Abstract
Purpose of the review
Acid suppression treatment has revolutionized the management of the acid-related disorders since the introduction of the H2-receptor antagonists (H2-RAs) and the proton pump inhibitors (PPIs). However, there has been increasing identification of needs for improvement in antisecretory therapy, especially in gastroesophageal reflux disease (GERD), the eradication of Helicobacter pylori (H. pylori), protection from aspirin (ASA) and non-steroidal inflammatory drug (NSAID) injury and the management of upper gastrointestinal (UGI) bleeding. There have also been increasing publications addressing safety concerns of antisecretory drugs.
Recent findings
The needs have been identified as shortcomings of the pharmacology of the delayed release-PPIs (DR-PPIs), which have short plasma half-lives, required to be given before a meal and show poor control of nocturnal acid secretion. New-generation PPIs have been developed, including dexlansoprazole modified release (MR), instant release omeprazole (IR-omeprazole), while metered release preparations such as Durasec™ or novel molecules such as tenatoprazole have also been developed and achieve superior control of intragastric pH especially at night. The major advance has been the development of the potassium channel acid blocking drugs, which block the K+,H+-ATPase K+ channel, are food independent, reversible, have a rapid onset of action, and maintain a prolonged and consistent elevation of intragastric pH. Vonoprazan, the first P-CAB, has so far been introduced only into a small number of Asian countries. Safety issues have been extensively addressed in numerous publications. This review sets the needs, individual new drug classes and key individual new treatments into clinical context.
Summary
Acid suppression treatment is reviewed including the pharmacology, the unmet clinical needs across the acid-related disorders, the place of new drug treatments, and where superiority exists. The safety of antisecretory drugs is broadly summarized with reference to several recent comprehensive reviews and set within the clinical context of patient management, particularly those on long-term treatment who are the greatest risk of some adverse events.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
With the need for improved antisecretory drugs, the relative efficacy of new treatments is usually compared by “potency.” However, this term is not defined and so, is not helpful for making rational and evidence-based clinical decisions. After more than two decades of delayed release proton pump inhibitors (DR-PPIs), which have been marketed and prescribed as “one drug, once a day for all acid related disorders,” we now have an increasing choice in our therapeutic armamentarium [1, 2, 3•]. The evolution of several novel formulations of existing PPIs and the introduction of the potassium-channel acid blockers (P-CABs) in several jurisdictions, now offer the opportunity to consider how we can best individualize the choice of drug and dose to both the patient and the underlying condition [1, 2, 3•].
In the treatment of acid-related disorders, the criteria for efficacy in healing duodenal and gastric ulcer as well as erosive esophagitis have been defined as the degree of acid suppression and the duration of suppression over 24 h and the length of treatment [4,5,6]. The duration of time that intragastric acidity is measured pH ≥ 3 correlates with the healing of DU and GU and pH ≥ 4 is correlated with healing of erosive esophagitis [4, 6]. These parameters are appropriate to compare antisecretory treatments when expressed as the pH holding time ratio: the ratio of the time period above a given pH threshold, e.g., pH 3, 4, or 6, to the total continuous period of monitoring. However, there is no evidence for any correlation with the control of symptoms and there are no studies relating pH to symptoms reported with the P-CAB class to date. Comparisons should also determine time after dosing to antisecretory effect, the impact on nocturnal acid secretion, duration of antisecretory effects, and if any meal effect is seen. The importance of considering the potency of antisecretory drugs is to relate the pharmacological properties to healing efficacy and symptom response. The studies referenced above all confirm a shallow dose-response relationship between the differing doses of antisecretory treatments and control of pH and healing of acid-related disorders. A recent publication has suggested that PPI’s are interchangeable based on relative potency [7•]. However, this study did not analyze potency but rather reviewed data from randomized clinical trials involving pH testing on day 5 of treatment with solid-dose formulations of PPIs. From this, the authors calculated omeprazole equivalency and employed percent time pH > 4 over 24 h to compare therapeutic effectiveness. As expected, the study concluded that at recommended doses the DR-PPI’s are functionally equivalent and confirmed that increasing a single daily dose achieves little or no increase in antisecretory effect.
Unmet needs in acid-related diseases
Careful studies in patients with poor response to antisecretory drugs have defined their shortcomings. The first generation of DR-PPIs, while superior to the H2-receptor antagonists (H2-RA), still show a significant failure rate in gastroesophageal reflux disease (GERD) [1, 8, 9]. Moreover, symptoms may still be problematic in 50% of patients 3 days after starting treatment [10].
The DR-PPIs are all pro-drugs and require activation in the secretory canaliculus of parietal cells and take 3–5 days to reach a steady antisecretory effect and hence symptom relief is slow. A key reason for failure with DR-PPIs is the short plasma residence time of 1–1.5 h and limited duration of effect. Little or no drug remains in the circulation to bind to proton pumps 5–7 h after dosing. The DR-PPIs also require ingestion 30–60 min before a meal, usually breakfast, to ensure appropriate drug levels ahead of proton pump activation. Consequently, the duration of antisecretory effect is partly dictated by the time of meals. Splitting the daily dose or increasing a DR-PPI dose to b.i.d. with a pre-dinner administration of the second dose does not achieve full control of nighttime acidity [1].
Three key pharmacological requirements for new antisecretory drugs are flexibility in time of dosing, rapid onset of acid suppression, and predictability of dose/antisecretory effect within the 24-h period, with the opportunity to better control time periods, especially the nighttime. These criteria are largely met by the P-CAB class which are not pro-drugs and can be taken independent of meals, exhibit rapid onset of antisecretory effect, and plasma levels are sustained with a more predictable degree and duration of antisecretory effect [2, 3•].
Unmet needs in GERD
Unmet needs in the treatment of GERD include a significant improvement in symptom control and faster assured healing, especially in patients with grades C and D erosive esophagitis, Barrett’s esophagus, and extra-esophageal complications of GERD [8, 11,12,13,14,15]. Patients with refractory GERD continue to pose a clinical problem for those with Los Angeles grade C or D esophagitis, where the rate of unhealed ulcers with DR-PPIs is 39.6% at 4 weeks and 15% after 8 weeks treatment for grade C disease and 58.4 after 4 weeks falling to 25.3% after 8 weeks treatment in grade D disease [16]. The promise of overcoming these high rates of non-response of erosive GERD to DR-PPIs is seen with the initial results with the P-CAB vonoprazan (see below).
The slow onset of symptom relief with DR-PPIs and nighttime and postprandial heartburn also remain a problem in ~ 20% patients with GERD. Dexlansoprazole-modified release (MR), a PPI with an extended plasma residence time when given once daily, was as effective as a PPI twice daily for controlling symptoms in patients with difficult-to-control reflux disease [1, 17].
Poor response of reflux patients to treatment with a PPI requires the clinician to determine that the symptoms are due to acid reflux rather than functional heartburn [9, 18, 19]. Management options have been recommended by an expert panel for patients with GERD and persistent symptoms on PPIs [20••].
Unmet needs in the treatment of Helicobacter pylori infection
PPIs display several pharmacological actions that give them a place in the eradication regimens [21••]:
-
1.
They exert an antibacterial action against H. pylori;
-
2.
By increasing intragastric pH, they allow the microorganism to reach the growth phase and become more sensitive to antibiotics such as amoxicillin and clarithromycin;
-
3.
They increase antibiotic stability and efficacy; and
-
4.
By reducing gastric emptying and mucus viscosity, they increase the gastric residence time and mucus penetration of antimicrobials.
The importance of pH control in the management of H. pylori infection is confirmed by the MACH-2 study [22], which showed that cure rates with omeprazole and two antibiotics are significantly higher than the same two antibiotics, given without omeprazole. A similar result was reported with clarithromycin and tinidazole given with or without lansoprazole [23].
Prolonged acid suppression especially during the night is crucial for H. pylori eradication [24, 25]. Confirmation of the importance of profound and long-lasting acid suppression is illustrated by two studies reporting significantly higher intragastric pH and lower percentage time spent at pH < 4 in patients successfully cured versus those with persisting infection [26, 27]. Furthermore, the eradication rate was higher in those without nocturnal acid breakthrough (NAB) than in NAB-positive patients [26].
Antisecretory drugs which offer superior control of both day and nighttime acidity could also improve compliance. Current PPIs must be taken at high doses, twice daily, while a long-acting PPI could be given once daily, simplifying treatment without impairing efficacy. Finally, it might be possible with a long-acting acid inhibitor to achieve high eradication rates with dual therapy (e.g., amoxicillin/acid suppressant) alone [28, 29].
Unmet needs in the prevention and treatment of NSAID-associated upper GI lesions
Gastro-duodenal mucosa possesses an array of defensive mechanisms and non-steroidal anti-inflammatory drugs (NSAIDs) and aspirin have a deleterious effect on most of them. This results in a mucosa less able to cope with even a reduced acid load. The presence of acid appears to be a conditio sine qua non for NSAID injury, which is pH-dependent [30, 31] Indeed, the higher the intragastric pH, the lower the extent and severity [31], as well as probability, of mucosal damage [30]. There is a strong rationale for PPI use in both treatment and prevention of NSAID-associated gastro-duodenal ulcers [21••, 32••]. Unlike H2-RAs, PPIs protect from NSAID-injury not only in the duodenum but also the stomach, where the majority of mucosal lesions occur [33, 34].
However, while DR-PPIs promote healing of NSAID-related lesions, gastric ulcers respond less well than duodenal ulcers. In the ASTRONAUT trial of omeprazole versus ranitidine [35], 81% of patients with gastric ulcers healed at 8 weeks with omeprazole compared with 92% with duodenal ulcers. This difference was more marked at 4 weeks. Most patients continue to require their NSAID, but there is loss of protection with time, even with continued PPI treatment [35,36,37].
Most NSAIDs are taken more than once daily, or are “sustained release” formulations to provide 24-h benefit. Some drugs (e.g., naproxen) undergo enterohepatic circulation, further extending GI exposure especially at night. Thus, patients on NSAIDs taking a DR-PPI once daily will have residual acid secretion during the 24-h period and continue to be at risk of GI injury [38]. All these do represent unmet clinical needs [39]. A once-daily antisecretory drug with true 24-h acid control would be expected to provide improved mucosal protection and clinical trial data with P-CABs in the primary prevention of NSAID ulcers are awaited with interest.
Unmet needs in upper GI bleeding
Treatment with PPIs is established in the management guidelines for upper GI bleeding with initial intervention at the time of resuscitation before endoscopy and then following endoscopic diagnosis and therapeutic intervention, if any [40••, 41, 42]. Theory argues for powerful, prolonged acid suppression to raise and maintain the pH of gastric juice to pH ≥ 6 since several studies have shown that platelet aggregation is normal at pH 7.4 but decreases rapidly below pH 6.8 and is abolished at pH 5.9 [43]. In addition, fibrinolytic activity is enhanced in patients with bleeding gastroduodenal ulcers and acid suppression decreases this increased activity [44]. Guidelines recommend high-dose iv PPI to downgrade stigmata of recent bleeding and, potentially, reduce the need for endoscopic treatment. This practice is endorsed although there is no evidence for a reduction in the rate of re-bleeding, need for surgical intervention, or mortality [41, 42].
Following endoscopy with or without therapeutic intervention, the objectives of acid suppression continues to be reducing re-bleeding risk. This will also heal any ulcer and prevent long-term ulcer recurrence. There is strong evidence from meta-analyses for a significant reduction in re-bleeding (RR 0.40, 95% CI 0.28–0.59); need for surgery (RR 0.43, 95% CI 0.24–0.76); and mortality (RR 0.41, 95% CI 0.20–0.84) when iv PPI treatment (bolus followed by continuous infusion) is compared for 72 h with no treatment [45]. In an iv comparative study [46], high-dose omeprazole was superior to the H2-RA, ranitidine, at maintaining intragastric pH > 6. During the study period, the time pH < 6 for omeprazole and ranitidine was 15.3 ± 5.9% and 61.8 ± 5.6% (p < 0.0001), respectively.
There is considerable variation in the pH threshold maintained by different DR-PPIs in this therapeutic indication and with the treatment target to maintain pH > 6 in [43] (vide supra). The new class of P-CAB drugs might fulfill the unmet need in upper gastrointestinal (UGI) bleeding and improve outcomes with respect to re-bleeding, transfusion requirements, surgical intervention, and mortality.
PPIs: new formulations and combinations
A new esomeprazole salt (esomeprazole strontium) DR capsules (49.3 mg) was recently approved in the USA via the 505(b)(2) NDA pathway. The compound is bioequivalent with esomeprazole magnesium and considered interchangeable. There are no specific clinical studies with esomeprazole strontium DR capsules or pharmacodynamic (PD) comparisons with the magnesium salt. The safety and efficacy data from the FDA’s approval of esomeprazole magnesium (40 mg) were used to support this new drug application (NDA) (https://www.accessdata.fda.gov/drugsatfdadocs/nda/2013/202342Orig1s000MedR.pdf).
Extended-release formulations of PPIs are increasingly important considerations for control of nocturnal acid secretion because of their sustained release [47]. The foremost is dexlansoprazole modified release (MR) formulation [48, 49], now marketed in the USA and some other countries. Pharmacokinetic studies show a plasma concentration-time profile, with two distinct peaks, occurring 1–2 h and 4–5 h after dosing, and 24-h intragastric pH-recordings confirm a prolonged acid inhibition across all doses (60, 90, and 120 mg) [48]. An extended release formulation of rabeprazole [50] has also been developed but not marketed.
While currently available DR-PPIs are orally administered as gastro-protected preparations [47], the immediate release (IR) omeprazole formulation consists of pure, non-enteric-coated (naked) omeprazole powder (40 mg or 20 mg per unit dose) with 1680 mg of sodium bicarbonate (containing 460 mg of sodium). This formulation displays different pharmacokinetics and pharmacodynamics compared with the standard, DR-omeprazole [51]. The antisecretory effect is faster than that observed with DR-omeprazole, is food-independent and a bedtime dose assures a better control of nocturnal acid secretion than lansoprazole or esomeprazole [51]. Immediate release formulations of esomeprazole have also been developed [52, 53].
DR-PPIs are the standard of care for prevention and treatment of NSAID-associated gastroduodenal ulcers in patients at GI risk [21••, 38]. However, adherence to concomitant gastroprotective therapy is paramount to reducing GI events among NSAID users and an inverse relationship between the incidence of complications and the adherence rate has been reported [54]. A naproxen-esomeprazole fixed combination is marketed in the USA, Europe, and Asia. In this formulation, esomeprazole magnesium immediate-release covers an enteric-coated core of naproxen [55] and a multilayer fixed-dose combination is currently under development [56].
DR-PPIs are a key component of current treatments for H. pylori eradication, as recommended by national and international guidelines [21••]. Given the complexity (number of pills and number of drug administrations) and duration of treatments, compliance is an issue [57]. Compliance packages containing daily administration card and the drugs (usually a PPI plus two antimicrobials) are now marketed (e.g., PrevPAC™ in USA and Heli-Kit™ in some African Countries). RHB-105 (also known as Talicia™, RedHill Biopharma) is a fixed-dose combination therapy of two antibiotics (amoxicillin 250 mg and rifabutin 12.5 mg) and a PPI (omeprazole 10 mg) in an all-in-one capsule, four of which are given three times daily. A phase III study (ERADICATE Hp trial) [58], showed this combination achieved superiority (89.4% cure rate) over an historical standard of care of 70% rate (p < 0.001).
Long-duration PPIs
Several new PPIs are still in preclinical development and five drugs are actively being studied in humans (Table 1), while ilaprazole is already on the market in South Korea and China. Ilaprazole (IY-81149) is a benzimidazole synthesized at Il-Yang (South Korea) and developed by Livzon Pharmaceutical Group Inc. (Zhuhai, China). Ilaprazole irreversibly inhibits H+,K+-ATPase and aminopyrine accumulation in a dose-dependent manner with a potency comparable to omeprazole. Compared with DR-PPIs, ilaprazole displays an extended plasma half-life (i.e., 3.6 h), a metabolism not significantly influenced by CYP2C19 genetic polymorphism and with similar drug safety. This results in a low inter-individual variability, particularly in Asian populations, a faster and improved control of gastric acid secretion, with better effect on nocturnal acidity and faster symptom relief [60]. However, ilaprazole has not shown significant improvements over DR-PPIs in GERD or H. pylori eradication.
AGN 201904-Z (Durasec™), developed by GI Logics Ltd., is the sodium salt of an acid stable prodrug of omeprazole while tenatoprazole (TU-199) synthesized by Mitsubishi Pharma (Japan) and developed by Sidem Pharma (France), is a long-acting PPI that is not a benzimidazole derivative. The clinical pharmacology of these compounds has previously been reviewed by Scarpignato and Hunt [61]. They both have a half-life longer than the current DR-PPIs (4.5 h and 9 h, respectively) and showed an extended acid suppression, especially during the night with almost no occurrence of NAB. A more recent dose-ranging study [62] showed that the Na salt of tenatoprazole S-stereoisomer produced significantly greater and more prolonged dose-dependent 24-h and nocturnal acid suppression than esomeprazole. Both PPIs are therefore promising antisecretory drugs for the treatment of acid-related diseases, where it has the potential to address unmet clinical needs [12].
Azeloprazole (E-3710 or Z-215) synthesized by Eisai (Japan) is currently at the end of phase II. Experimental studies [63] demonstrated a long-acting inhibition of gastric acid secretion and a pharmacokinetic (PK)/PD study [64] showed a linear PK profile and potent dose-dependent antisecretory effect. Plasma concentrations of azeloprazole were similar among CYP2C19 genotypes, suggesting that clinical efficacy could be independent from patients’ genetics. In a large, 8-week, multicenter, RCT [59], 503 patients with GERD taking azeloprazole (10, 20 or 40 mg daily) or rabeprazole (10 mg daily), the healing rate was above 95% in all arms. Healing rates and serum gastrin levels in the azeloprazole-treated patients were not influenced by CYP2C19 and the safety profile of both PPIs (at any dose) was similar.
Anaprazole, synthesized by Sihuan Pharmaceutical Holdings Group Ltd. (China) and developed by Xuan Zhu Pharma Co., Ltd. (Jinan, China), is a new long-acting, substituted benzimidazole [65] while DLBS-2411 (Redacid®) is a natural PPI. It is a bioactive fraction from Cinnamomum burmannii (Indonesian cinnamon, locally known as kayu manis) [66]. They are both in phase III in China and Indonesia, respectively.
Potassium-competitive acid blockers
As discussed above, several new PPIs (see above) or alternative formulations of existing drugs have been explored but only instant release omeprazole (i.e., IR-omeprazole) and dexlansoprazole-MR have been introduced in some countries. Both represent a measurable but small incremental advance in the pharmacological control of acid secretion over the DR-PPIs but fall short of achieving the ideal pharmacologic profile to control intragastric acidity in patients with more complex clinical problems [3•].
A more innovative approach has been the development of the of H+,K+, ATPase blockers, called potassium-competitive acid blockers (P-CABs) [2, 67, 68], which block the K+ exchange channel of the proton pump, resulting in a very fast, competitive, reversible inhibition of acid secretion (Table 2) [2]. A P-CAB offers a more rapid elevation of intra-gastric pH than a DR-PPI, while maintaining the same degree of antisecretory effect, the duration of which is dependent on the half-life and can be prolonged by extended release formulations (Fig. 1).
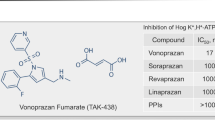
The P-CAB class has attracted several pharmaceutical companies to this avenue of drug development (Table 3). Almost all these new compounds display rapid and effective antisecretory activity, but not all favorable pharmacodynamic properties have translated into clinical benefits. The first P-CAB (revaprazan, YH1885) [69], marketed in South Korea and India, achieved healing rates in both duodenal [70] and gastric [71] ulcer, which were not significantly different from omeprazole. Similarly, large, randomized, controlled trials did not show superiority of linaprazan (AZD0865) over standard-dose esomeprazole, for both healing [72] or symptom relief [73] in GERD. However, the design of these studies as well as the P-CAB dose used were not appropriate considering the short half-life of linaprazan. These early poor results led to the conclusion that the P-CAB class was a “promise unfulfilled.” Furthermore, linaprazan was associated with transaminase elevation and development was stopped, as was the case for several other P-CABs (e.g., soraprazan, CS526, YH4808), withdrawn from development (Table 3).
Vonoprazan (TAK-438) is a novel and potent orally active P-CAB, developed by Takeda and marketed in Japan since 2015 [74]. The drug is a pyrrole derivative, displaying powerful inhibition of the proton pump compared to PPIs and other P-CABs [75].
Vonoprazan has been in clinical use for almost 4 years and considerable clinical data are available, detailed in extensive reviews [74, 76•, 77,78,79,80]. We summarize below the PK and PD properties of vonoprazan as well as the derived benefits in some acid-related diseases, referencing systematic reviews and meta-analyses, where available.
PK and PD studies [81], performed in Japanese or Caucasian healthy male volunteers, showed that vonoprazan displays almost linear pharmacokinetics and inhibits acid secretion (by 87 and 92% of the 24 h, respectively) in a dose-dependent fashion. Holding times above pH > 4 and pH > 5 after the vonoprazan 40-mg dose were 100 and 99%, respectively, 12–24 h post dose in the Japanese study and 90 and 79%, respectively, from 2000 to 0800 hours in the UK study. The increase in pH was reflected by an increase in serum gastrin and pepsinogen I concentrations. The drug was well-tolerated at all doses tested, with no changes in serum transaminase levels. In another study [82], these pharmacological effects persisted with repeated administration and, after 7 days of treatment, the mean 24-h intragastric pH > 4 holding time with vonozapran 40 mg was 100% in Japanese subjects and 93.2% in UK volunteers; mean nocturnal times spent at pH > 4 were 100 and 90.4%, respectively. Conversely from esomeprazole, the antisecretory activity of vonoprazan was independent of CYP2C19 genotype [83]. In H. pylori-negative healthy volunteers, the increase of intragastric pH with vonozapran (20 mg) was higher and faster compared to lansoprazole (30 mg) and similar to famotidine (20 mg) [84]. In CYP2C19 extensive metabolizers, vonoprazan (20 mg) induced a more rapid and sustained acid inhibitory effect than esomeprazole (20 mg) or rabeprazole (10 mg), showing virtually no NAB [85].
A large meta-analysis [86], including 95 studies (411 arms) providing intragastric pH data and 109 studies (243 arms) providing EE healing data for three H2RAs, five PPIs, and placebo, demonstrated that any increase in the time intragastric pH is pH < 4 during the 24 h (at steady state in healthy volunteers) is associated with a decrease in the erosive esophagitis healing rate at 4 and 8 weeks. The percent time that intragastric pH < 4 is a significant predictor for esophagitis non-healing. As confirmed by a dexlansoprazole-MR study [87], from the derived mathematical relationship, it is possible to predict the healing rate of any new antisecretory drug. As expected, the healing rate of reflux esophagitis after 8-week therapy with vonoprazan was almost 100%. Moreover, while there were no differences between vonoprazan (20 mg daily) and lansoprazole (30 mg daily) in grades A and B esophagitis, the healing rate of vonoprazan was significantly higher than with lansoprazole in grades C and D esophagitis [88], a superiority maintained in CYP2C19 extensive metabolizers [89]. Vonoprazan is also effective in patients with PPI-resistant esophagitis, inducing healing in 87.5% [90]. The efficacy of vonoprazan was maintained long-term, with post hoc analysis showing lower recurrence rates compared to lansoprazole [91].
In a randomized, double-blind, placebo-controlled, multicenter study in patients with non-erosive reflux disease (NERD) [92], the number of days heartburn-free with vonoprazan (10 mg or 20 mg daily) was not superior to placebo, although the mean severity of heartburn was lower. Despite these findings, the drug appears to be effective in PPI-resistant NERD. A small retrospective study [93] found that 69.2% of patients reported an improvement of symptoms and of quality of life (measured by GERD-Q score). However, P-CAB-resistant NERD does also exist and it is mainly ascribed to weakly acidic reflux or functional heartburn [94].
Helicobacter pylori infection and reinfection present serious challenges in management. H. pylori resistance to antimicrobials (especially clarithromycin, metronidazole, or levofloxacin) is high in most countries [95] and the selection of a regimen to eradicate H. pylori in more than 90% of infected patients, is critical. Control of intragastric pH, especially during the night vide supra, to maintain bactericidal activity of combined antimicrobials, is crucial [3•, 12] and vonoprazan may represent a significant advance over DR-PPI-based eradication therapies [96].
An early meta-analysis [97], including 10 studies and 10,644 patients, showed that vonoprazan-based triple therapy was superior to PPI-based triple therapy, with comparable tolerability and adverse events. This superiority, was only evident in first-line H. pylori triple eradication therapies but not in second-line treatments [98]. Moreover, a recent systematic review with meta-analysis [99] pointed out that vonoprazan is superior to conventional PPI-based therapy only for eradication of clarithromycin-resistant H. pylori strains while vonoprazan- and conventional PPI-based therapies are similarly effective in patients harboring clarithromycin-susceptible H. pylori strains. Moreover, a retrospective study [100] found that vonoprazan-based triple therapy was effective as susceptibility-guided triple therapy for H. pylori eradication. A small study [101] in unselected patients reported eradication rates with vonoprazan/amoxicillin therapy for first- and second-line treatments as high as 95% (19/20) and 90% (18/20), respectively. Therefore, provided this dual therapy can be optimized (dose, number of drug administrations, and duration [102•]), it could provide a simple, reliable, and effective first-line eradication treatment.
To date, only triple therapy is covered by the Japanese National Health Insurance System and almost all studies with vonoprazan for H. pylori eradication have been conducted with the triple regimen (PPI or vonoprazan, amoxicillin, and clarithromycin) [103]. More studies with vonoprazan-based alternative regimens will provide a better understanding of the efficacy of vonoprazan in the eradication of H. pylori.
NSAID-gastropathy is a pH-dependent phenomenon: the higher the intragastric pH, the lower the extent and severity, as well as the probability, of mucosal damage [21••, 38]. A once-daily antisecretory drug with prolonged control of acid secretion over the 24-h period is expected to display improved mucosal protection [12]. Vonoprazan prevented recurrence of both aspirin [104] and NSAID-associated [105] ulcers, but clinical trial data in the primary prevention of NSAID ulcers are still awaited.
A sustained intragastric pH > 6, to promote platelet aggregation, clot formation, and stability [106], should be of benefit in upper GI bleeding. Until now, it has not been possible to attain a consistent intragastric pH of ≥ 6 in fed patients, with oral PPIs, even two or three times daily [107]. The pharmacodynamic properties of oral vonoprazan are expected to achieve the same, or better outcomes to those obtained with intravenous PPIs [107].
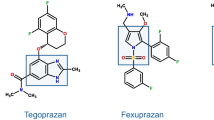
Tegoprazan (formerly RQ-00000004 or CJ-12420) is a benzimidazole derivative [108] developed by RaQualia Pharma and CJ HealthCare, for treatment of GERD and PU. The first human study [109] showed that single oral administration of tegoprazan provided rapid elevation of intragastric pH to > 6 under fasted condition in healthy subjects. A dose-ranging study [110] demonstrated a linear PK profile after single and multiple administrations and a fast and dose-dependent acid suppression. A phase III trial in GERD has been completed and one H. pylori eradication trial is ongoing in South Korea, where the drug should be launched during the 2018.
Like vonoprazan, DWP14012 is a pyrrole derivative, developed by Daewoong Pharmaceutical Co., Ltd. (Seoul, Korea). Preclinical pharmacology demonstrated a fast, dose-dependent acid suppression, similar or greater to vonoprazan [111]. A phase I study [112] showed that DWP14012 is well tolerated, and induces a rapid and long-lasting gastric acid suppression in healthy subjects. PK was linear after multiple ascending doses and the safety profile, including hepatic tolerability, overlapped that of placebo. Phase II has been completed (https://clinicaltrials.gov/ct2/show/NCT03184324) and phase III studies are planned.
X842 is a pro-drug of linaprazan, developed in Europe by Cinclus Pharma AG. The active metabolite has a comprehensive data base from 25 phase I studies, including more than 600 subjects, and 2 phase II studies, including 2.973 patients. All these investigations showed linaprazan was well tolerated, with a fast onset of action and full effect from the first dose. However, linaprazan did not control 24-h intragastric pH, likely because of its short plasma half-life [73]. In contrast, X842 has a longer half-life which provides effective 24-h pH control.
The first human study of X842 [113] evaluated the PK and PD after single and multiple ascending doses. Linaprazan rapidly appeared in plasma, with the Cmax at ~ 2 h after oral administration. Plasma half-life was ≥ 10 h, following doses of 1 mg/kg or higher. Linaprazan AUC linearly correlated with the X842 dose with a dose-dependent acid inhibition over the 24 h, and linear correlation between plasma concentrations of the active metabolite (i.e., linaprazan) and intragastric pH. At doses of 2 mg/kg, X842 achieved effective acid control over 24 h without NAB. A phase 2 randomized, double-blind, active comparator study with four treatment arms in some 310 patients with severe esophagitis, evaluating 4-week healing rates, is planned in Europe and the USA.
Overuse and misuse of acid suppression
Studies suggest PPIs are frequently prescribed for inappropriate indications or where there is little benefit [114]. The introduction of generic PPIs into the market has been followed by an increasing rate of PPI prescribing related to chronic treatments, unlicensed indications, and therapeutic substitutions [115]. Hospital patients are often started on PPIs inappropriately [116], and medications are continued, following discharge, by primary care physicians. Inadequate recommendations for PPIs in discharge letters are frequent [117] and prescription habit may lead to a continuation of PPI. An Italian study [118] found the persistence rate of PPI therapy is high after both appropriate and inappropriate prescriptions (62 and 71%, respectively). The primary care attitude to continuing or discontinuing PPIs depends on their knowledge and perceptions of hospital physicians’ competence and the threshold to prescribing in hospitals [119].
Inappropriate PPI use is a great concern, especially in the elderly, who often have multiple comorbidities and take multiple medications. Long-term PPI-related adverse outcomes and drug-to-drug interactions (DDIs) show a strong relationship between the number of drugs and clinically relevant DDIs [120], particularly in the elderly [121].
Opportunities exist to increase the effectiveness and safety of drug therapy as well as minimize overall healthcare costs. Education is key and guidelines and their implementation provide a rational approach [21••, 122]. Judicious surveillance of hospital use and prescription refills in the outpatient settings [114], with re-evaluation and justification for continued treatment, can minimize potential for adverse effects and achieve cost saving. However, surveillance must be close and continuous since benefits could be short lasting. In a Canadian study [123], deprescribing guidelines were associated with a decline in PPI use during the initial 6 months but prescription patterns subsequently began to climb.
Acid suppression: established benefits versus potential risks
Antisecretory drugs are among the most prescribed drugs worldwide and are also widely available over the counter (OTC). Over the past 50 years, there have been many concerns expressed about the potential adverse effects of antisecretory drugs and, in particular PPIs and also now the P-CABs, which have recently been introduced in some Asian countries.
The safety of long-term acid suppression has been long debated and focused on gastrin levels and the development enterochromaffin-like cell (ECL) hyperplasia and the risk of proliferation of gastric microflora and nitrate-reducing bacteria which might, theoretically, lead to gastric malignancy. These concerns were extensively addressed at the Hanbury Manor Workshop in 1995 [124] and there has been no convincing evidence of neoplastic change in subsequent years of follow-up.
At therapeutic doses, PPIs are more potent than H2-RAs for suppressing gastric acid secretion and comparable studies with P-CABs confirm the sustained and greater elevation of intragastric pH over the whole 24 h period [3•]. The effect on gastrin is greater than that seen with DR-PPIs, which may be at the upper limit of normal although invariably higher in patients colonized with H. pylori than in uninfected controls [3•].
Publications concerning safety with DR-PPIs have increased dramatically with many widely publicized topics appearing in high profile journals or the media. These focus on drug effects which alter the host physiology due to exaggerated pharmacological effects as above and less commonly to drug-related effects. Studies employ widely variable methodologies and extensive data dredging of large treatment data bases which were not designed to answer the question being asked and may also include confounding factors [125••]. Some adverse events are plausible and predictable while others are idiosyncratic and rare. Several important papers have shown the importance of critical evaluation and the application of strict criteria to determine the strength and validity, if any, of a reported association [125••, 126•, 127,128,129]. Overall, the concerns include rebound acid hypersecretion on stopping treatment, hypochlorhydria associated with an increasing risk of enteric pathogenic infections, C. difficile infection and small intestinal bacterial overgrowth (SIBO).; disturbed electrolyte and nutrient absorption; idiosyncratic reactions including microscopic colitis and pancreatitis; effects on bone metabolism, bone density, osteoporosis, and fracture risk; changes in drug absorption and interaction, e.g., with clopidogrel and drugs metabolized by the cytochrome P450 2C19 pathway; kidney function and interstitial nephritis; vitamin B12 deficiency, altered β-amyloid levels, and dementia; myocardial infarction and reduced nitrous oxide; pneumonia resulting from aerobic organisms colonizing the stomach and associated with micro-aspiration and myositis and rhabdomyolysis in association with statins which are metabolized by the CYP3A4 pathway.
When reading reports of adverse effects, it is important to apply critical appraisal to the population studied, seek evidence for biological plausibility and gradient, and explore the methods used to determine possible association and the risk estimate and zone of potential bias [125••]. Much of the evidence which has been reported to link PPI treatment with serious long-term conditions is weak with very low OR [130]. In clinical practice, therefore, it is important to balance the undoubted benefits of treatment with PPIs with their alleged risks and review the indications for the choice of drug and dose and to explain this carefully to the patient [21••, 122•, 126•].
Nearly all the adverse outcomes associated with PPIs occur among patients who receive long-term therapy; minimizing the duration of treatment by periodically reviewing a patient’s need for acid-suppressive therapy could eliminate or substantially reduce the risk of adverse outcomes. Therefore, during continued long-term use, the clinical effects should always be reviewed and attempts be made to stop any treatment that may not be needed. It is imperative to use the lowest dose of drug required to achieve the desired therapeutic goals. This may entail implementing discontinuation of treatment in asymptomatic patients as well as step-down, intermittent, or on-demand PPI therapy for maintenance of GERD.
The introduction of all new drugs is dependent on responsible marketing and thoughtful prescribing with careful monitoring of patients treated. The safety profile of vonoprazan to date has proved to be excellent but overuse and misuse can occur as with any new treatment. It is expected that AE’s related to long acting acid inhibition will be seen [131]. PPI-related adverse events are unlikely, due to the differing molecular structure of vonoprazan. In any event the indications for treatment with vonoprazan or other P-CABs should be for the difficult to treat acid-related disorders and unmet needs where the benefit to risk would be expected to be most favorable [2, 3•].
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Hunt R. Acid suppression for reflux disease: "off-the-peg" or a tailored approach? Clin Gastroenterol Hepatol. 2012;10(3):210–3. https://doi.org/10.1016/j.cgh.2011.11.018.
Scarpignato C, Hunt RH. Editorial: towards extended acid suppression--the search continues. Aliment Pharmacol Ther. 2015;42(8):1027–9. https://doi.org/10.1111/apt.13384.
• Hunt RH, Scarpignato C. Potassium-competitive acid blockers (P-CABs): are they finally ready for prime time in acid-related disease? Clin Transl Gastroenterol. 2015;6:e119. https://doi.org/10.1038/ctg.2015.39 Thoughtful review on the potential role of P-CABs in acid-related diseases.
Burget DW, Chiverton SG, Hunt RH. Is there an optimal degree of acid suppression for healing of duodenal ulcers? A model of the relationship between ulcer healing and acid suppression. Gastroenterology. 1990;99(2):345–51.
Howden CW, Jones DB, Peace KE, Burget DW, Hunt RH. The treatment of gastric ulcer with antisecretory drugs. Relationship of pharmacological effect to healing rates. Dig Dis Sci. 1988;33(5):619–24.
Bell NJ, Burget D, Howden CW, Wilkinson J, Hunt RH. Appropriate acid suppression for the management of gastro-oesophageal reflux disease. Digestion. 1992;51(Suppl 1):59–67. https://doi.org/10.1159/000200917.
• Graham DY, Tansel A. Interchangeable use of proton pump inhibitors based on relative potency. Clin Gastroenterol Hepatol. 2018;16(6):800–8 e7. https://doi.org/10.1016/j.cgh.2017.09.033 Interesting attempt to identify antisecretory equivalence among the currently available DR-PPIs.
Hunt RH. Review article: the unmet needs in delayed-release proton-pump inhibitor therapy in 2005. Aliment Pharmacol Ther. 2005;22(Suppl 3):10–9.
Scarpignato C. Poor effectiveness of proton pump inhibitors in non-erosive reflux disease: the truth in the end! Neurogastroenterol Motil. 2012;24(8):697–704. https://doi.org/10.1111/j.1365-2982.2012.01977.x.
Yuan Y, Wang C, Yuan Y, Hunt RH. The proportion of patients who are free of reflux symptoms during the initial days of treatment with proton pump inhibitors (PPIs) in GERD trials: a meta-analysis. Gastroenterology. 2008;134(4, Suppl 1):A-174–5. https://doi.org/10.1016/s0016-5085(08)60812-4.
Katz PO, Scheiman JM, Barkun AN. Review article: acid-related disease—what are the unmet clinical needs? Aliment Pharmacol Ther. 2006;23(Suppl 2):9–22. https://doi.org/10.1111/j.1365-2036.2006.02944.x.
Scarpignato C, Pelosini I. Review article: the opportunities and benefits of extended acid suppression. Aliment Pharmacol Ther. 2006;23(Suppl 2):23–34. https://doi.org/10.1111/j.1365-2036.2006.02945.x.
Dickman R, Maradey-Romero C, Gingold-Belfer R, Fass R. Unmet needs in the treatment of gastroesophageal reflux disease. J Neurogastroenterol Motil. 2015;21(3):309–19. https://doi.org/10.5056/jnm15105.
Goh KL, Choi MG, Hsu WP, Chun HJ, Mahachai V, Kachintorn U, et al. Unmet treatment needs of gastroesophageal reflux disease in Asia: gastroesophageal reflux disease in Asia Pacific survey. J Gastroenterol Hepatol. 2014;29(12):1969–75. https://doi.org/10.1111/jgh.12655.
Wright MR, Sharda R, Vaezi MF. Unmet needs in treating laryngo-pharyngeal reflux disease: where do we go from here? Expert Rev Gastroenterol Hepatol. 2016;10(9):995–1004. https://doi.org/10.1080/17474124.2016.1179576.
Yaghoobi M, Padol S, Yuan Y, Hunt RH. Impact of oesophagitis classification in evaluating healing of erosive oesophagitis after therapy with proton pump inhibitors: a pooled analysis. Eur J Gastroenterol Hepatol. 2010;22(5):583–90. https://doi.org/10.1097/MEG.0b013e328335d95d.
Fass R, Inadomi J, Han C, Mody R, O'Neil J, Perez MC. Maintenance of heartburn relief after step-down from twice-daily proton pump inhibitor to once-daily dexlansoprazole modified release. Clin Gastroenterol Hepatol. 2012;10(3):247–53. https://doi.org/10.1016/j.cgh.2011.11.021.
Yuan Y, Hunt RH. Evolving issues in the management of reflux disease? Curr Opin Gastroenterol. 2009;25(4):342–51. https://doi.org/10.1097/MOG.0b013e32832c1504.
Herregods TV, Troelstra M, Weijenborg PW, Bredenoord AJ, Smout AJ. Patients with refractory reflux symptoms often do not have GERD. Neurogastroenterol Motil. 2015;27(9):1267–73. https://doi.org/10.1111/nmo.12620.
•• Yadlapati R, Vaezi MF, Vela MF, Spechler SJ, Shaheen NJ, Richter J, et al. Management options for patients with GERD and persistent symptoms on proton pump inhibitors: recommendations from an expert panel. Am J Gastroenterol. 2018;113(7):980–6. https://doi.org/10.1038/s41395-018-0045-4 Expert consensus providing an evidence-based decision making in PPI-refractory GERD.
•• Scarpignato C, Gatta L, Zullo A, Blandizzi C. SIF-AIGO-FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases—a position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14(1):179. https://doi.org/10.1186/s12916-016-0718-z Comprehensive review on evidence-based PPI use.
Lind T, Megraud F, Unge P, Bayerdorffer E, O'Morain C, Spiller R, et al. The MACH2 study: role of omeprazole in eradication of helicobacter pylori with 1-week triple therapies. Gastroenterology. 1999;116(2):248–53.
Wheeldon TU, Hoang TT, Phung DC, Bjorkman A, Granstrom M, Sorberg M. Helicobacter pylori eradication and peptic ulcer healing: the impact of deleting the proton pump inhibitor and using a once-daily treatment. Aliment Pharmacol Ther. 2003;18(1):93–100.
Hunt RH. pH and Hp—gastric acid secretion and Helicobacter pylori: implications for ulcer healing and eradication of the organism. Am J Gastroenterol. 1993;88(4):481–3.
Furuta T, Graham DY. Pharmacologic aspects of eradication therapy for Helicobacter pylori infection. Gastroenterol Clin N Am. 2010;39(3):465–80. https://doi.org/10.1016/j.gtc.2010.08.007.
Kim JI, Park SH, Kim JK, Chung IS, Chung KW, Sun HS. The effects of nocturnal acid breakthrough on Helicobacter pylori eradication. Helicobacter. 2002;7(6):331–6. https://doi.org/10.1046/j.1523-5378.2002.00105.x.
Sugimoto M, Furuta T, Shirai N, Kodaira C, Nishino M, Ikuma M, et al. Evidence that the degree and duration of acid suppression are related to Helicobacter pylori eradication by triple therapy. Helicobacter. 2007;12(4):317–23. https://doi.org/10.1111/j.1523-5378.2007.00508.x.
Yang JC, Lin CJ, Wang HL, Chen JD, Kao JY, Shun CT, et al. High-dose dual therapy is superior to standard first-line or rescue therapy for Helicobacter pylori infection. Clin Gastroenterol Hepatol. 2015;13(5):895–905.e5. https://doi.org/10.1016/j.cgh.2014.10.036.
Zullo A, Ridola L, Francesco VD, Gatta L, Hassan C, Alvaro D, et al. High-dose esomeprazole and amoxicillin dual therapy for first-line helicobacter pylori eradication: a proof of concept study. Ann Gastroenterol. 2015;28(4):448–51.
Plachetka J, Morelli G, Hines C, et al. Integrated gastric acidity can predict the prevention of naxopren-induced gastroduodenal pathology in normal subjects. Gastroenterology. 2003;124(Suppl 1):A510.
Scarpignato C, Pelosini I. Prevention and treatment of non-steroidal anti-inflammatory drug-induced gastro-duodenal damage: rationale for the use of antisecretory compounds. Ital J Gastroenterol Hepatol. 1999;31(Suppl 1):S63–72.
•• Scally B, Emberson JR, Spata E, Reith C, Davies K, Halls H, et al. Effects of gastroprotectant drugs for the prevention and treatment of peptic ulcer disease and its complications: a meta-analysis of randomised trials. Lancet Gastroenterol Hepatol. 2018;3(4):231–41. https://doi.org/10.1016/s2468-1253(18)30037-2 The most comprehensive meta-analysis to date on the prevention and treatment of PUD and its complications with PPIs and other gastroprotective compounds.
Rostom A, Moayyedi P, Hunt R. Canadian consensus guidelines on long-term nonsteroidal anti-inflammatory drug therapy and the need for gastroprotection: benefits versus risks. Aliment Pharmacol Ther. 2009;29(5):481–96. https://doi.org/10.1111/j.1365-2036.2008.03905.x.
Scarpignato C, Lanas A, Blandizzi C, Lems WF, Hermann M, Hunt RH. Safe prescribing of non-steroidal anti-inflammatory drugs in patients with osteoarthritis—an expert consensus addressing benefits as well as gastrointestinal and cardiovascular risks. BMC Med. 2015;13:55. https://doi.org/10.1186/s12916-015-0285-8.
Yeomans ND, Tulassay Z, Juhasz L, Racz I, Howard JM, van Rensburg CJ, et al. A comparison of omeprazole with ranitidine for ulcers associated with nonsteroidal antiinflammatory drugs. Acid suppression trial: ranitidine versus omeprazole for NSAID-associated ulcer treatment (ASTRONAUT) study group. New Engl J Med. 1998;338(11):719–26. https://doi.org/10.1056/nejm199803123381104.
Hawkey CJ, Karrasch JA, Szczepanski L, Walker DG, Barkun A, Swannell AJ, et al. Omeprazole compared with misoprostol for ulcers associated with nonsteroidal antiinflammatory drugs. Omeprazole versus misoprostol for NSAID-induced ulcer management (OMNIUM) study group. New Engl J Med. 1998;338(11):727–34. https://doi.org/10.1056/nejm199803123381105.
Graham DY, Agrawal NM, Campbell DR, Haber MM, Collis C, Lukasik NL, et al. Ulcer prevention in long-term users of nonsteroidal anti-inflammatory drugs: results of a double-blind, randomized, multicenter, active- and placebo-controlled study of misoprostol vs lansoprazole. Arch Intern Med. 2002;162(2):169–75.
Scarpignato C, Hunt RH. Nonsteroidal antiinflammatory drug-related injury to the gastrointestinal tract: clinical picture, pathogenesis, and prevention. Gastroenterol Clin N Am. 2010;39(3):433–64. https://doi.org/10.1016/j.gtc.2010.08.010.
Scheiman JM. Unmet needs in non-steroidal anti-inflammatory drug-induced upper gastrointestinal diseases. Drugs. 2006;66(Suppl 1):15–21.
•• Lanas A, Dumonceau JM, Hunt RH, Fujishiro M, Scheiman JM, Gralnek IM, et al. Non-variceal upper gastrointestinal bleeding. Nat Rev Dis Primers. 2018;4:18020. https://doi.org/10.1038/nrdp.2018.20 Comprehensive review by leading experts on the pathophysiology and management of upper GI bleeding.
Gralnek IM, Dumonceau JM, Kuipers EJ, Lanas A, Sanders DS, Kurien M, et al. Diagnosis and management of nonvariceal upper gastrointestinal hemorrhage: European Society of Gastrointestinal Endoscopy (ESGE) guideline. Endoscopy. 2015;47(10):a1–46. https://doi.org/10.1055/s-0034-1393172.
Barkun AN, Bardou M, Kuipers EJ, Sung J, Hunt RH, Martel M, et al. International consensus recommendations on the management of patients with nonvariceal upper gastrointestinal bleeding. Ann Internal Med. 2010;152(2):101–13. https://doi.org/10.7326/0003-4819-152-2-201001190-00009.
Green FW Jr, Kaplan MM, Curtis LE, Levine PH. Effect of acid and pepsin on blood coagulation and platelet aggregation. A possible contributor prolonged gastroduodenal mucosal hemorrhage. Gastroenterology. 1978;74(1):38–43.
Vreeburg EM, Levi M, Rauws EA, Deventer SJ, Snel P, Bartelsman JW, et al. Enhanced mucosal fibrinolytic activity in gastroduodenal ulcer haemorrhage and the beneficial effect of acid suppression. Aliment Pharmacol Ther. 2001;15(5):639–46.
Laine L, McQuaid KR. Endoscopic therapy for bleeding ulcers: an evidence-based approach based on meta-analyses of randomized controlled trials. Clin Gastroenterol Hepatol. 2009;7(1):33–47. https://doi.org/10.1016/j.cgh.2008.08.016.
Lanas A, Artal A, Blas JM, Arroyo MT, Lopez-Zaborras J, Sainz R. Effect of parenteral omeprazole and ranitidine on gastric pH and the outcome of bleeding peptic ulcer. J Clin Gastroenterol. 1995;21(2):103–6.
Scarpignato C, Pelosini I, Di Mario F. Acid suppression therapy: where do we go from here? Dig Dis. 2006;24(1–2):11–46. https://doi.org/10.1159/000091298.
Fass R, Frazier R. The role of dexlansoprazole modified-release in the management of gastroesophageal reflux disease. Ther Adv Gastroenterol. 2017;10(2):243–51. https://doi.org/10.1177/1756283x16681701.
Oldfield Iv EC, Parekh PJ, Johnson DA. Dexlansoprazole: delayed-release orally disintegrating tablets for the treatment of heartburn associated with non-erosive gastroesophageal reflux disease and the maintenance of erosive esophagitis. Expert Rev Gastroenterol Hepatol. 2016;10:1083–9. https://doi.org/10.1080/17474124.2016.1230496.
Morelli G, Chen H, Rossiter G, Rege B, Lu Y. An open-label, parallel, multiple-dose study comparing the pharmacokinetics and gastric acid suppression of rabeprazole extended-release with esomeprazole 40 mg and rabeprazole delayed-release 20 mg in healthy volunteers. Aliment Pharmacol Ther. 2011;33(7):845–54. https://doi.org/10.1111/j.1365-2036.2011.04580.x.
Howden CW. Review article: immediate-release proton-pump inhibitor therapy—potential advantages. Aliment Pharmacol Ther. 2005;22(Suppl 3):25–30. https://doi.org/10.1111/j.1365-2036.2005.02709.x.
Benetti C, Flammini L, Vivo V, Colombo P, Colombo G, Elviri L, et al. Esomeprazole immediate release tablets: gastric mucosa ex vivo permeation, absorption and antisecretory activity in conscious rats. J Control Release. 2016;239:203–10. https://doi.org/10.1016/j.jconrel.2016.08.032.
Banerjee R, Reddy DN, Guda NM, Kalpala R, Mahurkar S, Darisetty S, et al. Oral buffered esomeprazole is superior to i.v. pantoprazole for rapid rise of intragastric pH: a wireless pH metry analysis. J Gastroenterol Hepatol. 2010;25(1):43–7. https://doi.org/10.1111/j.1440-1746.2009.05994.x.
Goldstein JL, Howard KB, Walton SM, McLaughlin TP, Kruzikas DT. Impact of adherence to concomitant gastroprotective therapy on nonsteroidal-related gastroduodenal ulcer complications. Clin Gastroenterol Hepatol. 2006;4(11):1337–45. https://doi.org/10.1016/j.cgh.2006.08.016.
Dhillon S. Naproxen/esomeprazole fixed-dose combination: for the treatment of arthritic symptoms and to reduce the risk of gastric ulcers. Drugs Aging. 2011;28(3):237–48. https://doi.org/10.2165/11207150-000000000-00000.
Vynckier AK, De Beer M, Monteyne T, Voorspoels J, De Beer T, Remon JP, et al. Enteric protection of naproxen in a fixed-dose combination product produced by hot-melt co-extrusion. Int J Pharmac. 2015;491(1–2):243–9. https://doi.org/10.1016/j.ijpharm.2015.06.010.
O'Connor JP, Taneike I, O'Morain C. Improving compliance with Helicobacter pylori eradication therapy: when and how? Ther Adv Gastroenterol. 2009;2(5):273–9. https://doi.org/10.1177/1756283x09337342.
Kalfus IN, Raday G, Graham DY. Randomized placebo-controlled phase III study to assess the safety and efficacy of rifabutin triple therapy (RHB-105) for Helicobacter pylori infection in dyspepsia patients. Gastroenterology. 2017; 152(5). doi:https://doi.org/10.1016/s0016-5085(17)31130-7.
Kinoshita Y, Kusano M, Iwakiri K, Fujishiro M, Tachikawa N, Haruma K. Efficacy and safety profile of Z-215 (azeloprazole sodium), a proton pump inhibitor, compared with rabeprazole sodium in patients with reflux esophagitis: a phase II, multicenter, randomized, double-blind, comparative study. Curr Ther Res Clin Exp. 2018;88:26–34. https://doi.org/10.1016/j.curtheres.2018.03.004.
de Bortoli N, Martinucci I, Giacchino M, Blandizzi C, Marchi S, Savarino V, et al. The pharmacokinetics of ilaprazole for gastro-esophageal reflux treatment. Expert Opin Drug Metab Toxicol. 2013;9(10):1361–9. https://doi.org/10.1517/17425255.2013.813018.
Scarpignato C, Hunt RH. Proton pump inhibitors: the beginning of the end or the end of the beginning? Curr Opin Pharmacol. 2008;8(6):677–84. https://doi.org/10.1016/j.coph.2008.09.004.
Hunt RH, Armstrong D, Yaghoobi M, James C. The pharmacodynamics and pharmacokinetics of S-tenatoprazole-Na 30 mg, 60 mg and 90 mg vs. esomeprazole 40 mg in healthy male subjects. Aliment Pharmacol Ther. 2010;31(6):648–57. https://doi.org/10.1111/j.1365-2036.2009.04219.x.
Kodama K, Fujisaki H, Kubota A, Kato H, Hirota K, Kuramochi H, et al. E3710, a new proton pump inhibitor, with a long-lasting inhibitory effect on gastric acid secretion. J Pharmacol Exp Ther. 2010;334(2):395–401. https://doi.org/10.1124/jpet.110.167783.
Toda R, Shiramoto M, Komai E, Yoshii K, Hirayama M, Kawabata Y. Pharmacokinetics and pharmacodynamics of azeloprazole sodium, a novel proton pump inhibitor, in healthy Japanese volunteers. J Clin Pharmacol. 2018;58(4):425–33. https://doi.org/10.1002/jcph.1038.
Cheng D, Dai X, Zhang Y, Wu Y, Shi C, Ma X, et al. Determination of anaprazole in human plasma by LC-MS/MS in pharmacokinetic study. Acta Pharmac Sin. 2016;51(12):1885–90.
Wulandari AS, Tandrasasmita OM, Tjandrawinata RR. Bioactive fraction DLBS2411 from Cinnamomum burmanii, (nees and t. nees) blume as colon and gastroprotector by stimulating muc5ac and cyclooxygenase-2 gene expression. Int J Pharmacy Pharmac Sci. 2016;8(8):202–7.
Huang JQ, Hunt RH. pH, healing rate, and symptom relief in patients with GERD. Yale J Biol Med. 1999;72(2–3):181–94.
Inatomi N, Matsukawa J, Sakurai Y, Otake K. Potassium-competitive acid blockers: advanced therapeutic option for acid-related diseases. Pharmacol Ther. 2016;168:12–22. https://doi.org/10.1016/j.pharmthera.2016.08.001.
Kim HK, Park SH, Cheung DY, Cho YS, Kim JI, Kim SS, et al. Clinical trial: inhibitory effect of revaprazan on gastric acid secretion in healthy male subjects. J Gastroenterol Hepatol. 2010;25(10):1618–25. https://doi.org/10.1111/j.1440-1746.2010.06408.x.
Chung IS, Choi MG, Park S-H, Kim S-K, Chang R, Hyun J-H, et al. Revaprazan (Revanex®), a novel acid pump antagonist, for duodenal ulcer: results of a double-blind, randomized, parallel, multi-center phase III clinical trial. Korean J Gastrointest Endosc. 2005;31(1):17–24.
Chang R, Chung IS, Park S-H, Kim S-K, Reyol Choi S-R, Song G-A, et al. Phase III clinical trial of Revaprazan (Revanex®) for gastric ulcer. Korean J Gastrointest Endosc. 2007;34(6):312–9.
Kahrilas PJ, Dent J, Lauritsen K, Malfertheiner P, Denison H, Franzen S, et al. A randomized, comparative study of three doses of AZD0865 and esomeprazole for healing of reflux esophagitis. Clin Gastroenterol Hepatol. 2007;5(12):1385–91. https://doi.org/10.1016/j.cgh.2007.08.014.
Dent J, Kahrilas PJ, Hatlebakk J, Vakil N, Denison H, Franzen S, et al. A randomized, comparative trial of a potassium-competitive acid blocker (AZD0865) and esomeprazole for the treatment of patients with nonerosive reflux disease. Am J Gastroenterol. 2008;103(1):20–6. https://doi.org/10.1111/j.1572-0241.2007.01544.x.
Garnock-Jones KP. Vonoprazan: first global approval. Drugs. 2015;75(4):439–43. https://doi.org/10.1007/s40265-015-0368-z.
Shin JM, Inatomi N, Munson K, Strugatsky D, Tokhtaeva E, Vagin O, et al. Characterization of a novel potassium-competitive acid blocker of the gastric H,K-ATPase, 1-[5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrol-3-yl]-N-methyl methanamin e monofumarate (TAK-438). J Pharmacol Exp Ther. 2011;339(2):412–20. https://doi.org/10.1124/jpet.111.185314.
• Echizen H. The first-in-class potassium-competitive acid blocker, vonoprazan fumarate: pharmacokinetic and pharmacodynamic considerations. Clin Pharmacokin. 2016;55(4):409–18. https://doi.org/10.1007/s40262-015-0326-7 Detailed review on the pharmacokinetics and pharmacodynamics of the first P-CAB, vonoprazan.
Otake K, Sakurai Y, Nishida H, Fukui H, Tagawa Y, Yamasaki H, et al. Characteristics of the novel potassium-competitive acid blocker vonoprazan fumarate (TAK-438). Adv Ther. 2016;33(7):1140–57. https://doi.org/10.1007/s12325-016-0345-2.
Martinucci I, Blandizzi C, Bodini G, Marabotto E, Savarino V, Marchi S, et al. Vonoprazan fumarate for the management of acid-related diseases. Expert Opin Pharmacother. 2017;18(11):1145–52. https://doi.org/10.1080/14656566.2017.1346087.
Yang X, Li Y, Sun Y, Zhang M, Guo C, Mirza IA, et al. Vonoprazan: a novel and potent alternative in the treatment of acid-related diseases. Dig Dis Sci. 2018;63(2):302–11. https://doi.org/10.1007/s10620-017-4866-6.
Sugano K. Vonoprazan fumarate, a novel potassium-competitive acid blocker, in the management of gastroesophageal reflux disease: safety and clinical evidence to date. Ther Adv Gastroenterol. 2018;11:1756283x17745776. https://doi.org/10.1177/1756283x17745776.
Sakurai Y, Nishimura A, Kennedy G, Hibberd M, Jenkins R, Okamoto H, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of single rising TAK-438 (vonoprazan) doses in healthy male Japanese/non-Japanese subjects. Clin Transl Gastroenterol. 2015;6:e94. https://doi.org/10.1038/ctg.2015.18.
Jenkins H, Sakurai Y, Nishimura A, Okamoto H, Hibberd M, Jenkins R, et al. Randomised clinical trial: safety, tolerability, pharmacokinetics and pharmacodynamics of repeated doses of TAK-438 (vonoprazan), a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2015;41(7):636–48. https://doi.org/10.1111/apt.13121.
Kagami T, Sahara S, Ichikawa H, Uotani T, Yamade M, Sugimoto M, et al. Potent acid inhibition by vonoprazan in comparison with esomeprazole, with reference to CYP2C19 genotype. Aliment Pharmacol Ther. 2016;43(10):1048–59. https://doi.org/10.1111/apt.13588.
Ohkuma K, Iida H, Inoh Y, Kanoshima K, Ohkubo H, Nonaka T, et al. Comparison of the early effects of vonoprazan, lansoprazole and famotidine on intragastric pH: a three-way crossover study. J Clin Biochem Nutr. 2018;63(1):80–3. https://doi.org/10.3164/jcbn.17-128.
Sakurai Y, Mori Y, Okamoto H, Nishimura A, Komura E, Araki T, et al. Acid-inhibitory effects of vonoprazan 20 mg compared with esomeprazole 20 mg or rabeprazole 10 mg in healthy adult male subjects—a randomised open-label cross-over study. Aliment Pharmacol Ther. 2015;42(6):719–30. https://doi.org/10.1111/apt.13325.
Yuan Y, Hunt RH. Intragastric pH holding time of pH <4 predicts low erosive esophagitis (EE) healing rate. Gastroenterology. 2010;138(Suppl 1):S-651.
Yuan Y, Hunt RH. An updated model of intragastric pH and erosive esophagitis (EE) healing using 24-hour intragastric pH values to predict EE healing: validation by data from TAK-390MR. Gastroenterology. 2009;136(Suppl 1):A440–A1.
Ashida K, Sakurai Y, Nishimura A, Kudou K, Hiramatsu N, Umegaki E, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther. 2015;42(6):685–95. https://doi.org/10.1111/apt.13331.
Ashida K, Sakurai Y, Hori T, Kudou K, Nishimura A, Hiramatsu N, et al. Randomised clinical trial: vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the healing of erosive oesophagitis. Aliment Pharmacol Ther. 2016;43(2):240–51. https://doi.org/10.1111/apt.13461.
Hoshino S, Kawami N, Takenouchi N, Umezawa M, Hanada Y, Hoshikawa Y, et al. Efficacy of vonoprazan for proton pump inhibitor-resistant reflux esophagitis. Digestion. 2017;95(2):156–61. https://doi.org/10.1159/000456072.
Ashida K, Iwakiri K, Hiramatsu N, Sakurai Y, Hori T, Kudou K, et al. Maintenance for healed erosive esophagitis: phase III comparison of vonoprazan with lansoprazole. World J Gastroenterol. 2018;24(14):1550–61. https://doi.org/10.3748/wjg.v24.i14.1550.
Kinoshita Y, Sakurai Y, Shiino M, Kudou K, Nishimura A, Miyagi T, et al. Evaluation of the efficacy and safety of Vonoprazan in patients with nonerosive gastroesophageal reflux disease: a phase III, randomized, double-blind, placebo-controlled, multicenter study. Curr Ther Res Clin Exp. 2016;81-82:1–7. https://doi.org/10.1016/j.curtheres.2016.12.001.
Niikura R, Yamada A, Hirata Y, Hayakawa Y, Takahashi A, Shinozaki T et al. Efficacy of Vonoprazan for Gastroesophageal Reflux Symptoms in Patients with Proton Pump Inhibitor-resistant Non-erosive Reflux Disease. Intern Med (Tokyo, Japan). 2018. doi:https://doi.org/10.2169/internalmedicine.0492-17.
Kawami N, Hoshino S, Hoshikawa Y, Takenouchi N, Umezawa M, Hanada Y, et al. Pathogenesis of potassium-competitive acid blocker-resistant non-erosive reflux disease. Digestion. 2018;98(3):194–200. https://doi.org/10.1159/000488530.
Thung I, Aramin H, Vavinskaya V, Gupta S, Park JY, Crowe SE, et al. Review article: the global emergence of Helicobacter pylori antibiotic resistance. Aliment Pharmacol Ther. 2016;43(4):514–33. https://doi.org/10.1111/apt.13497.
Gatta L, Scarpignato C. Editorial: Helicobacter pylori resistance and sequential therapy-authors’ reply. Aliment Pharmacol Ther. 2018;48(1):96–7. https://doi.org/10.1111/apt.14676.
Jung YS, Kim EH, Park CH. Systematic review with meta-analysis: the efficacy of vonoprazan-based triple therapy on Helicobacter pylori eradication. Aliment Pharmacol Ther. 2017;46(2):106–14. https://doi.org/10.1111/apt.14130.
Dong SQ, Singh TP, Wei X, Yao H, Wang HL. Review: a Japanese population-based meta-analysis of vonoprazan versus PPI for Helicobacter pylori eradication therapy: Is superiority an illusion? Helicobacter. 2017; 22(6). doi:https://doi.org/10.1111/hel.12438.
Li M, Oshima T, Horikawa T, Tozawa K, Tomita T, Fukui H, et al. Systematic review with meta-analysis: vonoprazan, a potent acid blocker, is superior to proton-pump inhibitors for eradication of clarithromycin-resistant strains of Helicobacter pylori. Helicobacter. 2018;23(4):e12495. https://doi.org/10.1111/hel.12495.
Tanabe H, Yoshino K, Ando K, Nomura Y, Ohta K, Satoh K, et al. Vonoprazan-based triple therapy is non-inferior to susceptibility-guided proton pump inhibitor-based triple therapy for helicobacter pylori eradication. Ann Clin Microbiol Antimicrob. 2018;17(1):29. https://doi.org/10.1186/s12941-018-0281-x.
Furuta T, Sahara S, Ichikawa H, Kagami T, Uotani T, Yamade M et al. Dual therapy with vonoprazan and amoxicillin is as effective as standard PPI-based triple therapy with amoxicillin and clarithromycin or metronidazole in Japan. Gastroenterology. 2016; 150(4). https://doi.org/10.1016/s0016-5085(16)32958-4.
• Graham DY, Dore MP. Update on the use of vonoprazan: a competitive acid blocker. Gastroenterology. 2018;154(3):462–6. https://doi.org/10.1053/j.gastro.2018.01.018 Thoughtful remarks on current and potential clinical use of vonoprazan.
Kim EH, Park CH. Vonoprazan-based Helicobacter pylori eradication therapy: time to get competitive? Dig Dis Sci. 2017;62(11):2955–7. https://doi.org/10.1007/s10620-017-4699-3.
Kawai T, Oda K, Funao N, Nishimura A, Matsumoto Y, Mizokami Y, et al. Vonoprazan prevents low-dose aspirin-associated ulcer recurrence: randomised phase 3 study. Gut. 2018;67(6):1033–41. https://doi.org/10.1136/gutjnl-2017-314852.
Mizokami Y, Oda K, Funao N, Nishimura A, Soen S, Kawai T, et al. Vonoprazan prevents ulcer recurrence during long-term NSAID therapy: randomised, lansoprazole-controlled non-inferiority and single-blind extension study. Gut. 2018;67(6):1042–51. https://doi.org/10.1136/gutjnl-2017-314010.
Leontiadis GI, Howden CW. The role of proton pump inhibitors in the management of upper gastrointestinal bleeding. Gastroenterol Clin N Am. 2009;38(2):199–213. https://doi.org/10.1016/j.gtc.2009.03.008.
Greenspoon J, Barkun A. The pharmacological therapy of non-variceal upper gastrointestinal bleeding. Gastroenterol Clin N Am. 2010;39(3):419–32. https://doi.org/10.1016/j.gtc.2010.08.002.
Takahashi N, Take Y. Tegoprazan, a novel potassium-competitive acid blocker to control gastric acid secretion and motility. J Pharmacol Exp Ther. 2018;364(2):275–86. https://doi.org/10.1124/jpet.117.244202.
Tajimi M, Nii T, Takahashi N, Yamamoto T, Koizumi S. First-in-human study of the novel acid pump antagonist, RQ-00000004, demonstrated a rapid elevation of gastric pH following single oral administration in healthy subjects. Gastroenterology. 2011; 140(5). doi:https://doi.org/10.1016/s0016-5085(11)60324-7.
Han S, Choi HY, Kim YH, Nam JY, Song GS, Lim HS, et al. Safety, tolerability, pharmacokinetics, and pharmacodynamics of escalating single and multiple Oral doses of CJ-12420 (tegoprazan), a novel potassium-competitive acid blocker (P-CAB) in healthy male subjects. Clin Ther. 2017;39(8):e97–e8. https://doi.org/10.1016/j.clinthera.2017.05.306.
Lee C-H, Lee S-C, Lee Y-I,EOM D-K,Han RR, Koh EJ. Novel 4-methoxy pyrrole derivatives or salts thereof and pharmaceutical composition comprising the same. WO 2016/175555 A2.
Sunwoo J, Oh J, Moon SJ, Ji SC, Lee SH, Yu KS, et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of DWP14012, a novel potassium-competitive acid blocker, in healthy male subjects. Aliment Pharmacol Ther. 2018;48(2):206–18. https://doi.org/10.1111/apt.14818.
Unge P, Andersson K. A first-in-human, open-label, healthy volunteer study of the new P-CAB X842 demonstrating 24h acid control for treatment of acid related diseases. Gastroenterology. 2017;154(6, Suppl 1):S-238. https://doi.org/10.1016/S0016-5085(18)31174-0.
Heidelbaugh JJ, Kim AH, Chang R, Walker PC. Overutilization of proton-pump inhibitors: what the clinician needs to know. Ther Adv Gastroenterol. 2012;5(4):219–32. https://doi.org/10.1177/1756283x12437358.
Cammarota S, Bruzzese D, Sarnelli G, Citarella A, Menditto E, Riegler S, et al. Proton pump inhibitors prescribing following the introduction of generic drugs. Eur J Clin Investig. 2012;42(10):1068–78. https://doi.org/10.1111/j.1365-2362.2012.02696.x.
Gupta R, Garg P, Kottoor R, Munoz JC, Jamal MM, Lambiase LR, et al. Overuse of acid suppression therapy in hospitalized patients. South Med J. 2010;103(3):207–11. https://doi.org/10.1097/SMJ.0b013e3181ce0e7a.
Ahrens D, Chenot JF, Behrens G, Grimmsmann T, Kochen MM. Appropriateness of treatment recommendations for PPI in hospital discharge letters. Eur J Clin Pharmacol. 2010;66(12):1265–71. https://doi.org/10.1007/s00228-010-0871-9.
Parente F, Cucino C, Gallus S, Bargiggia S, Greco S, Pastore L, et al. Hospital use of acid-suppressive medications and its fall-out on prescribing in general practice: a 1-month survey. Aliment Pharmacol Ther. 2003;17(12):1503–6.
Wermeling M, Himmel W, Behrens G, Ahrens D. Why do GPs continue inappropriate hospital prescriptions of proton pump inhibitors? A qualitative study. Eur J Gen Pract. 2014;20(3):174–80. https://doi.org/10.3109/13814788.2013.844787.
Johnell K, Klarin I. The relationship between number of drugs and potential drug-drug interactions in the elderly: a study of over 600,000 elderly patients from the Swedish prescribed drug register. Drug Saf. 2007;30(10):911–8.
Marengoni A, Pasina L, Concoreggi C, Martini G, Brognoli F, Nobili A, et al. Understanding adverse drug reactions in older adults through drug-drug interactions. Eur J Intern Med. 2014;25(9):843–6. https://doi.org/10.1016/j.ejim.2014.10.001.
• Savarino V, Tosetti C, Benedetto E, Compare D, Nardone G. Appropriateness in prescribing PPIs: a position paper of the Italian Society of Gastroenterology (SIGE)—study section “digestive diseases in primary care”. Dig Liver Dis. 2018. https://doi.org/10.1016/j.dld.2018.07.004. Position paper of the Italian Society of Gastroenterology on PPI appropriateness.
Thompson W, Hogel M, Li Y, Thavorn K, O'Donnell D, McCarthy L et al. Effect of a proton pump inhibitor deprescribing guideline on drug usage and costs in long-term care. J Am Med Dir Assoc. 2016; 17(7): 673 e1–4. https://doi.org/10.1016/j.jamda.2016.04.020.
Hunt R, Sachs G. A review of the status of omeprazole: the Hambury workshop. Dig Dis Sci. 1995;40(2 Suppl):1s–131s.
•• Vaezi MF, Yang YX, Howden CW. Complications of proton pump inhibitor therapy. Gastroenterology. 2017;153(1):35–48. https://doi.org/10.1053/j.gastro.2017.04.047 The most critical and detailed appraisal of the reported PPI adverse events, with main focus on biological plausibility and strength of association.
• Freedberg DE, Kim LS, Yang YX. The risks and benefits of long-term use of proton pump inhibitors: expert review and best practice advice from the American Gastroenterological Association. Gastroenterology. 2017;152(4):706–15. https://doi.org/10.1053/j.gastro.2017.01.031 Best practice recommendations from the AGA on PPI use, taking into account benefits and risks.
Kinoshita Y, Ishimura N, Ishihara S. Advantages and disadvantages of long-term proton pump inhibitor use. J Neurogastroenterol Motil. 2018;24(2):182–96. https://doi.org/10.5056/jnm18001.
Savarino E, Marabotto E, Zentilin P, Furnari M, Bodini G, Pellegatta G, et al. A safety review of proton pump inhibitors to treat acid-related digestive diseases. Exp Opin Drug Saf. 2018;17(8):785–94. https://doi.org/10.1080/14740338.2018.1497155.
•• Malfertheiner P, Kandulski A, Venerito M. Proton-pump inhibitors: understanding the complications and risks. Nature Rev Gastroenterol Hepatol. 2017;14(12):697–710. https://doi.org/10.1038/nrgastro.2017.117 Detailed review analyzing the level of evidence for the association between PPIs and the reported adverse events, with recommendations on management of some undesirable effects.
Laine L, Nagar A. Long-term PPI use: balancing potential harms and documented benefits. Am J Gastroenterol. 2016;111(7):913–5. https://doi.org/10.1038/ajg.2016.156.
Yeomans ND, Dent J. Personal review: alarmism or legitimate concerns about long-term suppression of gastric acid secretion? Aliment Pharmacol Ther. 2000;14(3):267–71. https://doi.org/10.1046/j.1365-2036.2000.00750.x.
Author information
Authors and Affiliations
Contributions
Both authors designed the flow chart and methodology of the paper. Each of them carried out an independent systematic search of the relevant literature using Medline/PubMed, Embase, and the Cochrane databases. Search outputs were discussed and distilled, paying more attention to systematic reviews and meta-analyses (where available). Each author then wrote given sections, amended by the other co-author. The final manuscript was then prepared, revised critically, and approved before its submission,
Corresponding author
Ethics declarations
Conflict of Interest
Carmelo Scarpignato has served as a speaker, consultant and/or advisory board member for Alfasigma, Pfizer, Takeda, Reckitt-Benkiser and Shionogi, and has, in the past, received funding from Giuliani Pharmaceuticals and Pfizer. Richard H Hunt has served as a speaker, a consultant, and an advisory board member for AstraZeneca, Danone, GSK, Merck, Pfizer, and Takeda.
Human and Animal Rights and Informed Consent
This article does not contain (but only mention) any specific studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Stomach
Rights and permissions
About this article
Cite this article
Hunt, R.H., Scarpignato, C. Potent Acid Suppression with PPIs and P-CABs: What’s New?. Curr Treat Options Gastro 16, 570–590 (2018). https://doi.org/10.1007/s11938-018-0206-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11938-018-0206-y