Abstract
Purpose of review
The anthracycline (AC) group of drugs is widely used for cancer chemotherapy and has improved outcomes in many childhood malignancies. However, cardiovascular complications are major causes of morbidity and mortality in AC recipients, with the greatest risk factor being a higher cumulative dosage. The purpose of this review is to describe the etio-pathogenesis and risk factors of AC induced cardiotoxicity, with emphasis on currently available and emerging modalities of non-invasive imaging in its surveillance, and to review guidelines on its prevention and treatment.
Recent findings
Presently, ejection fraction and shortening fraction derived from two-dimensional echocardiography are the most widely used parameter for monitoring of cardiac function in childhood cancer survivors. The newer speckle tracking echocardiography has shown potential to detect abnormalities in ventricular function prior to the conventional measures such as ejection fraction and shortening fraction. When available, three-dimensional echocardiography should be used as it allows for more accurate estimation of ejection fraction. Newer magnetic resonance imaging (MRI) techniques, such as delayed enhancement and T1 mapping, are useful adjuncts for cardiac evaluation in cancer survivors, especially in patients with poor echocardiographic windows.
Summary
Early detection and management of cardiovascular diseases is one of the major goals in the long-term follow-up of childhood cancer survivors. In addition to conventional two-dimensional echocardiography, newer techniques such as speckle tracking echocardiography and three-dimensional echocardiography should be incorporated due to its ability to detect early changes in anthracycline-induced cardiotoxicity. However further research are needed to guide changes in management due to abnormalities in speckle tracking echocardiography.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The incidence of childhood cancer has increased by 0.6% each year since 1975, with an estimate of 10,590 newly diagnosed malignancies in 2018 in children below 14 years of age [1]. Fortunately, the childhood cancer death rate has decreased by more than two thirds from 6.5 per 100,000 cases in 1969 to 1.5 per 100,000 cases in 2015 [1]. The estimated prevalence of cancer survivors in 2016 was 15.5 million, 429,000 of whom were diagnosed under the age of 20 years [2]. Due to the rapid increase in the number of childhood cancer survivors (CCSs), there is a critical need to focus on long-term outcomes in this population. The CCS study reports that 62% of 10,397 cancer survivors had chronic health conditions and 27.5% had life threatening or disabling conditions by a mean age of 26.6 years and 9.1 times (95% CI 8.9 to 9.4) higher risk for death, compared to the general population [3, 4]. At 5 years of follow-up, cardiovascular disorders constituted the 2nd most common cause of morbidity and mortality in this population, following secondary or recurrent malignancies [4]. According to the British CCS study, in survivors aged 60 years or older, the leading cause of mortality was cardiovascular disease (37%) [4]. Hence, the primary goals in the long-term follow-up of the CCS are prevention, early identification, and prompt treatment of cardiotoxicity. In this review, we describe the etio-pathogenesis and risk factors of cardiotoxicity, with emphasis on currently available and emerging modalities of non-invasive imaging for surveillance, and review guidelines on its prevention and treatment.
Risk factors of cardiotoxicity
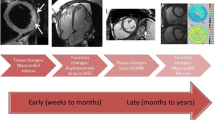
A major risk factor for cardiotoxicity in CCSs is exposure to chemotherapeutic agents, of which the anthracycline (AC) group of drugs poses the highest risk. Chemotherapy-related cardiotoxicity can be acute (within 1 week), early onset (within 1 year), or late onset (more than 1 year of exposure) [5]. While a higher AC cumulative dose increases the risk for development of cardiotoxicity, there is no safe dose [6]. Concomitant mediastinal or spinal radiotherapy is an independent risk factor for late cardiotoxicity and is associated with premature coronary artery disease (due to atherosclerosis), diastolic dysfunction, restrictive cardiomyopathy, and systolic dysfunction [7, 8]. Valvular heart disease and conduction abnormalities are noted in survivors of Hodgkin’s disease exposed to radiation therapy [8]. Concomitant exposure to other chemotherapeutic agents such as alkylating agents (cyclophosphamide, busulfan, ifosfamide), antimetabolites (5-fluorouracil, cytarabine, capecitabine), antimicrotubular agents (vinca alkaloids), monoclonal antibodies (trastuzumab, bevacizumab, rituximab), and tyrosine kinase inhibitors (imatinib, sorafenib, sunitinib, dasatinib) increases the risk of cardiotoxicity [9]. Younger age at diagnosis, female gender, longer duration of follow-up, and presence of preexisting cardiovascular disease are additional risk factors [6, 10]. Traditional cardiovascular risk factors such as diabetes, hypertension, obesity, dyslipidemia, metabolic syndrome, and smoking, alcohol, stimulant drug abuse, and physical inactivity also increase the risk of long-term chemotherapy-related cardiotoxicity in CCSs [11].
Mechanism of cardiotoxicity
Even though the exact mechanism of AC-mediated cardiotoxicity is not fully understood, experimental models suggest several possibilities. Cardiomyocyte damage could be related to iron mediated generation of free radicals or disruption of sarcomere maintenance [12]. Deletion of topoisomerase IIb specific to cardiac myocytes in mice models has been shown to protect from doxorubicin induced double-stranded DNA damage [13]. Other putative mechanisms include defective mitochondrial function, mitochondrial iron accumulation, and impairment of the cardiac progenitor cells [14].
Surveillance of cardiotoxicity
Echocardiography
Evaluation of the left ventricle
Systolic function
Conventional echocardiography
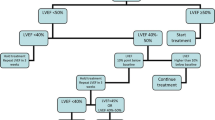
Echocardiography is a widely used tool for cardiac function evaluation before, during, and after the completion of chemotherapy. It is easily available, less expensive, non-invasive, and safe. Fractional shortening (FS) and ejection fraction (EF) on M-mode or two-dimensional echocardiograms (2D) are the most commonly used measures of left ventricular (LV) function assessment. However, these measures are load dependent (preload and afterload) and altered by anemia, fever, and sepsis. The FS and EF are additionally affected by ventricular geometry, have measurement variability, and may not detect early changes in the LV function [15]. Rather, decrease in LVEF may occur only after substantial and potentially irreversible myocardial damage. In addition, LVEF as measured by the biplane disc method (modified Simpson’s) from the apical four and two chamber views relies on the visualization of the endocardial borders [16]. The empiric guidelines published by the Children’s Cancer Study Group suggest that decrease in LVEF by 10% or below 55% or decrease in LVFS by 10% or below 29% on two successive occasions, preferably using echocardiogram and radio nucleotide angiography, indicates significant deterioration of myocardial function and that the chemotherapeutic agents should be discontinued [17]. A decline in LVEF occurs only after substantial myocardial damage, wherein the changes are likely to be irreversible. The recovery of LVEF and reduction in adverse cardiac events may be possible with early detection of cardiac dysfunction and prompt initiation of ACE inhibitor and beta blocker [18].
In children with Hodgkin’s disease exposed to mediastinal irradiation, LV mass (p < 0.001) and LV end diastolic dimension (p < 0.001) were found to be decreased [9]. LV myocardial mass can be measured by M-mode and two- and three-dimensional (2D and 3D) echocardiographies [15, 19]. The relationship of end systolic wall stress to velocity of circumferential shortening has been studied as a load-independent measure in CCSs. Doxorubicin therapy has shown to cause progressive increase in end systolic wall stress (64.6 ± 22.4 g per square centimeter compared to normal, 47.5 ± 7.0, p < 0.001) and is attributed to decreased (0.68 ± 0.2 vs. 0.81 ± 0.09, p < 0.001) LV wall thickness in cancer patients, compared to controls [10]. However, obtaining this parameter is cumbersome and hence not used in every day clinical practice.
Speckle tracking echocardiography
Recently, speckle tracking echocardiography (STE) has gained popularity in the evaluation of ventricular function. Myocardial strain, the ratio of change in length (ΔL) to the baseline length (L), is a measure of regional myocardial deformation during different phases of cardiac cycle. It is a dimensionless measure, expressed as percentage, where negative strain indicates shortening and positive strain indicates lengthening [20]. Longitudinal strain (LS) denotes deformation along the long axis, circumferential strain (CS) along the circumference, and radial strain (RS) measures thickening of the LV during cardiac cycle [21]. Strain can be measured by tissue Doppler imaging (TDI), 2D and 3D STE, tagged cardiac MRI, or velocity vector imaging. Unlike TDI, STE is easy to perform, independent of angle, and has greater reproducibility [22]. Studies have shown that LS does not show maturational changes or alter with varying heart rates in growing children. [23]. In adults with heart failure and reduced EF, global longitudinal strain (GLS) is an independent predictor of adjusted mortality and has superior prognostic value compared to other echocardiographic measures [24••]. A meta-analysis of 2325 children has defined the reference values for strain measures by 2D-STE [25••].
In a study of 19 pediatric cancer patients receiving AC, the LV GLS decreased at 4 months (− 18.1 ± 2.5% vs − 20.5 ± 1.5%, p = 0.011) and at 8 months (4.360.1%, p = .044), whereas LVEF decreased only at 8 months (4.360.1%, P = .044). The average LS in the mid and the apical segments correlated significantly with change in EF [26]. Among 25 pediatric cancer patients (aged 9.8 ± 5.8 years), GLS was abnormal in 60% (15 patients) and peak CS was abnormal in 76% (19 patients) compared to age-matched controls immediately after completion of chemotherapy [27]. The St. Jude lifetime cohort study analyzed data from 1820 CCSs at a median age of 31 (range 18–65) years; age at diagnosis of cancer was 22.6 (range 10.4–48.3) years. Again, rates of abnormal GLS (31.8%) and abnormal GCS (23.1%) were higher than of abnormal LVEF (5.8%) measured by 3D echocardiography; 28% of survivors with normal LVEF (> 50%) had abnormal GLS [28••]. A recent meta-analysis of 14 studies evaluating the natural history of myocardial strain in CCSs exposed to AC therapy showed that abnormalities in GLS are common during or within one year of completion of chemotherapy [29••]. After 1 year of completion of chemotherapy, GLS was not significantly different between survivors and controls but reduction in CS and RS was more consistent [29••]. However, studies have shown that longitudinal strain has greatest reproducibility followed by CS and RS [30]. Also LS derived by STE had greater agreement to MRI derived LS compared to CS [31]. A summary of the studies evaluating STE in CCSs is shown in Table 1.
We reviewed echocardiograms of 41 asymptomatic CCSs aged 12.7 ± 3.8 years at a median follow-up of 4.73 (inter quartile range 2.15–8) years. The GLS (− 17.6 ± 2.7 vs. − 21.3 ± 2.0, p < 0.001) and LS measured from two-chamber (− 18.6 ± 3.2 vs. − 21.3 ± 2.5, p < 0.001), three-chamber (− 16.3 ± 6.0 vs. − 21.7 ± 3.0, p < 0.001), and four-chamber views (− 17.6 ± 2.7 vs. − 20.8 ± 2.0, p < 0.001) were lower compared to 72 healthy controls matched for age, weight and height. The CS was also decreased compared to controls (− 20.8 ± 4.3 vs. − 23.5 ± 2.6, p < 0.001) [39]. The CCSs had lower systolic LS measurements in all but four of the 16 segments of the LV (basal inferior, basal anterior, mid inferior, and mid-inferolateral) [39]. In a small study of 22 CCSs (median age 14.8 years) at 1.9 years after completion of chemotherapy, an improvement in GLS (− 13.83 ± 0.74% to − 15.94 ± 0.74%, p = 0.002), GCS (− 18.79 ± 1.21% to − 20.74 ± 0.84%, p = 0.027), LS rate (− 0.78 ± 0.04 to − 0.88 ± 0.04/s, p = 0.022), and CS rate (− 1.08 ± 0.07 to − 1.21 ± 0.06/s, p = 0.027) was noted after a median duration of 41 (range 14–379) days of angiotensin converting enzyme inhibitors/angiotensin receptor blockers, which persisted for more than a year [40••].
We performed strain analysis in 89 cancer patients aged 8.4 ± 5.2 years prior to receiving chemotherapy and found a lower LS in two-chamber (− 19.8 ± 3.0 vs. − 23.5 ± 4.0, p < 0.001), three-chamber (− 19.4 ± 3.2 vs. − 23.4 ± 4.0, p < 0.001), and four-chamber views (− 19.7 ± 3.4 vs. − 22.5 ± 3.0, p < 0.001) and lower GLS (− 19.8 ± 2.9 vs. − 23.4 ± 3.2, p < 0.001) compared to 82 matched healthy controls, although FS was normal (35.8 ± 5.2 vs. 36.1 ± 6.1. p − 0.75) [41]. A recent report from the American Society of Echocardiography (ASE) and European Society of Cardiovascular Imaging (ESCVI) recommends measurement of GLS before, during, and after chemotherapy in the long-term follow-up of cancer survivors. A decrease in GLS of more than 15% from the baseline value is likely abnormal, whereas a decrease less than 8% is unlikely to be meaningful [42]. There is increasing evidence that CCSs have strain abnormalities before changes in EF. STE is emerging as a useful imaging modality in identifying CCSs at high risk for cardiotoxicity. However, high-quality studies on management based on strain abnormalities in CCSs are not available.
Diastolic function
Diastolic dysfunction has been reported in several studies in CCSs exposed to AC. Among 151 children aged 11.0 ± 5.6 years with various malignancies, the mitral valve E/A ratio was found to be abnormal and decreased compared to 151 healthy controls (2.5 ± 0.8 vs. 3.0 ± 0.9, p < 0.001) at a median follow-up of 8 months (1 day to 16 years). However, the tricuspid valve E/A ratio (1.5 ± 0.7 vs. 1.7 ± 0.7, p < 0.001) was similar to controls [43]. At a follow-up of 5.3 ± 4 years in 63 CCSs exposed to a median dose of 165 mg/m2 of AC, diastolic function measured by transmitral Doppler velocities and TDI velocities was within normal range [44]. At 22.3 years from diagnosis, the diastolic function assessed by e′ was decreased in 138 survivors of childhood acute lymphoid leukemia exposed to AC (median dose of 120 mg/m2), compared to 138 matched controls (11 vs. 12.6 cm/s, p < 0.001) [45•]. In the St. Jude lifetime cohort study, 5.8% CCSs had decreased LVEF by 3D echocardiography, whereas diastolic dysfunction was reported in 11%. Among survivors with normal LVEF (> 50%) by 3D echocardiography, abnormal diastolic function (ASE grades 1–3) was present in 8.7%. The prevalence of diastolic dysfunction was 22.4% in survivors exposed to radiotherapy alone [28••]. The presence of metabolic syndrome increases the risk of diastolic dysfunction (RR 1.68, 95%CI 1.39–2.03) [28••]. At a median duration of 8–9 years from chemotherapy, the high-risk group (mean ACT dose 344 mg/m2) among 80 CCSs had low E/A ratio at rest compared to controls (1.43 vs. 1.93, p = 0.008) despite similar systolic FS and EF. Aerobic capacity during exercise was normal with augmentation of systolic and diastolic function [46].
Global function
Myocardial performance index (MPI) is used to assess global LV function and is calculated as the ratio of sum of times spent in isovolumetric contraction phase and isovolumetric relaxation phase to the LV ejection time. There are relatively few studies on MPI in CCSs with equivocal results. In CCSs aged 11.0 ± 5.6 years, at a median duration of 8.1 months (range 1 day–16 years) after completion of AC (mean dose 200 ± 101 mg/m2), LV MPI was similar to controls (0.34 ± 0.1 vs. 0.34 ± 0.07, p not significant); right ventricle (RV) MPI was lower (0.21 ± 0.1 vs. 0.28 ± 0.08, p − 0.001) although the difference was unlikely to be clinically significant [43]. A cross-sectional study of 100 high-risk survivors (> 300 mg/m2 AC) had higher MPI compared to 50 low risk survivors (exposed to < 300 mg/m2) and healthy controls (0.51 vs. 0.46 vs. 0.46, p < 0.01) [47].
Evaluation of the RV
While LV function has been extensively studied in CCSs, little is known about the effect of chemotherapeutic agents on RV function. About 30% of 246 adult survivors of childhood lymphoma and acute lymphoid leukemia had RV systolic dysfunction at a mean follow-up of 21.7 years after diagnosis. Echocardiographic measures such as RV fractional area change (44.5 vs. 48.6%, p < 0.001), tricuspid annular planar systolic excursion (TAPSE) (2.24 vs. 2.49 cm, p < 0.001), tissue Doppler derived peak systolic tricuspid annular velocity (12.1 vs. 13.0 cm/s, p 0.001), and RV-free wall strain (− 26.5 vs. − 28.4%, p < 0.001) were significantly lower in survivors compared to 211 matched controls [48•]. RV dysfunction occurred three times more in survivors with LV dysfunction, possibly due to interventricular dependence [48•]. Murbraech et al. reported impaired RV function in 6.2% of lymphoma survivors (n = 274) compared to 0.7% of controls (n = 222). Decreases in TAPSE (22.9 ± 4.1 vs. 27.0 ± 4.2 mm, p < 0.001), RV fractional area change (44 ± 5 vs. 48 ± 5, p < 0.001), and RV-free wall strain (− 27.1 ± 4.0 vs. − 30.0 ± 2.6, p < 0.001) compared to healthy controls were also noted. Most of the patients with RV dysfunction (15 out of 17) also had LV dysfunction, although the overall prevalence of RV dysfunction (6.2%) was less than LV dysfunction (30.8%) [49•]. Abnormalities in RV-free wall strain in the basal segments (− 33 ± 13* vs.40 ± 16, p < 0.05) compared to controls, although not in the apical (− 38 ± 18 vs. − 41 ± 13) and mid portion (− 43 ± 15 vs. − 47 ± 12) segments, were seen at a median duration of 5.2 (range 2 to 15.2) years following exposure to a cumulative AC dose of 240 (range 90–300) mg/m2 [50]. Armstrong et al. studied 10 adult cancer survivors exposed to radiation therapy who had elevated tricuspid regurgitation velocity of > 2.8 m/s and decreased exercise tolerance (dyspnea and/or less than 600 m on 6 min walk test) [51]. Agha et al. reported decrease in tricuspid valve E:A ratio (1.03 ± 0.37 vs. 1.29 ± 0.27, p < 0.01) and increase in MPI (0.36 ± 0.08 vs. 0.32 ± 0.06, p < 0.01), after exposure to chemotherapy [33•]. The RV MPI correlated with the LV GLS (r = − 0.44, p < 0.001) indicating the role of interventricular dependence in RV dysfunction [33•]. Longitudinal studies are needed to evaluate RV function including strain analysis at baseline, during and long after completion of chemotherapy and the correlation of RV dysfunction with adverse cardiac outcomes.
Three-dimensional echocardiography
The 3D echocardiography overcomes the geometric limitations of 2D echocardiography. For the assessment of ventricular volumes and EF, three-dimensional echocardiography has been shown to be superior to 2D echocardiography and comparable to the volumes derived by cardiac MRI [52]. 3D real-time echocardiography-derived LV volumes, EF, and mass have good intra and interobserver correlation. LV function analysis in 50 patients by 2D and 3D real-time echocardiography and MRI showed that the LV end diastolic volume derived by 3D differed only slightly from that of MRI (mean difference − 4, p = 0.31), whereas 2D-derived LV end-diastolic volume differed significantly (mean difference − 28, p value < 0.001) [52]. Measurement of strain using 3D echocardiography has allowed tracking of the myocardial speckle in three dimensions and enabled the assessment of all LV segments from the same data set. In 53 CCSs aged 18.6 ± 5.1 years exposed to a median dose of 229 mg/m2 of AC, global 3D strain was lower compared to healthy controls (35.4 ± 7.5 vs. 44.6 ± 7.8, p < 0.001) at a median follow-up of 7.2 years. All segments except the basal anteroseptal had decreased 3D strain compared to controls (p < 0.05) [38]. In addition to measuring the GLS, CS, and RS, 3D echocardiography is useful to estimate measures of LV mechanics such as LV torsion and LV systolic dyssynchrony index (shown in Fig. 1) [53].
Evaluation of ventricular mechanics
The arrangement of the myofibers in the LV changes from a right handed helix pattern in the sub endocardial layer to a left-handed helix arrangement in the sub epicardium. This allows for efficient contraction with minimal loss of energy [54]. During systole, the LV contracts with the base rotating in the clockwise direction and the apex in the counterclockwise direction to achieve a wringing motion [55]. Torsion is defined as the difference in the base to apex gradient in rotational angle along the long axis of the LV and is expressed in degrees per centimeter or radians per meter [56]. These mechanics augment ejection of the blood from the LV [55]. Similarly during diastole, untwisting of the LV aids in relaxation and filling. Cheung et al. studied 36 survivors of childhood cancer aged 15.6 ± 5.5 years at a median duration of 7 years (range 3.1–24.3 years) following completion of AC chemotherapy (median dose of 240 mg/m2, range 120–470 mg/m2). CCSs had decreased LV torsion (8.0 ± 4.1 vs.11.8 ± 4.5, p = 0.003), systolic twisting velocity (68.1 ± 20.3 vs.91.0 ± 22.3, p < 0.001) and diastolic untwisting velocity (− 90.1 ± 34.3 vs. − 109.6 ± 33.4 0.04) compared to controls. These findings were noted to be secondary to reduced LV apical rotation (p = 0.003) and apical untwisting rate (p = 0.002). Even survivors (78%) with LVEF > 50% had evidence of deceased LV torsion, apical untwisting rate (p = 0.01) and LV systolic twisting velocity (p = 0.001) [57]. LV dyssynchrony index is calculated as the mean standard deviation of time to peak systolic strain of the 16 segments of the LV divided by RR interval [58•]. CCSs with wall motion abnormalities had higher LV systolic dyssynchrony index derived from RS compared to those without wall motion abnormalities (16.5 ± 5.1vs. 8.5 ± 6.0, p − 0.01) [58•]. The global CS (− 23.5 ± 3.8 vs. − 31.3 ± 8.1, p = 0.025) and global RS (14.3 ± 6.1 vs. 33.1 ± 10.1, p < 0.001) were also lower in CCS with wall motion abnormalities compared to those without abnormalities [58•].
Magnetic resonance imaging
Cardiac MRI is emerging as a useful modality for the diagnosis of various cardiovascular diseases including cardiotoxicity in CCSs. Although echocardiography is the most commonly used modality in the assessment of LVEF, it has limited utility in patients with poor acoustic windows. In such cases, MRI is useful in assessment of cardiac structure and function. It is the gold standard for assessment of ventricular volumes and EF. Cardiac MRI also has great utility in imaging the right ventricle, which is often inadequately visualized by echocardiography. In addition, MRI provides information on not only ventricular function but also tissue characterization. One of the mechanisms of AC-mediated cardiotoxicity is myocyte loss leading to myocardial fibrosis [59], which is evident on histological studies obtained from RV biopsies and autopsy specimens of AC-exposed cancer patients [17]. Focal myocardial fibrosis can be evaluated by late enhancement after administration of gadolinium chelated contrast agent [60]. Contrast-enhanced MRI also allows differentiation between ischemic (involving subendocardial layer) and non-ischemic (affecting other layers) causes of fibrosis such as AC-mediated cardiac fibrosis [61].
In a study of 62 CCSs aged 14.6 ± 3.2 years, at a median duration of 7.8 years (4.9 to 18.0 years) following exposure to AC (median cumulative dose of 222, 80 to 419 mg/m2), 11 (18%) survivors had decreased LVEF (< 45%) and 38 (61%) had subnormal LV function (LVEF 45–55%) [62]. RV function was decreased in 17 (27%) and subnormal in 33 (53%) survivors. Surprisingly, late gadolinium enhancement was not present in any of the patients [62]. Lunning et al. evaluated cardiac MRI in 10 non-Hodgkin’s lymphoma patients before and 3 months after chemotherapy [63]. Five patients developed a significant (≥ 10%) drop in LVEF, and three patients had at least one new or progressive segment of gadolinium delayed enhancement [63].
More recently, the use of myocardial T1 mapping has gained popularity due to its ability to detect intrinsic myocardial properties. In addition, the T1 relaxation time can be used to calculate the extracellular volume of distribution of gadolinium in the myocardium, which has been found to be increased in the presence of diffuse myocardial fibrosis [64]. In a study of 30 CCSs aged 15.2 ± 2.7 years exposed to a cumulative dose of 197.2 ± 84.3 (mg/m2) of AC, at 7.6 ± 4.5 years follow-up, T1 relaxation times (1155.3 ± 56.5) were increased and T1 values were longer in those exposed to higher AC dose (r = 0.52, p = 0.052) and those with decreased LV mass/volume ratio ((r = − 0.54, p − 0.027). The increased extracellular volume also correlated with higher AC dose (r = 0.40), decreased peak VO2(r = − 0.52), decreased LV wall thickness/height ratio (r = − 0.72), and decreased LV mass/volume ratio (r = − 0.64). There was no late gadolinium enhancement [65]. The limitations of cardiac MRI are that it is time-consuming, more costly compared to echocardiography, less easily available, and often needs sedation and trained physicians for interpretation.
Computed tomography
Computed tomographic (CT) scans are useful in the surveillance of cardiotoxicity. They provide greater spatial resolution, with good visualization of vessels and calcified tissue, in a short exam time. CT scan is the only non invasive imaging modality that can assess the coronary arteries. Its disadvantage is exposure to ionizing radiation and the need for contrast which limits its utility in patients with renal failure. Among nine long-term survivors of Hodgkin’s disease, eight had coronary artery disease detected by CT scan. Long segment disease and focal stenosis due to plaque were demonstrated, and calcium scores were higher than the reference range for this age group [66]. CT scan allows visualization of pericardial calcification and hence is useful in the diagnosis of constrictive pericarditis, which is a dreaded complication in cancer survivors exposed to mediastinal radiotherapy [66]. In survivors with valvular heart disease, CT scan can provide three-dimensional information about the pathology of valvular apparatus and the mechanism of stenosis or regurgitation [67••].
Biomarkers for detection of LV dysfunction
Both NT-pro BNP and troponin, cardiac myocyte proteins can be elevated during or shortly after receiving chemotherapy [68]. Elevated troponin in the first 3 months of doxorubicin therapy was found to be associated with lower LV mass and lower LV posterior wall thickness at 4.1 years follow-up [68]. Therefore, an acute rise in troponin during chemotherapy may predict the risk of late onset LV dysfunction. However, troponin is not a useful biomarker for detection of late onset cardiotoxicity [69•]. NT-pro B type natriuretic peptide was shown to have low sensitivity and positive predictive value, with high specificity and negative predictive value for late onset cardiotoxicity [69•]. In a study of 63 long-term CCSs, plasma B natriuretic peptide levels showed mild elevation in survivors with cardiac dysfunction compared to those without dysfunction (23.4 ± 25.3 vs. 14.2 ± 8.9 pg/ml, p 0.02), although the values were not clinically significant [70]. In a systematic review of eight studies, both NT-pro B natriuretic peptide and troponin were shown to have limited utility as diagnostic biomarkers of late onset LV systolic dysfunction (reduced LVEF) in CCSs.
Cardiopulmonary exercise response
In the St. Jude lifetime cohort study, 17.6% of CCSs had reduced exercise tolerance (< 490 m on 6 min walk test). Survivors with abnormal GLS had lower exercise tolerance determined by 6 min walk test compared to those with normal GLS (560 vs. 590 m, p = 0.0002). A reduced maximum volume of oxygen consumption (VO2 max) was demonstrated in 47% of 138 survivors of acute lymphoid leukemia at a median duration of 23.4 years after diagnosis and was more common in those exposed to AC (56%) compared to those who were not exposed to AC (17%, p < 0.001) [45•]. Thirty percent of survivors of Hodgkin’s disease had severe decrease of VO2 max to below 20 ml/kg/m2 at 14.3 years from diagnosis [8]. They were also noted to have blunted blood pressure response (27%), monotonous heart rate response (57%), and persistent tachycardia (31%) indicating autonomic dysfunction [8]. In a 10-year follow-up of CCSs who underwent AC chemotherapy and had normal baseline LVEF, the biventricular systolic and diastolic response (strain and tissue Doppler indexed) to exercise were similar to that of healthy controls [71].
Prevention and treatment of cardiotoxicity
Due to the progressive nature of the myocardial damage, vigilant surveillance and closer follow-up of high-risk survivors are essential to detect, treat, and prevent worsening of late onset cardiotoxicity. Dexrazoxane is a cardioprotective agent which acts by iron chelation, decreases free radical generation, and alters the topoisomerase activity to decrease binding to AC [72,72,73,74,76]. Lipshultz et al. have shown higher troponin T in the group treated with Doxorubicin alone compared to the group treated with dexrazoxane and doxorubicin [68]. In addition, studies have shown that dexrazoxane does not interfere with oncologic efficacy and hence is not associated with an increased risk of secondary malignancies, while providing cardio protection [77••]. The Children’s Oncology group administers dexrazoxane routinely in patients who are exposed to > 150 mg/m2 of AC or any AC dose with concomitant radiotherapy [78] and the use of dexrazoxane for cardioprotection has been endorsed by the American Heart Association and the American Academy of Pediatrics [79]. Doxil, a liposomal form of doxorubicin, has been shown to decrease myocardial damage due to decreased free circulating levels of doxorubicin [80]. Animal studies have shown cardioprotection with nutritional supplements such as coenzyme Q, selenium, vitamin C, and vitamin E, but their usage in humans is not approved [81,81,83]. Altering the modifiable risk factors for cardiovascular disease in survivors such as physical inactivity, obesity, smoking and alcohol consumption and prevention and management of hypertension, diabetes, metabolic syndrome, and dyslipidemia is beneficial in CCSs and would also delay the onset of cardiotoxicity.
In asymptomatic patients with decreased LVEF, the use of angiotensin converting enzyme inhibitors or beta blockers has not shown to be beneficial [84]. In patients with symptomatic heart failure, enalapril decreases afterload and has been shown to cause transient improvement in LV function but did not prevent progression of thinning of the LV [85]. In patients with symptomatic heart failure, diuretic therapy can provide symptomatic relief. The use of carvedilol was not associated with improvement in clinical outcomes [86]. In patients with decompensated heart failure, refractory to medical therapy, cardiac transplantation is indicated [87]. Mechanical devices such as pulsatile or continuous flow ventricular assist device or extracorporeal membranous oxygenation can be used to support these patients as a bridge to cardiac transplantation [87, 88].
Conclusion
The field of cardio-oncology has undergone rapid advancements over the last three decades due to increase in cancer survivorship. Due to the irreversible nature of chemotherapy-related cardiotoxicity, the goals in the long-term care of these patients are early detection of subclinical cardiovascular dysfunction and prevention of progression of late cardiotoxicity in addition to treatment of cardiotoxicity. Although most commonly used, the conventional echocardiographic parameters such as ejection fraction and shortening fraction may not detect early cardiovascular damage in childhood cancer survivors. Speckle tracking echocardiography is a useful adjunct and has been shown to detect decline in cardiac function prior to conventional echocardiography. Use of three-dimensional echocardiography is superior to 2D echocardiography in the assessment of ventricular function and should be used when available. However, in patients with poor acoustic windows, the estimation of EF and strain analysis is inaccurate. In this scenario, cardiac MRI provides accurate assessment of cardiac volumes and function. With the use of T1 mapping and extracellular volume assessment, cardiac MRI detects myocardial tissue changes such as fibrosis. Despite significant advancements in early detection of cardiac damage, evidence-based recommendations for prevention or altering cardiotoxicity in pediatric cancer patients are lacking.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Society AC. Cancer facts & figures 2018. Atlanta. 2018.
Institute NC. Childhood Cancer Survivor Study: an overview https://www.cancer.gov/types/childhood-cancers/ccss. 2018 Accessed December 19, 2018.
Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–82. https://doi.org/10.1056/NEJMsa060185.
Mertens AC, Liu Q, Neglia JP, Wasilewski K, Leisenring W, Armstrong GT, et al. Cause-specific late mortality among 5-year survivors of childhood cancer: the Childhood Cancer Survivor Study. J Natl Cancer Inst. 2008;100(19):1368–79. https://doi.org/10.1093/jnci/djn310.
Adams MJ, Lipshultz SE. Pathophysiology of anthracycline- and radiation-associated cardiomyopathies: implications for screening and prevention. Pediatr Blood Cancer. 2005;44(7):600–6. https://doi.org/10.1002/pbc.20352.
Lipshultz SE, Lipsitz SR, Mone SM, Goorin AM, Sallan SE, Sanders SP, et al. Female sex and higher drug dose as risk factors for late cardiotoxic effects of doxorubicin therapy for childhood cancer. N Engl J Med. 1995;332(26):1738–43. https://doi.org/10.1056/NEJM199506293322602.
Orzan F, Brusca A, Conte MR, Presbitero P, Figliomeni MC. Severe coronary artery disease after radiation therapy of the chest and mediastinum: clinical presentation and treatment. Br Heart J. 1993;69(6):496–500.
Adams MJ, Lipsitz SR, Colan SD, Tarbell NJ, Treves ST, Diller L, et al. Cardiovascular status in long-term survivors of Hodgkin’s disease treated with chest radiotherapy. J Clin Oncol. 2004;22(15):3139–48. https://doi.org/10.1200/JCO.2004.09.109.
Lipshultz SE, Cochran TR, Franco VI, Miller TL. Treatment-related cardiotoxicity in survivors of childhood cancer. Nat Rev Clin Oncol. 2013;10(12):697–710. https://doi.org/10.1038/nrclinonc.2013.195.
Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324(12):808–15. https://doi.org/10.1056/NEJM199103213241205.
Akam-Venkata J, Franco VI, Lipshultz SE. Late cardiotoxicity: issues for childhood cancer survivors. Curr Treat Options Cardiovasc Med. 2016;18(7):47. https://doi.org/10.1007/s11936-016-0466-6.
Canzoneri JC, Oyelere AK. Interaction of anthracyclines with iron responsive element mRNAs. Nucleic Acids Res. 2008;36(21):6825–34. https://doi.org/10.1093/nar/gkn774.
Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, et al. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18(11):1639–42. https://doi.org/10.1038/nm.2919.
Huang C, Zhang X, Ramil JM, Rikka S, Kim L, Lee Y, et al. Juvenile exposure to anthracyclines impairs cardiac progenitor cell function and vascularization resulting in greater susceptibility to stress-induced myocardial injury in adult mice. Circulation. 2010;121(5):675–83. https://doi.org/10.1161/CIRCULATIONAHA.109.902221.
Borow KM, Neumann A, Marcus RH, Sareli P, Lang RM. Effects of simultaneous alterations in preload and afterload on measurements of left ventricular contractility in patients with dilated cardiomyopathy: comparisons of ejection phase, isovolumetric and end-systolic force-velocity indexes. J Am Coll Cardiol. 1992;20(4):787–95.
Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18(12):1440–63. https://doi.org/10.1016/j.echo.2005.10.005.
Steinherz LJ, Graham T, Hurwitz R, Sondheimer HM, Schwartz RG, Shaffer EM, et al. Guidelines for cardiac monitoring of children during and after anthracycline therapy: report of the Cardiology Committee of the Childrens Cancer Study Group. Pediatrics. 1992;89(5 Pt 1):942–9.
Cardinale D, Colombo A, Lamantia G, Colombo N, Civelli M, De Giacomi G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55(3):213–20. https://doi.org/10.1016/j.jacc.2009.03.095.
Lopez L, Colan SD, Frommelt PC, Ensing GJ, Kendall K, Younoszai AK, et al. Recommendations for quantification methods during the performance of a pediatric echocardiogram: a report from the Pediatric Measurements Writing Group of the American Society of Echocardiography Pediatric and Congenital Heart Disease Council. J Am Soc Echocardiogr. 2010;23(5):465–95; quiz 576-467. https://doi.org/10.1016/j.echo.2010.03.019.
Mirsky I, Parmley WW. Assessment of passive elastic stiffness for isolated heart muscle and the intact heart. Circ Res. 1973;33(2):233–43.
Bansal M, Kasliwal RR. How do I do it? Speckle-tracking echocardiography. Indian Heart J. 2013;65(1):117–23. https://doi.org/10.1016/j.ihj.2012.12.004.
Amundsen BH, Helle-Valle T, Edvardsen T, Torp H, Crosby J, Lyseggen E, et al. Noninvasive myocardial strain measurement by speckle tracking echocardiography: validation against sonomicrometry and tagged magnetic resonance imaging. J Am Coll Cardiol. 2006;47(4):789–93. https://doi.org/10.1016/j.jacc.2005.10.040.
Lorch SM, Ludomirsky A, Singh GK. Maturational and growth-related changes in left ventricular longitudinal strain and strain rate measured by two-dimensional speckle tracking echocardiography in healthy pediatric population. J Am Soc Echocardiogr. 2008;21(11):1207–15. https://doi.org/10.1016/j.echo.2008.08.011.
•• Sengelov M, Jorgensen PG, Jensen JS, Bruun NE, Olsen FJ, Fritz-Hansen T, et al. Global longitudinal strain is a superior predictor of all-cause mortality in heart failure with reduced ejection fraction. JACC Cardiovasc Imaging. 2015;8(12):1351–9. https://doi.org/10.1016/j.jcmg.2015.07.013 A study indicating that decreased global longitudinal strain is an independent predictor of all-cause mortality in 1065 heart failure patients with reduced ejection fraction.
•• Levy PT, Machefsky A, Sanchez AA, Patel MD, Rogal S, Fowler S, et al. Reference ranges of left ventricular strain measures by two-dimensional speckle-tracking echocardiography in children: a systematic review and meta-analysis. J Am Soc Echocardiogr. 2016;29(3):209–225 e206. https://doi.org/10.1016/j.echo.2015.11.016 Meta-analysis of 2325 healthy children providing reference range for left ventricular global longitudinal strain and global circumferential strain.
Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25(7):733–40. https://doi.org/10.1016/j.echo.2012.04.007.
Pignatelli RH, Ghazi P, Reddy SC, Thompson P, Cui Q, Castro J, et al. Abnormal myocardial strain indices in children receiving anthracycline chemotherapy. Pediatr Cardiol. 2015;36(8):1610–6. https://doi.org/10.1007/s00246-015-1203-8.
•• Armstrong GT, Joshi VM, Ness KK, Marwick TH, Zhang N, Srivastava D, et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J Am Coll Cardiol. 2015;65(23):2511–22. https://doi.org/10.1016/j.jacc.2015.04.013 St. Jude lifetime cohort study evaluating 1820 adult survivors of childhood cancer exposed to chemotherapy and radiotherapy. One third of the survivors with normal left ventricular ejection fraction had either decraesed global longitudinal strain or decreased diastolic function.
•• Tuzovic M, Wu PT, Kianmahd S, Nguyen KL. Natural history of myocardial deformation in children, adolescents, and young adults exposed to anthracyclines: systematic review and meta-analysis. Echocardiography. 2018;35(7):922–34. https://doi.org/10.1111/echo.13871 A meta-analysis reviewing the natural history of myocardial deformation in childhood cancer survivors exposed to anthracycline. The global longitudinal strain was decreased before and immediately after chemotherapy, where as the global circumferential strain was decreased in the long-term follow-up.
Leischik R, Dworrak B, Hensel K. Intraobserver and interobserver reproducibility for radial, circumferential and longitudinal strain echocardiography. Open Cardiovasc Med J. 2014;8:102–9. https://doi.org/10.2174/1874192401408010102.
Singh GK, Cupps B, Pasque M, Woodard PK, Holland MR, Ludomirsky A. Accuracy and reproducibility of strain by speckle tracking in pediatric subjects with normal heart and single ventricular physiology: a two-dimensional speckle-tracking echocardiography and magnetic resonance imaging correlative study. J Am Soc Echocardiogr. 2010;23(11):1143–52. https://doi.org/10.1016/j.echo.2010.08.010.
•• Assuncao BMBL, Handschumacher MD, Brunner AM, Yucel E, Bartko PE, Cheng KH, et al. Acute leukemia is associated with cardiac alterations before chemotherapy. J Am Soc Echocardiogr. 2017;30(11):1111–8. https://doi.org/10.1016/j.echo.2017.07.016 Retrospective study reporting abnormal left ventricular global longitudinal strain in adult acute leukemia patients even before exposure to chemotherapy.
• Agha H, Shalaby L, Attia W, Abdelmohsen G, Aziz OA, Rahman MY. Early ventricular dysfunction after anthracycline chemotherapy in children. Pediatr Cardiol. 2016;37(3):537–44. https://doi.org/10.1007/s00246-015-1311-5 A retrospective study evluating the conventoinal and speckle tracking echocardiography in pre- and post-chemotherapy evaluation of left and right ventricles. Study showed that compared to baseline, the post-chemotherapy left ventricular systolic function such as global longitudinal strain and right ventricular diastolic function were decreased.
Al-Biltagi M, Abd Rab Elrasoul Tolba O, El-Shanshory MR, Abd El-Aziz El-Shitany N, El-Sayed El-Hawary E. Strain echocardiography in early detection of doxorubicin-induced left ventricular dysfunction in children with acute lymphoblastic leukemia. ISRN Pediatrics. 2012;2012:9. https://doi.org/10.5402/2012/870549.
Ganame J, Claus P, Eyskens B, Uyttebroeck A, Renard M, D’hooge J, et al. Acute cardiac functional and morphological changes after anthracycline infusions in children. Am J Cardiol. 2007;99:974–7. https://doi.org/10.1016/j.amjcard.2006.10.063.
Cheung YF, Hong WJ, Chan GC, Wong SJ, Ha SY. Left ventricular myocardial deformation and mechanical dyssynchrony in children with normal ventricular shortening fraction after anthracycline therapy. Heart. 2010;96(14):1137–41. https://doi.org/10.1136/hrt.2010.194118.
Mavinkurve-Groothuis AM, Groot-Loonen J, Marcus KA, Bellersen L, Feuth T, Bokkerink JP, et al. Myocardial strain and strain rate in monitoring subclinical heart failure in asymptomatic long-term survivors of childhood cancer. Ultrasound Med Biol. 2010;36(11):1783–91. https://doi.org/10.1016/j.ultrasmedbio.2010.08.001.
Yu AF, Raikhelkar J, Zabor EC, Tonorezos ES, Moskowitz CS, Adsuar R, et al. Two-dimensional speckle tracking echocardiography detects subclinical left ventricular systolic dysfunction among adult survivors of childhood, adolescent, and young adult cancer. Biomed Res Int. 2016;2016:9363951. https://doi.org/10.1155/2016/9363951.
Venkata JA, Kadiu G, Aggarwal S Effect of presence of cancer in pre-chemotherapy stage in children on left ventricle function as assessed by speckle echocardiography. In: American Society of Echocardiography—annual scientific sessions, Baltimore, MD. 2017. Journal of the American Society of Echocardiography,
•• Harrington JK, Richmond ME, Fein AW, Kobsa S, Satwani P, Shah A. Two-dimensional speckle tracking echocardiography-derived strain measurements in survivors of childhood cancer on angiotensin converting enzyme inhibition or receptor blockade. Pediatr Cardiol. 2018;39(7):1404–12. https://doi.org/10.1007/s00246-018-1910-z Study reporting improvement in the left ventricular global longitudinal strain and strain rate and improvement in global circumferential strain and strain rate in after treatment with angiotensin converting enzyme inhibitor/angiotensin receptor blockers in childhood cancer survivors.
Kadiu G, Venkata JA, Aggarwal S. Speckle tracking abnormalities of global left ventricle strain in children following chemotherapy. J Am Soc Echocardiogr 2016;29.
Plana JC, Galderisi M, Barac A, Ewer MS, Ky B, Scherrer-Crosbie M, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27(9):911–39. https://doi.org/10.1016/j.echo.2014.07.012.
Stapleton GE, Stapleton SL, Martinez A, Ayres NA, Kovalchin JP, Bezold LI, et al. Evaluation of longitudinal ventricular function with tissue Doppler echocardiography in children treated with anthracyclines. J Am Soc Echocardiogr. 2007;20(5):492–7. https://doi.org/10.1016/j.echo.2006.10.011.
Harahsheh A, Aggarwal S, Pettersen MD, L’Ecuyer T. Diastolic function in anthracycline-treated children. Cardiol Young. 2015;25(6):1130–5. https://doi.org/10.1017/S1047951114001760.
• Christiansen JR, Kanellopoulos A, Lund MB, Massey R, Dalen H, Kiserud CE, et al. Impaired exercise capacity and left ventricular function in long-term adult survivors of childhood acute lymphoblastic leukemia. Pediatr Blood Cancer. 2015;62(8):1437–43. https://doi.org/10.1002/pbc.25492 A cross-sectional study of 138 survivors of childhood cancer survivors reporting LV diastolic dysfunction, impaired aerboic capacity, and their association with anthracycline exposure.
Ryerson AB, Border WL, Wasilewski-Masker K, Goodman M, Meacham L, Austin H, et al. Assessing anthracycline-treated childhood cancer survivors with advanced stress echocardiography. Pediatr Blood Cancer. 2015;62(3):502–8. https://doi.org/10.1002/pbc.25328.
Armenian SH, Gelehrter SK, Vase T, Venkatramani R, Landier W, Wilson KD, et al. Screening for cardiac dysfunction in anthracycline-exposed childhood cancer survivors. Clin Cancer Res. 2014;20(24):6314–23. https://doi.org/10.1158/1078-0432.CCR-13-3490.
• Christiansen JR, Massey R, Dalen H, Kanellopoulos A, Hamre H, Ruud E, et al. Right ventricular function in long-term adult survivors of childhood lymphoma and acute lymphoblastic leukaemia. Eur Heart J Cardiovasc Imaging. 2016;17(7):735–41. https://doi.org/10.1093/ehjci/jew018 A study reporting decreased right ventricular function in 30% of 246 survivors of childhood cancer survivors. The RV dysfunction was three times more likely in survivors with LV dysfunction.
• Murbraech K, Holte E, Broch K, Smeland KB, Holte H, Rosner A, et al. Impaired right ventricular function in long-term lymphoma survivors. J Am Soc Echocardiogr. 2016;29(6):528–36. https://doi.org/10.1016/j.echo.2016.02.014 A study of 274 lymphoma survivors reporting impaired right ventriuclar systolic dysfunction compared to 222 controls. The RV function was associated with left ventricular systolic function indicating the interventricular dependence.
Ganame J, Claus P, Uyttebroeck A, Renard M, D’Hooge J, Bijnens B, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr. 2007;20(12):1351–8. https://doi.org/10.1016/j.echo.2007.04.007.
Armstrong GT, Tolle JJ, Piana R, Santucci A, Leathers J, Ness KK, et al. Exercise right heart catheterization for pulmonary hypertension identified on screening echocardiography in adult survivors of childhood cancer: a report from the St. Jude Lifetime Cohort. Pediatr Blood Cancer. 2018;65(1). https://doi.org/10.1002/pbc.26769.
Jenkins C, Bricknell K, Hanekom L, Marwick TH. Reproducibility and accuracy of echocardiographic measurements of left ventricular parameters using real-time three-dimensional echocardiography. J Am Coll Cardiol. 2004;44(4):878–86. https://doi.org/10.1016/j.jacc.2004.05.050.
Kapetanakis S, Kearney MT, Siva A, Gall N, Cooklin M, Monaghan MJ. Real-time three-dimensional echocardiography: a novel technique to quantify global left ventricular mechanical dyssynchrony. Circulation. 2005;112(7):992–1000. https://doi.org/10.1161/CIRCULATIONAHA.104.474445.
Sengupta PP, Tajik AJ, Chandrasekaran K, Khandheria BK. Twist mechanics of the left ventricle: principles and application. JACC Cardiovasc Imaging. 2008;1(3):366–76. https://doi.org/10.1016/j.jcmg.2008.02.006.
Nakatani S. Left ventricular rotation and twist: why should we learn? J Cardiovasc Ultrasound. 2011;19(1):1–6. https://doi.org/10.4250/jcu.2011.19.1.1.
Geyer H, Caracciolo G, Abe H, Wilansky S, Carerj S, Gentile F, et al. Assessment of myocardial mechanics using speckle tracking echocardiography: fundamentals and clinical applications. J Am Soc Echocardiogr. 2010;23(4):351–69; quiz 453-355. https://doi.org/10.1016/j.echo.2010.02.015.
Cheung YF, Lam WW, Ip JJ, Cheuk DK, Cheng FW, Yang JY, et al. Myocardial iron load and fibrosis in long term survivors of childhood leukemia. Pediatr Blood Cancer. 2015;62(4):698–703. https://doi.org/10.1002/pbc.25369.
• Okuma H, Noto N, Tanikawa S, Kanezawa K, Hirai M, Shimozawa K, et al. Impact of persistent left ventricular regional wall motion abnormalities in childhood cancer survivors after anthracycline therapy: assessment of global left ventricular myocardial performance by 3D speckle-tracking echocardiography. J Cardiol. 2017;70(4):396–401. https://doi.org/10.1016/j.jjcc.2016.12.015 A case–control study reporting reduced left ventricular myocardial performance in patients with regional wall motion abnormalities compared to those without regional wall motion abnormalities.
Sawyer DB, Peng X, Chen B, Pentassuglia L, Lim CC. Mechanisms of anthracycline cardiac injury: can we identify strategies for cardioprotection? Prog Cardiovasc Dis. 2010;53(2):105–13. https://doi.org/10.1016/j.pcad.2010.06.007.
Wu E, Judd RM, Vargas JD, Klocke FJ, Bonow RO, Kim RJ. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction. Lancet. 2001;357(9249):21–8. https://doi.org/10.1016/S0140-6736(00)03567-4.
Hunold P, Schlosser T, Vogt FM, Eggebrecht H, Schmermund A, Bruder O, et al. Myocardial late enhancement in contrast-enhanced cardiac MRI: distinction between infarction scar and non-infarction-related disease. AJR Am J Roentgenol. 2005;184(5):1420–6. https://doi.org/10.2214/ajr.184.5.01841420.
Ylanen K, Poutanen T, Savikurki-Heikkila P, Rinta-Kiikka I, Eerola A, Vettenranta K. Cardiac magnetic resonance imaging in the evaluation of the late effects of anthracyclines among long-term survivors of childhood cancer. J Am Coll Cardiol. 2013;61(14):1539–47. https://doi.org/10.1016/j.jacc.2013.01.019.
Lunning MA, Kutty S, Rome ET, Li L, Padiyath A, Loberiza F, et al. Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am J Clin Oncol. 2013.
Wassmuth R, Lentzsch S, Erdbruegger U, Schulz-Menger J, Doerken B, Dietz R, et al. Subclinical cardiotoxic effects of anthracyclines as assessed by magnetic resonance imaging—a pilot study. Am Heart J. 2001;141(6):1007–13.
Tham EB, Haykowsky MJ, Chow K, Spavor M, Kaneko S, Khoo NS, et al. Diffuse myocardial fibrosis by T1-mapping in children with subclinical anthracycline cardiotoxicity: relationship to exercise capacity, cumulative dose and remodeling. J Cardiovasc Magn Reson. 2013;15:48. https://doi.org/10.1186/1532-429X-15-48.
Nielsen KM, Offersen BV, Nielsen HM, Vaage-Nilsen M, Yusuf SW. Short and long term radiation induced cardiovascular disease in patients with cancer. Clin Cardiol. 2017;40(4):255–61. https://doi.org/10.1002/clc.22634.
•• Lancellotti P, Nkomo VT, Badano LP, Bergler-Klein J, Bogaert J, Davin L, et al. Expert consensus for multi-modality imaging evaluation of cardiovascular complications of radiotherapy in adults: a report from the European Association of Cardiovascular Imaging and the American Society of Echocardiography. J Am Soc Echocardiogr. 2013;26(9):1013–32. https://doi.org/10.1016/j.echo.2013.07.005 Expert opinion from the American Society of Echocardiography and European Society of Cardiovascular Imaging on multimodality imaging in evaluation of radiotherapy-related cardiovascular complications in adult cancer patients.
Lipshultz SE, Miller TL, Scully RE, Lipsitz SR, Rifai N, Silverman LB, et al. Changes in cardiac biomarkers during doxorubicin treatment of pediatric patients with high-risk acute lymphoblastic leukemia: associations with long-term echocardiographic outcomes. J Clin Oncol. 2012;30(10):1042–9. https://doi.org/10.1200/JCO.2010.30.3404.
• Leerink JM, Verkleij SJ, Feijen EAM, Mavinkurve-Groothuis AMC, Pourier MS, Ylanen K, et al. Biomarkers to diagnose ventricular dysfunction in childhood cancer survivors: a systematic review. Heart. 2018. https://doi.org/10.1136/heartjnl-2018-313634 A systematic review of eight studies evaluating the utility of biomarkers in asymptomatic childhood cancer survivors. It shows that NT-pro BNP and troponin T have limited utility in the detection of late cardiotoxicity.
Aggarwal S, Pettersen MD, Bhambhani K, Gurczynski J, Thomas R, L’Ecuyer T. B-type natriuretic peptide as a marker for cardiac dysfunction in anthracycline-treated children. Pediatr Blood Cancer. 2007;49(6):812–6. https://doi.org/10.1002/pbc.21100.
Cifra B, Chen CK, Fan CS, Slorach C, Manlhiot C, McCrindle BW, et al. Dynamic myocardial response to exercise in childhood cancer survivors treated with anthracyclines. J Am Soc Echocardiogr. 2018;31(8):933–42. https://doi.org/10.1016/j.echo.2018.02.003.
Wouters KA, Kremer LC, Miller TL, Herman EH, Lipshultz SE. Protecting against anthracycline-induced myocardial damage: a review of the most promising strategies. Br J Haematol. 2005;131(5):561–78. https://doi.org/10.1111/j.1365-2141.2005.05759.x.
Pai VB, Nahata MC. Cardiotoxicity of chemotherapeutic agents: incidence, treatment and prevention. Drug Saf. 2000;22(4):263–302. https://doi.org/10.2165/00002018-200022040-00002.
Schlitt A, Jordan K, Vordermark D, Schwamborn J, Langer T, Thomssen C. Cardiotoxicity and oncological treatments. Dtsch Arztebl Int. 2014;111(10):161–8. https://doi.org/10.3238/arztebl.2014.0161.
Vejpongsa P, Yeh ET. Prevention of anthracycline-induced cardiotoxicity: challenges and opportunities. J Am Coll Cardiol. 2014;64(9):938–45. https://doi.org/10.1016/j.jacc.2014.06.1167.
Lyu YL, Kerrigan JE, Lin CP, Azarova AM, Tsai YC, Ban Y, et al. Topoisomerase IIbeta mediated DNA double-strand breaks: implications in doxorubicin cardiotoxicity and prevention by dexrazoxane. Cancer Res. 2007;67(18):8839–46. https://doi.org/10.1158/0008-5472.CAN-07-1649.
•• Asselin BL, Devidas M, Chen L, Franco VI, Pullen J, Borowitz MJ, et al. Cardioprotection and safety of dexrazoxane in patients treated for newly diagnosed T-cell acute lymphoblastic leukemia or advanced-stage lymphoblastic non-Hodgkin lymphoma: a report of the Children’s Oncology Group Randomized Trial Pediatric Oncology Group 9404. J Clin Oncol. 2016;34(8):854–62. https://doi.org/10.1200/JCO.2015.60.8851 Prospective study proving the cardioprotective effect of dexrazoxane without decreasing the antitumor efficacy and without increasing the frequency of secondary malignancies.
Lipshultz SE, Franco VI, Sallan SE, Adamson PC, Steiner RK, Swain SM, et al. Dexrazoxane for reducing anthracycline-related cardiotoxicity in children with cancer: an update of the evidence. Prog Pediatr Cardiol. 2014;36(1–2):10.
Lipshultz SE, Adams MJ, Colan SD, Constine LS, Herman EH, Hsu DT, et al. Long-term cardiovascular toxicity in children, adolescents, and young adults who receive cancer therapy: pathophysiology, course, monitoring, management, prevention, and research directions: a scientific statement from the American Heart Association. Circulation. 2013;128(17):1927–95. https://doi.org/10.1161/CIR.0b013e3182a88099.
O’Brien ME, Wigler N, Inbar M, Rosso R, Grischke E, Santoro A, et al. Reduced cardiotoxicity and comparable efficacy in a phase III trial of pegylated liposomal doxorubicin HCl (CAELYX/Doxil) versus conventional doxorubicin for first-line treatment of metastatic breast cancer. Ann Oncol. 2004;15(3):440–9.
Danesi F, Malaguti M, Di Nunzio M, Maranesi M, Biagi PL, Bordoni A. Counteraction of adriamycin-induced oxidative damage in rat heart by selenium dietary supplementation. J Agric Food Chem. 2006;54(4):1203–8. https://doi.org/10.1021/jf0518002.
Shimpo K, Nagatsu T, Yamada K, Sato T, Niimi H, Shamoto M, et al. Ascorbic acid and adriamycin toxicity. Am J Clin Nutr. 1991;54(6 Suppl):1298S–301S. https://doi.org/10.1093/ajcn/54.6.1298s.
Wang YM, Madanat FF, Kimball JC, Gleiser CA, Ali MK, Kaufman MW, et al. Effect of vitamin E against adriamycin-induced toxicity in rabbits. Cancer Res. 1980;40(4):1022–7.
Silber JH, Cnaan A, Clark BJ, Paridon SM, Chin AJ, Rychik J, et al. Enalapril to prevent cardiac function decline in long-term survivors of pediatric cancer exposed to anthracyclines. J Clin Oncol. 2004;22(5):820–8. https://doi.org/10.1200/JCO.2004.06.022.
Lipshultz SE, Lipsitz SR, Sallan SE, Simbre VC 2nd, Shaikh SL, Mone SM, et al. Long-term enalapril therapy for left ventricular dysfunction in doxorubicin-treated survivors of childhood cancer. J Clin Oncol. 2002;20(23):4517–22. https://doi.org/10.1200/JCO.2002.12.102.
Shaddy RE, Boucek MM, Hsu DT, Boucek RJ, Canter CE, Mahony L, et al. Carvedilol for children and adolescents with heart failure: a randomized controlled trial. JAMA. 2007;298(10):1171–9. https://doi.org/10.1001/jama.298.10.1171.
Dipchand AI, Naftel DC, Feingold B, Spicer R, Yung D, Kaufman B, et al. Outcomes of children with cardiomyopathy listed for transplant: a multi-institutional study. J Heart Lung Transplant. 2009;28(12):1312–21. https://doi.org/10.1016/j.healun.2009.05.019.
Brancaccio G, Filippelli S, Michielon G, Iacobelli R, Alfieri S, Gandolfo F, et al. Ventricular assist devices as a bridge to heart transplantation or as destination therapy in pediatric patients. Transplant Proc. 2012;44(7):2007–12. https://doi.org/10.1016/j.transproceed.2012.06.034.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Pediatric and Congenital Heart Disease
Rights and permissions
About this article
Cite this article
Akam-Venkata, J., Galas, J. & Aggarwal, S. Cardiovascular Evaluation of Children With Malignancies. Curr Treat Options Cardio Med 21, 14 (2019). https://doi.org/10.1007/s11936-019-0719-2
Published:
DOI: https://doi.org/10.1007/s11936-019-0719-2