladder and pelvic floor through the spinal cord. Descending sympathetic and parasympathetic tracts run down the spinal cord in between the corticospinal tracts and the gray matter bilaterally. Sympathetic nerves destined for the urethra and pelvic floor synapse in the intermediolateral nucleus of the lumbar cord function to close the sphincters to prevent urine leaks. The parasympathetic serves destined for the bladder wall synapse in the lateral horn of the gray matter in the lumbar cord and function to contract the bladder during voiding. An acute lesion of the cervical spinal cord would cause loss of function of both autonomic systems which causes both bladder flaccidity and incontinence. Lower thoracic and lumbar lesions on the spinal cord cause acute urinary retention because of unbalanced sympathetic stimulation to the urethral outlet that exited the spinal cord rostral to the lesion. In these situations, patients are usually unable to sense that their bladder is full which can delay appropriate treatment.
There are two options for patients with urinary retention. The most widely recommended option by urologists is clean, intermittent self-catheterization at least three times daily or more often depending on bladder volumes throughout the day [56]. In longer term, some patients opt for an indwelling suprapubic catheter but all catheters tend to become sources of infection over time [57]. The second option is bethanechol, a parasympathetic agonist that increases bladder muscle tone and contraction at a dose of 25 mg three or four times daily [58]. Bethanechol is best for those who have some retained ability to relax the urethral sphincter. A trial of bethanechol can be performed within 1–2 h to determine whether it works for an individual patient. Side effects due to parasympathetic overstimulation are generally mild and include upset stomach, dizziness, and sweating/flushing.
There are several options for patients with urinary incontinence [59]. One approach involves scheduling frequent bathroom trips to keep the bladder from overfilling and thereby avoid leaks. In combination with pelvic floor muscle exercises, this method can provide long-term control without medication or intervention. The problem with this approach is that patients cannot always plan a bathroom break especially while at work or traveling. A second method to treatment urinary incontinence uses anti-cholinergic medications to block the parasympathetic innervation of the bladder wall therapy preventing spasms and allowing the bladder to expand without increasing pressure on the urethral sphincter. There are six FDA-approved medications that can be tried for patients that work in this way. A third method involves multiple injections of onabolutinumtoxinA (Botox) into the bladder wall to achieve a 3–6-month period of bladder wall relaxation. Botox is somewhat more effective in blocking bladder spasms associated with urinary incontinence than medication (ref) and avoids medication side effects, but it does require repeat intervention 2–3 times per year when the Botox wears off.
Fatigue/depression/cognition
Psychological issues related to NMO may be due to direct inflammatory influence on higher neuronal circuitry or may be related to complications from optic neuritis and transverse myelitis. Among the most disabling of these psychological issues are fatigue, depression, and cognitive problems [60].
The work-up for fatigue includes a thorough assessment of sleep habits, medication side effects, and depression. NMOSD patients can have primary sleep disorders, as well as sleep disorders secondary to repeat awakenings due to nocturia, chronic pain, and obstructive sleep apnea. Sleep disorders not only lead to fatigue, but they also impact a patient’s recovery potential and overall neurological well-being [61, 62]. Many medications that patients use for pain control and muscle spasticity cause fatigue. Sometimes, a transient side effect of fatigue is worth the benefit provided by the medication, but if not, alternative medications can be considered or the dose of the sedating medication can be reduced. Stimulants such as modafinil have been used empirically in autoimmune neurological conditions and may be helpful in certain circumstances [63]. Depression in NMOSD has been recognized as both primary and secondary etiologies [61, 62]. In either circumstance, a combination of behavioral therapy, psychotherapy, and medication may be helpful. Cognitive problems in NMOSD may be due to depression, fatigue, and medication but may occasionally be due to primary involvement of the subcortical or cortical brain matter. Research in cognition of NMO patients is ongoing, and specific therapies have not yet been developed [64, 65].
Summary
Research in NMOSD has come very far since the first trial of azathioprine 17 years ago. For a rare disease, NMOSD has the focus of over 30 treatment studies and many more epidemiology and case series highlighting important aspects of the disease. The popularity of NMOSD is due in large part to the discovery of the highly specific blood biomarker that effectively identifies NMOSD patients and distinguishes them from other diseases. The biomarker and its immune target have also been subjects of intense basic science investigations that have directly led to new clinical trials in acute, preventive, and symptomatic treatment of NMOSD. This review provides a snapshot of the current standards of care, as well as empiric therapy, which are likely to be updated frequently over the coming years.
References and Recommended Reading
Oh J, Levy M. Neuromyelitis optica: an antibody-mediated disorder of the central nervous system. Neurol Res Int. 2012;2012:460825. doi:10.1155/2012/460825.
Waters PJ, McKeon A, Leite MI, Rajasekharan S, Lennon VA, Villalobos A, et al. Serologic diagnosis of NMO: a multicenter comparison of aquaporin-4-IgG assays. Neurology. 2012;78(9):665–71. doi:10.1212/WNL.0b013e318248dec1. discussion 9.
Mealy MA, Wingerchuk DM, Greenberg BM, Levy M. Epidemiology of neuromyelitis optica in the United States: a multicenter analysis. Arch Neurol. 2012;69(9):1176–80. doi:10.1001/archneurol.2012.314.
Pereira WL, Reiche EM, Kallaur AP, Kaimen-Maciel DR. Epidemiological, clinical, and immunological characteristics of neuromyelitis optica: a review. J Neurol Sci. 2015;355(1–2):7–17. doi:10.1016/j.jns.2015.05.034.
Lennon VA, Kryzer TJ, Pittock SJ, Verkman AS, Hinson SR. IgG marker of optic-spinal multiple sclerosis binds to the aquaporin-4 water channel. J Exp Med. 2005;202(4):473–7. doi:10.1084/jem.20050304.
Wingerchuk DM, Pittock SJ, Lucchinetti CF, Lennon VA, Weinshenker BG. A secondary progressive clinical course is uncommon in neuromyelitis optica. Neurology. 2007;68(8):603–5. doi:10.1212/01.wnl.0000254502.87233.9a.
Burton JM, O’Connor PW, Hohol M, Beyene J. Oral versus intravenous steroids for treatment of relapses in multiple sclerosis. Cochrane Database Syst Rev. 2012;12:CD006921. doi:10.1002/14651858.CD006921.pub3.
Kimbrough DJ, Fujihara K, Jacob A, Lana-Peixoto MA, Leite MI, Levy M, et al. Treatment of neuromyelitis optica: review and recommendations. Mult Scler Relat Disord. 2012;1(4):180–7. doi:10.1016/j.msard.2012.06.002.
Le Page E, Veillard D, Laplaud DA, Hamonic S, Wardi R, Lebrun C, et al. Oral versus intravenous high-dose methylprednisolone for treatment of relapses in patients with multiple sclerosis (COPOUSEP): a randomised, controlled, double-blind, non-inferiority trial. Lancet. 2015;386(9997):974–81. doi:10.1016/S0140-6736(15)61137-0.
Bonnan M, Valentino R, Olindo S, Mehdaoui H, Smadja D, Cabre P. Plasma exchange in severe spinal attacks associated with neuromyelitis optica spectrum disorder. Mult Scler. 2009;15(4):487–92. doi:10.1177/1352458508100837.
Weinshenker BG, O’Brien PC, Petterson TM, Noseworthy JH, Lucchinetti CF, Dodick DW, et al. A randomized trial of plasma exchange in acute central nervous system inflammatory demyelinating disease. Ann Neurol. 1999;46(6):878–86.
Abboud H, Petrak A, Mealy M, Sasidharan S, Siddique L, Levy M. Treatment of acute relapses in neuromyelitis optica: steroids alone versus steroids plus plasma exchange. Mult Scler. 2015. doi:10.1177/1352458515581438.
Shemin D, Briggs D, Greenan M. Complications of therapeutic plasma exchange: a prospective study of 1,727 procedures. J Clin Apher. 2007;22(5):270–6. doi:10.1002/jca.20143.
Calis M, Kirnap M, Calis H, Mistik S, Demir H. Rehabilitation results of patients with acute transverse myelitis. Bratisl Lek Listy. 2011;112(3):154–6.
Lunemann JD, Quast I, Dalakas MC. Efficacy of intravenous immunoglobulin in neurological diseases. Neurotherapeutics. 2015. doi:10.1007/s13311-015-0391-5.
Elsone L, Panicker J, Mutch K, Boggild M, Appleton R, Jacob A. Role of intravenous immunoglobulin in the treatment of acute relapses of neuromyelitis optica: experience in 10 patients. Mult Scler. 2014;20(4):501–4. doi:10.1177/1352458513495938.
Absoud M, Gadian J, Hellier J, Brex PA, Ciccarelli O, Giovannoni G, et al. Protocol for a multicentre randomiSed controlled TRial of IntraVEnous immunoglobulin versus standard therapy for the treatment of transverse myelitis in adults and children (STRIVE). BMJ Open. 2015;5(5):e008312. doi:10.1136/bmjopen-2015-008312.
Saison J, Costedoat-Chalumeau N, Maucort-Boulch D, Iwaz J, Marignier R, Cacoub P, et al. Systemic lupus erythematosus-associated acute transverse myelitis: manifestations, treatments, outcomes, and prognostic factors in 20 patients. Lupus. 2015;24(1):74–81. doi:10.1177/0961203314547795.
Yaguchi H, Sakushima K, Takahashi I, Nishimura H, Yashima-Yamada M, Nakamura M, et al. Efficacy of intravenous cyclophosphamide therapy for neuromyelitis optica spectrum disorder. Intern Med. 2013;52(9):969–72.
Jones MV, Fox-Talbot K, Levy M. Evidence for classic complement activity in neuromyelitis optica. Clin Neuropathol. 2014;33(3):251–2. doi:10.5414/NP300697.
Nytrova P, Potlukova E, Kemlink D, Woodhall M, Horakova D, Waters P, et al. Complement activation in patients with neuromyelitis optica. J Neuroimmunol. 2014;274(1–2):185–91. doi:10.1016/j.jneuroim.2014.07.001.
Levy M, Mealy MA. Purified human C1-esterase inhibitor is safe in acute relapses of neuromyelitis optica. Neurol Neuroimmunol Neuroinflamm. 2014;1(1):e5. doi:10.1212/NXI.0000000000000005.
Mandler RN, Ahmed W, Dencoff JE. Devic’s neuromyelitis optica: a prospective study of seven patients treated with prednisone and azathioprine. Neurology. 1998;51(4):1219–20.
Costanzi C, Matiello M, Lucchinetti CF, Weinshenker BG, Pittock SJ, Mandrekar J, et al. Azathioprine: tolerability, efficacy, and predictors of benefit in neuromyelitis optica. Neurology. 2011;77(7):659–66. doi:10.1212/WNL.0b013e31822a2780.
Elsone L, Kitley J, Luppe S, Lythgoe D, Mutch K, Jacob S, et al. Long-term efficacy, tolerability and retention rate of azathioprine in 103 aquaporin-4 antibody-positive neuromyelitis optica spectrum disorder patients: a multicentre retrospective observational study from the UK. Mult Scler. 2014;20(11):1533–40. doi:10.1177/1352458514525870.
Qiu W, Kermode AG, Li R, Dai Y, Wang Y, Wang J, et al. Azathioprine plus corticosteroid treatment in Chinese patients with neuromyelitis optica. J Clin Neurosci. 2015;22(7):1178–82. doi:10.1016/j.jocn.2015.01.028.
Larvol L, Soule JC, Le Tourneau A. Reversible lymphoma in the setting of azathioprine therapy for Crohn’s disease. N Engl J Med. 1994;331(13):883–4. doi:10.1056/NEJM199409293311321.
Eisen HJ, Kobashigawa J, Keogh A, Bourge R, Renlund D, Mentzer R, et al. Three-year results of a randomized, double-blind, controlled trial of mycophenolate mofetil versus azathioprine in cardiac transplant recipients. J Heart Lung Transplant. 2005;24(5):517–25. doi:10.1016/j.healun.2005.02.002.
Houssiau FA, D’Cruz D, Sangle S, Remy P, Vasconcelos C, Petrovic R, et al. Azathioprine versus mycophenolate mofetil for long-term immunosuppression in lupus nephritis: results from the MAINTAIN Nephritis Trial. Ann Rheum Dis. 2010;69(12):2083–9. doi:10.1136/ard.2010.131995.
Huh SY, Kim SH, Hyun JW, Joung AR, Park MS, Kim BJ, et al. Mycophenolate mofetil in the treatment of neuromyelitis optica spectrum disorder. JAMA Neurol. 2014;71(11):1372–8. doi:10.1001/jamaneurol.2014.2057.
Jacob A, Matiello M, Weinshenker BG, Wingerchuk DM, Lucchinetti C, Shuster E, et al. Treatment of neuromyelitis optica with mycophenolate mofetil: retrospective analysis of 24 patients. Arch Neurol. 2009;66(9):1128–33. doi:10.1001/archneurol.2009.175.
Mealy MA, Wingerchuk DM, Palace J, Greenberg BM, Levy M. Comparison of relapse and treatment failure rates among patients with neuromyelitis optica: multicenter study of treatment efficacy. JAMA Neurol. 2014;71(3):324–30. doi:10.1001/jamaneurol.2013.5699.
Edwards JC, Szczepanski L, Szechinski J, Filipowicz-Sosnowska A, Emery P, Close DR, et al. Efficacy of B-cell-targeted therapy with rituximab in patients with rheumatoid arthritis. N Engl J Med. 2004;350(25):2572–81. doi:10.1056/NEJMoa032534.
Tony HP, Burmester G, Schulze-Koops H, Grunke M, Henes J, Kotter I, et al. Safety and clinical outcomes of rituximab therapy in patients with different autoimmune diseases: experience from a national registry (GRAID). Arthritis Res Ther. 2011;13(3):R75. doi:10.1186/ar3337.
Kelesidis T, Daikos G, Boumpas D, Tsiodras S. Does rituximab increase the incidence of infectious complications? A narrative review. Int J Infect Dis. 2011;15(1):e2–16. doi:10.1016/j.ijid.2010.03.025.
Dillman RO, Hendrix CS. Unique aspects of supportive care using monoclonal antibodies in cancer treatment. Support Cancer Ther. 2003;1(1):38–48. doi:10.3816/SCT.2003.n.003.
Vultaggio A, Matucci A, Nencini F, Pratesi S, Petroni G, Cammelli D, et al. Drug-specific Th2 cells and IgE antibodies in a patient with anaphylaxis to rituximab. Int Arch Allergy Immunol. 2012;159(3):321–6. doi:10.1159/000336839.
Watanabe S, Misu T, Miyazawa I, Nakashima I, Shiga Y, Fujihara K, et al. Low-dose corticosteroids reduce relapses in neuromyelitis optica: a retrospective analysis. Mult Scler. 2007;13(8):968–74. doi:10.1177/1352458507077189.
Kitley J, Elsone L, George J, Waters P, Woodhall M, Vincent A, et al. Methotrexate is an alternative to azathioprine in neuromyelitis optica spectrum disorders with aquaporin-4 antibodies. J Neurol Neurosurg Psychiatry. 2013;84(8):918–21. doi:10.1136/jnnp-2012-304774.
Ramanathan RS, Malhotra K, Scott T. Treatment of neuromyelitis optica/neuromyelitis optica spectrum disorders with methotrexate. BMC Neurol. 2014;14:51. doi:10.1186/1471-2377-14-51.
Cabre P, Olindo S, Marignier R, Jeannin S, Merle H, Smadja D, et al. Efficacy of mitoxantrone in neuromyelitis optica spectrum: clinical and neuroradiological study. J Neurol Neurosurg Psychiatry. 2013;84(5):511–6. doi:10.1136/jnnp-2012-303121.
Weinstock-Guttman B, Ramanathan M, Lincoff N, Napoli SQ, Sharma J, Feichter J, et al. Study of mitoxantrone for the treatment of recurrent neuromyelitis optica (Devic disease). Arch Neurol. 2006;63(7):957–63. doi:10.1001/archneur.63.7.957.
Roemer SF, Parisi JE, Lennon VA, Benarroch EE, Lassmann H, Bruck W, et al. Pattern-specific loss of aquaporin-4 immunoreactivity distinguishes neuromyelitis optica from multiple sclerosis. Brain. 2007;130(Pt 5):1194–205. doi:10.1093/brain/awl371.
Pittock SJ, Lennon VA, McKeon A, Mandrekar J, Weinshenker BG, Lucchinetti CF, et al. Eculizumab in AQP4-IgG-positive relapsing neuromyelitis optica spectrum disorders: an open-label pilot study. Lancet Neurol. 2013;12(6):554–62. doi:10.1016/S1474-4422(13)70076-0.
Barros PO, Cassano T, Hygino J, Ferreira TB, Centuriao N, Kasahara TM, et al. Prediction of disease severity in neuromyelitis optica by the levels of IL-6 produced during remission phase. Clin Exp Immunol. 2015. doi:10.1111/cei.12733.
Chihara N, Aranami T, Sato W, Miyazaki Y, Miyake S, Okamoto T, et al. Interleukin 6 signaling promotes anti-aquaporin 4 autoantibody production from plasmablasts in neuromyelitis optica. Proc Natl Acad Sci U S A. 2011;108(9):3701–6. doi:10.1073/pnas.1017385108.
Matsushita T, Tateishi T, Isobe N, Yonekawa T, Yamasaki R, Matsuse D, et al. Characteristic cerebrospinal fluid cytokine/chemokine profiles in neuromyelitis optica, relapsing remitting or primary progressive multiple sclerosis. PLoS ONE. 2013;8(4):e61835. doi:10.1371/journal.pone.0061835.
Araki M, Matsuoka T, Miyamoto K, Kusunoki S, Okamoto T, Murata M, et al. Efficacy of the anti-IL-6 receptor antibody tocilizumab in neuromyelitis optica: a pilot study. Neurology. 2014;82(15):1302–6. doi:10.1212/WNL.0000000000000317.
Ringelstein M, Ayzenberg I, Harmel J, Lauenstein AS, Lensch E, Stogbauer F, et al. Long-term therapy with interleukin 6 receptor blockade in highly active neuromyelitis optica spectrum disorder. JAMA Neurol. 2015;72(7):756–63. doi:10.1001/jamaneurol.2015.0533.
Chen D, Blazek M, Ireland S, Ortega S, Kong X, Meeuwissen A, et al. Single dose of glycoengineered anti-CD19 antibody (MEDI551) disrupts experimental autoimmune encephalomyelitis by inhibiting pathogenic adaptive immune responses in the bone marrow and spinal cord while preserving peripheral regulatory mechanisms. J Immunol. 2014;193(10):4823–32. doi:10.4049/jimmunol.1401478.
Mealy MA, Shin K, John G, Levy M. Bevacizumab is safe in acute relapses of neuromyelitis optica. Clin Exp Neuroimmunol. 2015. doi:10.1111/cen3.12239.
Levy M, Boulis N, Rao M, Svendsen CN. Regenerative cellular therapies for neurologic diseases. Brain Res. 2015. doi:10.1016/j.brainres.2015.06.053.
Qian P, Lancia S, Alvarez E, Klawiter EC, Cross AH, Naismith RT. Association of neuromyelitis optica with severe and intractable pain. Arch Neurol. 2012;69(11):1482–7. doi:10.1001/archneurol.2012.768.
Zhao S, Mutch K, Elsone L, Nurmikko T, Jacob A. Neuropathic pain in neuromyelitis optica affects activities of daily living and quality of life. Mult Scler. 2014;20(12):1658–61. doi:10.1177/1352458514522103.
Carnero Contentti E, Leguizamon F, Hryb JP, Celso J, Pace JL, Ferrari J, et al. Neuromyelitis optica: association with paroxysmal painful tonic spasms. Neurologia. 2015. doi:10.1016/j.nrl.2014.12.001.
Panicker JN, de Seze M, Fowler CJ. Rehabilitation in practice: neurogenic lower urinary tract dysfunction and its management. Clin Rehabil. 2010;24(7):579–89. doi:10.1177/0269215509353252.
Tenke P, Koves B, Johansen TE. An update on prevention and treatment of catheter-associated urinary tract infections. Curr Opin Infect Dis. 2014;27(1):102–7. doi:10.1097/QCO.0000000000000031.
Yoshimura N, Chancellor MB. Differential diagnosis and treatment of impaired bladder emptying. Rev Urol. 2004;6 Suppl 1:S24–31.
Wood LN, Anger JT. Urinary incontinence in women. BMJ. 2014;349:g4531. doi:10.1136/bmj.g4531.
Chanson JB, Zephir H, Collongues N, Outteryck O, Blanc F, Fleury M, et al. Evaluation of health-related quality of life, fatigue and depression in neuromyelitis optica. Eur J Neurol. 2011;18(6):836–41. doi:10.1111/j.1468-1331.2010.03252.x.
Akaishi T, Nakashima I, Misu T, Fujihara K, Aoki M. Depressive state and chronic fatigue in multiple sclerosis and neuromyelitis optica. J Neuroimmunol. 2015;283:70–3. doi:10.1016/j.jneuroim.2015.05.007.
Pan J, Zhao P, Cai H, Su L, Wood K, Shi FD, et al. Hypoxemia, sleep disturbances, and depression correlated with fatigue in neuromyelitis optica spectrum disorder. CNS Neurosci Ther. 2015;21(7):599–606. doi:10.1111/cns.12411.
Sheng P, Hou L, Wang X, Wang X, Huang C, Yu M, et al. Efficacy of modafinil on fatigue and excessive daytime sleepiness associated with neurological disorders: a systematic review and meta-analysis. PLoS ONE. 2013;8(12):e81802. doi:10.1371/journal.pone.0081802.
Nechemia Y, Moreh E, Weingarden H, Bloch A, Givon U, Vaknin-Dembinsky A, et al. Effectiveness of multi-disciplinary rehabilitation for patients with neuromyelitis optica. J Spinal Cord Med. 2015. doi:10.1179/2045772315Y.0000000060.
Vanotti S, Cores EV, Eizaguirre B, Melamud L, Rey R, Villa A. Cognitive performance of neuromyelitis optica patients: comparison with multiple sclerosis. Arq Neuropsiquiatr. 2013;71(6):357–61. doi:10.1590/0004-282X20130038.
Acknowledgments
This work was funded by NINDS grant NS078555, PI Levy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Remi A. Kessler and Maureen A. Mealy each declare no potential conflicts of interest.
Michael Levy receives research support from NIH, Guthy Jackson Charitable Foundation, Viropharma, Acorda, Sanofi, NeuralStem and Genentech, and serves as a consultant for Chugai Pharmaceuticals, GlaxoSmithKline and Medimmune.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Neurologic Ophthalmology and Otology
Rights and permissions
About this article
Cite this article
Kessler, R.A., Mealy, M.A. & Levy, M. Treatment of Neuromyelitis Optica Spectrum Disorder: Acute, Preventive, and Symptomatic. Curr Treat Options Neurol 18, 2 (2016). https://doi.org/10.1007/s11940-015-0387-9
Published:
DOI: https://doi.org/10.1007/s11940-015-0387-9