Abstract
Cardiotoxicity from chemotherapy is a leading cause of morbidity and mortality in cancer survivors. Cardiotoxic effects include left ventricular systolic dysfunction, coronary artery disease, hypertension, bradycardia, arrhythmias, pericardial disease, valvular disease, and radiation-induced restrictive cardiomyopathy. Noninvasive cardiac imaging has been at the forefront of detecting cardiotoxicity in patients receiving chemotherapeutic agents known to adversely affect cardiac structure and function. Regimens for cardiotoxicity surveillance prior to and during chemotherapy administration have been proposed; however, optimal screening for and treatment of long-term cancer survivors have yet to be clarified. This review focuses on the most common imaging modalities for assessing cardiac dysfunction along with newer imaging technologies, and reviews suggested long-term surveillance strategies in cancer survivors following chemotherapy and radiation therapy.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Cardiotoxicity from chemotherapy is a leading cause of morbidity and mortality in cancer survivors [1]. Certain chemotherapeutic agents such as anthracyclines (used to treat solid and hematologic malignancies), taxanes, alkylating agents, tyrosine kinase inhibitors, the monoclonal antibody trastuzumab (used to treat and thoracic radiation therapy are associated with the development of a wide range of cardiovascular disease manifestations [2, 3]. Cardiotoxic effects include left ventricular systolic dysfunction, coronary artery disease, hypertension, bradycardia, arrhythmias, pericardial disease, valvular disease, and radiation-induced restrictive cardiomyopathy [2]. Chemotherapeutic cardiotoxicity is generally characterized by two types: type I toxicity which is caused by myocyte death resulting in irreversibility and type II toxicity which is caused by myocyte dysfunction thus potentially reversible [4, 5]. These classifications are by no means concrete, as timely institution of therapy has been shown to lead to resolution of the cardiomyopathy observed with type 1 toxicity. There also appears to be preliminary evidence that certain cancers may directly negatively impact cardiac function prior to the initiation of cardiotoxic agents [6].
While there is no universally accepted definition of cardiotoxicity, according to the 2014 Expert Consensus Statement for Multimodality Imaging Evaluation of Adult Patients during and after cancer therapy from the American Society of Echocardiography (ASE) and the European Association of Cardiovascular Imaging (EACVI), cancer therapeutics-related cardiac dysfunction (CTRCD) is defined as a decrease in the left ventricular ejection fraction (LVEF) of >10 % to a value <53 % as measured by 2D echocardiography [7••]. The development of CTRCD often occurs within the first year of therapy [8], but can present as a late complication of cancer therapy and is an important predictor of long-term outcomes [9, 10]. In patients who develop heart failure from therapy, mortality rate as high as 60 % [11] and may result in termination or modification of life-saving cancer therapy.
Noninvasive cardiac imaging has been at the forefront of detecting cardiotoxicity in patients receiving chemotherapeutic agents known to affect cardiac structure and function, particularly anthracycline and trastuzumab which are the most commonly cited agents known to cause CTRCD. Most research has focused on identifying patients at risk for cardiomyopathy and early initiation of treatment prior to the development of clinical symptoms. Importantly, because cardiotoxicity is a well-known late manifestation of certain chemotherapeutic agents (anthracyclines, platinum-based chemotherapy, and/or radiation therapy) serial monitoring of high-risk patients long after therapy has been discontinued is required. Currently, there is a lack of consensus regarding long-term follow-up testing for adult patients exposed to cardiotoxic chemotherapeutic agents and/or radiation therapy.
This review focuses on the most common cardiac imaging modalities used to assess cardiac dysfunction and reviews possible approaches for long-term surveillance strategies in cancer survivors following chemotherapy and radiation therapy.
Imaging Modalities
Nuclear Imaging
Planar equilibrium radionuclide angiocardiography (ERNA), also known as multiple-gated acquisition (MUGA), is an established method to quantify LVEF and was widely used prior to the availability of echocardiography. A patient’s red blood cells are labeled with a technetium-99m blood pool radionuclide agent in vitro and are re-injected followed by image acquisition using ECG-gating. Several studies investigated the role of ERNA in monitoring for cardiotoxicity during and after chemotherapeutic administration [12–14]. Based on this data, roughly 15–20 % of patients have been shown to be at risk for developing cardiotoxicity [14, 15] following chemotherapy.
Guidelines for serial LVEF monitoring using radionuclide imaging suggest that the development of cardiotoxicity, defined by a LVEF decrease by ≥10 % EF from baseline and reaches a final LVEF ≤ 50 % (or to a final LVEF ≤ 30 % in patients with baseline resting LVEF < 50 %), should guide doxorubicin therapy duration (Table 1) [14]. A proposed algorithm (Fig. 1) for trastuzumab monitoring similarly recommends serial monitoring of cardiac function with cessation if LVEF decreases by >10 %, if final LVEF < 40 % or if symptoms of congestive heart failure (CHF) develop [16].
A proposed algorithm for monitoring trastuzumab cardiotoxicity. Adapted from Curigliano et al. [16], with permission from Oxford University Press
Adherence to treatment guidelines with serial ERNA monitoring of LVEF is effective to prevent or limit CHF by early detection and therapy [15], with 87 % of patients treated medically for CHF showing some improvement in the degree of left ventricular systolic dysfunction [14]. The majority of patients in whom screening led to early discontinuation of therapy were asymptomatic, emphasizing the importance of routine monitoring to diagnose subclinical cardiotoxicity.
A major advantage of ERNA is that it is based on radioisotope tracer counts and thus does not rely on LV geometric assumptions or LV endocardial detection as is used in echocardiography. Limitations however, include radiation exposure, difficulty with optimal image acquisition angles, lack of ability to report on pericardial and valvular disease, and ECG gating difficulties in patients with baseline arrhythmias when compared to the gold standard of endomyocardial biopsy [17]. Although ERNA-derived exercise LVEF improves overall sensitivity for detecting patients at moderate or high risk of developing congestive heart failure, it is not frequently performed in clinical practice.
While ERNA remains an effective approach for monitoring LV systolic dysfunction with chemotherapeutic regimens, repeated exposure to ionizing radiation, above-mentioned limitations, and higher costs compared to echocardiography are reasons for the tendency towards using echocardiography for routine screening of cancer patients.
Echocardiography
Echocardiography is the most common cardiac imaging modality for serial monitoring of cardiac function owing to its accessibility, versatility, and lack of radiation exposure. Echocardiography has the additional benefits of assessing both left and ventricular systolic and diastolic function, ventricular wall mechanics, pericardial and valvular disease [7••, 18]. Echocardiography is now commonly used prior to, during, and for short and long term surveillance following cardiotoxic chemotherapy administration depending on the agent used. Algorithms for surveillance during chemotherapy have been suggested and Fig. 2 shows an example of echocardiographic monitoring for type 1 cardiotoxicity [7••].
A proposed algorithm for surveillance in patients with chemotherapy regimens known to cause type 1 cardiotoxicity. Baseline evaluation with echocardiographic imaging is recommended. If there is any abnormality, cardiology should be consulted. If there is no abnormality, follow-up at the completion of therapy and at 6 months should be obtained. Reprinted from Plana et al. [7], with permission from Elsevier
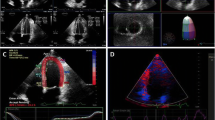
The drawback of 2D echocardiography stems from inherent limitations in how ejection fraction is calculated. Echocardiographic LV volumes are best estimated using the modified Simpson’s (or disk summation) method. In the modified Simpson’s method, orthogonal apical four- and two-chamber views are obtained (Fig. 3). The endocardial borders are traced either automatically or manually and the LV is divided into disks of equal heights. The volume of each cylinder is calculated from the four- and two-chamber image derived diameters and then the volumes are summed to give the total systolic and diastolic LV volume. This method is highly dependent on reliable visualization of the endocardial borders and absence of foreshortening. In addition, this method extrapolates a volume from two 2D slices by assuming that the LV is symmetric which is frequently not the case.
2D echocardiography demonstrating the modified Simpson's method, in the orthogonal apical two-chamber view. The left ventricular endocardial border is traced in end-systole (a) and end-diastole (b) to create disks forming a cylinder. Volumetric change during cardiac cycle allows calculation of left ventricular ejection fraction
Contrast Echocardiography
Ultrasound-based contrast agents improve LV endocardial border visualization thus improving LVEF volumetric assessments (Video 1). Contrast-enhanced 2D echocardiography is superior to 2D non-contrast echocardiography regarding agreements of LV volume and LVEF measurements [19]. In addition, the LV volumes measured with contrast-enhanced 2D echocardiography are larger and closer to the corresponding CMR measurements than those obtained with non-contrast echocardiography. This is probably because tissue harmonic imaging does not track the true endocardial surface as well as contrast echocardiography, resulting in tracking noise in the LV cavity that is perceived as the endocardial border. Addition of contrast echo produced a change in LVEF ≥ 10 % in 17 % of patients with the vast majority of these were due to underestimations of LVEF on the unenhanced echocardiogram [20]. Additional benefits of contrast echocardiography include thrombus detection and more accurate Doppler measurements. Contrast-enhanced 2D echocardiography is particularly useful in patients with poor acoustic windows which are estimated to comprise a large portion of the approximately 20 % suboptimal rate of echocardiography in the USA. Current American Society of Echocardiography (ASE) societal recommendations include the use of contrast agents when suboptimal studies are encountered, as defined by inability to visualize two contiguous LV segments in any apical view [7••].
3D Echocardiography (3DE)
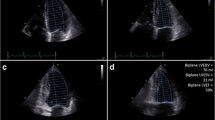
3DE (Fig. 4) adds reliability of volumetric assessment by acquiring pyramidal-shaped volumes which avoid the volumetric assumptions and foreshortening that are inherent to conventional 2D echocardiography [21]. Reliability and reproducibility of LVEF and LV volume assessment is increased with 3D imaging [22, 23]. 3DE is a sensitive modality for monitoring long term survivors of anthracycline and radiation exposure [24]. Operator dependence and adequate endocardial detection are major limitations of 3DE. Nevertheless, assessment of LVEF with 3DE is comparable to cardiac magnetic resonance imaging (CMR) and, based on the 2014 joint statement by the ASE and the European Association of Cardiovascular Imaging, is the preferred technique for monitoring LV function in patients with cancer [7••].
Myocardial Strain Imaging
There has been a growing interest in using myocardial strain imaging to detect subclinical deteriorations in LV systolic function prior to overt changes in LVEF. Detecting subtle myocardial dysfunction has important clinical implications due to a high rate of failure—up to 58 %—for complete recovery of LVEF if a significant delay occurs from the time of diagnosis of LV dysfunction to initiation of pharmacologic therapy [25–27].
Myocardial strain, a physical deformation property of the myocardium, can be assessed using speckle-tracking echocardiography (STE) which tracks natural acoustic reflectors within the myocardium, also known as “speckles”, from frame to frame to determine deformation (strain) and the speed of deformation (strain rate) (Fig. 5). STE has the ability to detect chemotherapy-induced cardiotoxicity through early detection in subtle decreases in ventricular function. Of particular importance, speckle tracking shows cardiotoxic effect of cancer therapy at doses much lower than were previously thought.
Speckle tracking echocardiography illustrating global longitudinal strain in apical four-chamber view (a), apical three-chamber view (b), apical two-chamber view (c), and polar plot (d) in a patient with a normal left ventricular systolic function with a global longitudinal strain of -21.1 % pre-chemotherapy/radiation
Three strain components are generally used to describe left ventricular deformation: longitudinal, circumferential, and radial strain. A systematic review summarized the literature for the use of myocardial strain in patients receiving chemotherapy [25]. The majority of data show earlier recognition of subtle myocardial decline, most commonly in global longitudinal strain prior to a decrease in LVEF [28–30]. A 10–15 % early reduction in GLS by speckle tracking during chemotherapy is the most useful parameter for the prediction of cardiotoxicity (Fig. 6) [7••, 25]. In long-term cancer survivors, circumferential and radial strain is consistently is also abnormal after chemotherapy administration, but is less reliable and are not currently recommended for screening purposes [25].
Early detection of subclinical LV dysfunction using speckle tracking echocardiography. In patients without CTRCD (defined as a drop of 10 points to LVEF < 53 %), strain echocardiography is for the identification of subclinical LV dysfunction. A relative percentage decrease of >15 % compared with baseline is of clinical significance. Reprinted from Plana et al. [7], with permission from Elsevier
Recent data presented in abstract form indicate that patients with untreated cancer have equally reduced myocardial strain as compared to patents post-chemotherapy [6]. This data, together with data showing elevated cardiac biomarker levels prior to chemotherapy [31], highlight the potential direct effect (in the absence of cardiotoxic agents) certain malignancies may have on subclinical cardiac dysfunction.
Stress Echocardiography (SE)
Both exercise and dobutamine SE are established techniques for detection of coronary disease [32] for at-risk patients who are undergoing chemotherapeutic regimens associated with coronary ischemia [2]. Additionally, SE is useful in the evaluation of subclinical LV dysfunction and evaluation of contractile reserve in patients with established cardiotoxicity-related LV dysfunction. Contractile reserve represents the normal physiological augmentation that occurs with exercise or pharmacological stress. A 5-unit decline in LV contractile reserve with dobutamine SE was found to be predictive of subsequent LVEF reduction <50 % due to high-dose chemotherapy-induced cardiotoxicity [33]. Asymptomatic doxorubicin-treated long-term survivors of childhood cancer may have latent decreased cardiac performance that is not detected by resting echocardiography, and evaluation of posterior wall dimension, posterior wall thickness, and end-systolic fractional shortening with dobutamine infusion is a sensitive technique for examining cardiac function in asymptomatic individuals [34]. Although used infrequently for monitoring of chemotherapy-induced cardiotoxicity, SE can potentially allow earlier identification of LV systolic dysfunction following chemotherapy and/or radiation.
Current guidelines of practice recommend either echocardiography (with strain measurements or 3D echocardiography) or ERNA imaging for evaluation of cardiotoxicity as long as the chosen modality is used consistently throughout serial monitoring [10].
Cardiac Magnetic Resonance (CMR)
Cardiac MRI (CMR) has superseded planar ERNA to become the noninvasive reference standard for volumetric measurement of cardiac chamber size and ventricular function [35]. In small subsets, CMR demonstrates the potential to detect subclinical cardiotoxic effects of anthracyclines on cardiac structure and function [36, 37]. Advantages of CMR include highly accurate and reproducible measurements of LVEF, ability to measure myocardial deformation (strain assessment), evaluation of valvular regurgitation, and the detection of myocardial scar by delayed gadolinium enhancement [38]. CMR is valuable in evaluating primary cardiac tumors with or without pericardial involvement and for evaluation of constrictive pericarditis [7••]. While the American College of Cardiology and American Heart Association Guidelines identify CMR as a valid imaging modality for cardiotoxicity screening [39], most centers are not using CMR as the primary imaging modality for screening to characterize effects of chemotherapy-induced cardiotoxicity. In patients for whom standard surveillance imaging (echocardiography, ERNA) demonstrates a decline in LV systolic function, the use of CMR may be a useful approach for further more accurate LVEF evaluation adding tissue characterization, myocardial edema or fibrosis assessment [40]. Overall, CMR should be viewed as a second-line modality in the evaluation of cardiotoxicity, particularly when cardiopulmonary symptoms are present in the absence of systolic dysfunction by echocardiography or ERNA, or if there is equivocal and/or suboptimal assessment in LVEF. However, availability of expertise, higher cost, and exam duration limit its wide clinical utility. The 2014 ASE/ESCAI expert consensus statement advises follow-up imaging with CMR for LVEF <53 % for more precise assessment [5].
Comparison between Echocardiography, Cardiac MRI, and ERNA
Echocardiography was compared to cardiac magnetic resonance imaging (CMR) for detection of cardiomyopathy in 114 adult survivors of childhood cancer (mean age 39 years) treated with anthracycline chemotherapy and/or chest directed radiation therapy. Compared with CMR, 2D echocardiography (biplane method) had a sensitivity of 25 % and a false-negative rate of 75 % for detection of EF less than 50 %, and 3D echocardiography appeared slightly more accurate with 53 and 47 %, respectively. Detection of cardiomyopathy was improved (sensitivity 75 %) using a higher 2D echocardiography cutoff (EF >60 %) to detect an EF less than 50 % by the reference standard CMR. The authors recommend that cancer survivors with exposure to cardiotoxic therapies with an EF 50–59 % by 2D echocardiography should be considered for comprehensive cardiac assessment, which may include CMR [41].
In previous studies, echocardiography was less sensitive and specific compared to ERNA although echocardiographic assessment was limited by non-contemporary echocardiographic assessment such as 3D imaging, myocardial strain imaging, and echo contrast use [42–45].
These include two older studies with small sample sizes have compared LVEF assessment by echocardiography and ERNA. The first (n = 21, median age of 6.9 years) found that ERNA was more sensitive than echocardiography (ERNA detected 29 % patients with a decreased LVEF, compared with 14 % patients with decreased LVEF with echocardiography, p = 0.003) [44]. The second (n = 28 adult patients) showed similar findings for M-mode and 2D echocardiography compared to ERNA [43].
The most comprehensive study from a multi-modality cardiac imaging perspective examined ERNA, 2D-echo, 3D-echo and cardiac magnetic resonance imaging (CMR) for the evaluation of cardiac function during trastuzumab therapy after doxorubicin. All patients received serial imaging using all four modalities at baseline, 6 and 12 months after beginning trastuzumab. The results indicated a weak correlation between 2D-echo and CMR (r = 0.31 at baseline and r = 0.42 at 12 months), whereas the correlation for both 3D-echo and ERNA versus CMR were strong (r = 0.91 at baseline; r = 0.90 at 12 months, respectively [45].
Cardiac Computed Tomography Angiography (CCTA)
Cardiac computed tomography angiography (CCTA) has emerged as a powerful tool for the non-invasive evaluation of the coronary arteries and an alternative to invasive diagnostic angiography. Due to its lower temporal resolution, LVEF assessment by CCTA is felt to be less accurate than conventional cardiac imaging techniques and, to our knowledge, there are no reports of CCTA for the evaluation of cardiotoxicity-induced LV systolic dysfunction. Preliminary data show that CCTA may be useful in evaluating patients with structural or ischemic disease prior to initiation of cancer therapy [46]. CCTA may also have a utility in the evaluation of coronary artery disease in long-term cancer survivors who were previously treated with thoracic radiation or known agents that induce coronary ischemia [47]. This is of particular interest in patients post-radiation who are at risk for developing premature coronary artery disease, particularly ostial coronary disease, manifesting years after radiation therapy. [48, 49]. Further research is necessary to elucidate the precise role of CCTA in monitoring this high-risk population. Coronary artery calcium measurement may have a role in screening for coronary disease in post-chemotherapy and/or radiation survivors [48, 50], but again also requires further validating studies.
Serum Biomarkers
Biomarkers, such as troponin, BNP, and NT-pro-BNP, have the ability to identify patients at risk for cardiotoxicity and have been found to be elevated prior to echocardiographic evidence of LV systolic dysfunction [51]. Even prior to cancer treatment, elevated cardiovascular hormone levels (e.g., NT-proBNP, proadrenomedullin, high sensitivity troponin-T, pro-endothelin-1) in cancer patients are associated with an increase in all-cause mortality [31].
While serum biomarkers can aid in early diagnosis and treatment of patients with potential cardiotoxicity, data shows that isolated biomarker measurements are not reliable predictors of cardiotoxicity [52]. A multimodality approach with integration of biomarkers and cardiac imaging findings has been proposed as an optimal strategy for the early detection of chemotherapy and/or radiation-induced cardiotoxicity. For example, myocardial strain using speckle tracking echocardiography combined with high-sensitive cardiac troponin T is a reliable method to predict cardiac dysfunction [53]. However, further large-scale studies are needed to answer these questions prior to incorporating troponin biomarker levels as part of a routine cardiotoxicity screening protocol.
Approach to Monitoring Cardiac Function Post-Chemotherapy and/or Radiation
In childhood cancer, post-chemotherapy cardiac monitoring showed an increasing incidence of abnormal echocardiographic findings reaching 43 % at 20 years post-doxorubicin therapy, a finding that also appeared to have dose dependence [54]. The Children’s Oncology Group have developed long-term guidelines for post-therapy cardiac screening [55]. Depending on age of therapy, serial LVEF assessment is recommended at differential intervals. For example, in children older than the age of 5 at time of therapy should receive LVEF monitoring every year to 5 years depending on a variety of factors including dose of anthracycline administered and concomitant chest irradiation.
There have been several clinical practice guidelines for surveillance of chemotherapy-induced cardiotoxicy developed independently. The International Late Effects of Childhood Cancer Guideline Harmonization Group [56] created consensus recommendations for cardiomyopathy surveillance in survivors of childhood cancer. Surveillance for cardiotoxicity should be pursued in patients treated with anthracyclines and/or radiation depending on cumulative dose. Echocardiography is the recommended primary surveillance modality, although ERNA or CMR may be reasonable alternatives.
While collaborative effort for long-term surveillance strategies for childhood cancer survivors were created, there are no approved long-term surveillance recommendations for asymptomatic adult cancer survivors at this time.
One proposed screening algorithm [57•], is based upon four assessments: prior cancer therapy, risk factors (such as age > 65 years old, female gender, obesity, hypertension, hyperlipidemia, left ventricular dysfunction, coronary artery disease, acute cardiotoxicity during treatment, cumulative anthracycline dose of >350 mg/m2, chest radiation ≥30 Gy, ≥10 years post-treatment follow up), functional status at initial and follow up visits, and cardiac structure. All survivors are recommended to receive a baseline ECG, echocardiogram, and BNP level. If all three are normal, then the patient is considered stage A, at risk of heart failure but without structural disease or symptoms of heart failure. If a patient is low risk without any new symptoms, then re-evaluation every 2 years with history, physical and BNP is recommended. Only if there is a change in symptoms or abnormality in BNP or ECG then an echocardiogram should be obtained.
High-risk patients, based on risk factors, should have annual re-evaluation. In the absence of any new symptoms or BNP abnormality, an echocardiogram can be performed every 5 years [57•]. A patient with an abnormal echocardiogram at baseline is considered stage B, structural heart disease but without symptoms of heart failure. Treatment should be pursued with re-evaluation every 6 months along with cardiology follow-up [57•]. In addition to the above screening, aggressive management of cardiac risk factors such as weight control, smoking cessation, and lipid control should be emphasized.
Conclusion
CTRCD is associated with elevated cardiac morbidity and mortality in patients during and following cardiotoxic chemotherapy. Several non-invasive cardiac imaging techniques are available for the accurate screening, detection and follow-up of patients at high risk for cardiotoxicity. However, further collaboration between respective cardiology, oncology, and multimodality imaging specialists is required to formulate guidelines that seek to optimize and standardize surveillance regimens in cancer patients. The optimal long-term screening approach for cancer survivors has also yet to be clarified. With greater understanding of tumor biology, similarities in signaling pathways in cancer and heart disease are being uncovered. Close attention should therefore be paid to the potentially deleterious cardiac effects of all new cancer therapeutic agents in development. Solving the burden of CTRCD will require much collaborative work in the fields of cardio-oncology and cardiovascular imaging.
References
Papers of particular interest, published recently, have been highlighted as • Of importance •• Of major importance
Doyle JJ, Neugut AI, Jacobson JS, Grann VR, Hershman DL. Chemotherapy and cardiotoxicity in older breast cancer patients: a population-based study. J Clin Oncol. 2005;23:8597–605.
Yeh ET, Bickford CL. Cardiovascular complications of cancer therapy: incidence, pathogenesis, diagnosis, and management. J Am Coll Cardiol. 2009;53:2231–47.
Khouri MG, Douglas PS, Mackey JR, et al. Cancer therapy-induced cardiac toxicity in early breast cancer: addressing the unresolved issues. Circulation. 2012;126:2749–63.
Ewer MS, Ewer SM. Cardiotoxicity of anticancer treatments: what the cardiologist needs to know. Nat Rev Cardiol. 2010;7:564–75.
Volkova M, Russell 3rd R. Anthracycline cardiotoxicity: prevalence, pathogenesis and treatment. Curr Cardiol Rev. 2011;7:214–20.
Venneri LC F, Manivarmane R, Pareek N, Baksi J, Rosen S, Senior R, et al. Subclinical myocardial dysfunction in cancer patients: is there a direct effect of tumour growth? Eur Heart J Cardiovasc Imag Abstracts Suppl. 2015;16(Supplement 2):ii127.
Plana JC, Galderisi M, Barac A, et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. J Am Soc Echocardiogr. 2014;27:911–39. Expert consensus which provides a detailed review of the imaging modalities in the evalution of cancer patients.
Cardinale D, Colombo A, Bacchiani G, et al. Early detection of anthracycline cardiotoxicity and improvement with heart failure therapy. Circulation. 2015;131:1981–8.
Reulen RC, Winter DL, Frobisher C, et al. Long-term cause-specific mortality among survivors of childhood cancer. JAMA. 2010;304:172–9.
Steinherz LJ, Steinherz PG, Tan CT, Heller G, Murphy ML. Cardiac toxicity 4 to 20 years after completing anthracycline therapy. JAMA. 1991;266:1672–7.
Felker GM, Thompson RE, Hare JM, et al. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy. N Engl J Med. 2000;342:1077–84.
Alexander J, Dainiak N, Berger HJ, et al. Serial assessment of doxorubicin cardiotoxicity with quantitative radionuclide angiocardiography. N Engl J Med. 1979;300:278–83.
Choi BW, Berger HJ, Schwartz PE, et al. Serial radionuclide assessment of doxorubicin cardiotoxicity in cancer patients with abnormal baseline resting left ventricular performance. Am Heart J. 1983;106:638–43.
Schwartz RG, McKenzie WB, Alexander J, et al. Congestive heart failure and left ventricular dysfunction complicating doxorubicin therapy. Seven-year experience using serial radionuclide angiocardiography. Am J Med. 1987;82:1109–18.
Mitani I, Jain D, Joska TM, Burtness B, Zaret BL. Doxorubicin cardiotoxicity: prevention of congestive heart failure with serial cardiac function monitoring with equilibrium radionuclide angiocardiography in the current era. J Nucl Cardiol. 2003;10:132–9.
Curigliano G, Cardinale D, Suter T. Cardiovascular toxicity induced by chemotherapy, targeted agents and radiotherapy: ESMO Clinical Practice Guidelines. Ann Oncol. 2012;23 Suppl 7:vii155–66.
McKillop JH, Bristow MR, Goris ML, Billingham ME, Bockemuehl K. Sensitivity and specificity of radionuclide ejection fractions in doxorubicin cardiotoxicity. Am Heart J. 1983;106:1048–56.
Jiji RS, Kramer CM, Salerno M. Non-invasive imaging and monitoring cardiotoxicity of cancer therapeutic drugs. J Nucl Cardiol. 2012;19:377–88.
Wood PW, Choy JB, Nanda NC, Becher H. Left ventricular ejection fraction and volumes: it depends on the imaging method. Echocardiography. 2014;31:87–100.
Kurt M, Shaikh KA, Peterson L, et al. Impact of contrast echocardiography on evaluation of ventricular function and clinical management in a large prospective cohort. J Am Coll Cardiol. 2009;53:802–10.
Hung J, Lang R, Flachskampf F, et al. 3D echocardiography: a review of the current status and future directions. J Am Soc Echocardiogr. 2007;20:213–33.
Jacobs LD, Salgo IS, Goonewardena S, et al. Rapid online quantification of left ventricular volume from real-time three-dimensional echocardiographic data. Eur Heart J. 2006;27:460–8.
Mor-Avi V, Jenkins C, Kuhl HP, et al. Real-time 3-dimensional echocardiographic quantification of left ventricular volumes: multicenter study for validation with magnetic resonance imaging and investigation of sources of error. JACC Cardiovasc Imag. 2008;1:413–23.
Ylanen K, Eerola A, Vettenranta K, Poutanen T. Three-dimensional echocardiography and cardiac magnetic resonance imaging in the screening of long-term survivors of childhood cancer after cardiotoxic therapy. Am J Cardiol. 2014;113:1886–92.
Thavendiranathan P, Poulin F, Lim KD, Plana JC, Woo A, Marwick TH. Use of myocardial strain imaging by echocardiography for the early detection of cardiotoxicity in patients during and after cancer chemotherapy: a systematic review. J Am Coll Cardiol. 2014;63:2751–68.
Cardinale D, Colombo A, Lamantia G, et al. Anthracycline-induced cardiomyopathy: clinical relevance and response to pharmacologic therapy. J Am Coll Cardiol. 2010;55:213–20.
Cardinale D, Colombo A, Torrisi R, et al. Trastuzumab-induced cardiotoxicity: clinical and prognostic implications of troponin I evaluation. J Clin Oncol. 2010;28:3910–6.
Poterucha JT, Kutty S, Lindquist RK, Li L, Eidem BW. Changes in left ventricular longitudinal strain with anthracycline chemotherapy in adolescents precede subsequent decreased left ventricular ejection fraction. J Am Soc Echocardiogr. 2012;25:733–40.
Ganame J, Claus P, Uyttebroeck A, et al. Myocardial dysfunction late after low-dose anthracycline treatment in asymptomatic pediatric patients. J Am Soc Echocardiogr. 2007;20:1351–8.
Jurcut R, Wildiers H, Ganame J, et al. Strain rate imaging detects early cardiac effects of pegylated liposomal Doxorubicin as adjuvant therapy in elderly patients with breast cancer. J Am Soc Echocardiogr. 2008;21:1283–9.
Pavo N, Raderer M, Hulsmann M, et al. Cardiovascular biomarkers in patients with cancer and their association with all-cause mortality. Heart. 2015;101:1874–80.
Douglas PS, Carr JJ, Cerqueira MD, et al. Developing an action plan for patient radiation safety in adult cardiovascular medicine: proceedings from the Duke University Clinical Research Institute/American College of Cardiology Foundation/American Heart Association Think Tank held on February 28, 2011. J Am Coll Cardiol. 2012;59:1833–47.
Civelli M, Cardinale D, Martinoni A, et al. Early reduction in left ventricular contractile reserve detected by dobutamine stress echo predicts high-dose chemotherapy-induced cardiac toxicity. Int J Cardiol. 2006;111:120–6.
Klewer SE, Goldberg SJ, Donnerstein RL, Berg RA, Hutter Jr JJ. Dobutamine stress echocardiography: a sensitive indicator of diminished myocardial function in asymptomatic doxorubicin-treated long-term survivors of childhood cancer. J Am Coll Cardiol. 1992;19:394–401.
Cranney GB, Lotan CS, Dean L, Baxley W, Bouchard A, Pohost GM. Left ventricular volume measurement using cardiac axis nuclear magnetic resonance imaging. Validation by calibrated ventricular angiography. Circulation. 1990;82:154–63.
Lunning MA, Kutty S, Rome ET, et al. Cardiac magnetic resonance imaging for the assessment of the myocardium after doxorubicin-based chemotherapy. Am J Clin Oncol. 2015;38:377–81.
Drafts BC, Twomley KM, D’Agostino Jr R, et al. Low to moderate dose anthracycline-based chemotherapy is associated with early noninvasive imaging evidence of subclinical cardiovascular disease. JACC Cardiovasc Imag. 2013;6:877–85.
Iles L, Pfluger H, Phrommintikul A, et al. Evaluation of diffuse myocardial fibrosis in heart failure with cardiac magnetic resonance contrast-enhanced T1 mapping. J Am Coll Cardiol. 2008;52:1574–80.
Hendel RC, Patel MR, Kramer CM. ACCF/ACR/SCCT/SCMR/ASNC/NASCI/SCAI/SIR 2006 appropriateness criteria for cardiac computed tomography and cardiac magnetic resonance imaging: a report of the American College of Cardiology Foundation Quality Strategic Directions Committee Appropriateness Criteria Working Group, American College of Radiology, Society of Cardiovascular Computed Tomography, Society for Cardiovascular Magnetic Resonance, American Society of Nuclear Cardiology, North American Society for Cardiac Imaging, Society for Cardiovascular Angiography and Interventions, and Society of Interventional Radiology. J Am Coll Cardiol. 2006;48:1475–97.
Meyersohn NM, Pursnani A, Neilan TG. Detection of cardiac toxicity due to cancer treatment: role of cardiac MRI. Curr Treat Options Cardiovasc Med. 2015;17:396.
Armstrong GT, Plana JC, Zhang N, et al. Screening adult survivors of childhood cancer for cardiomyopathy: comparison of echocardiography and cardiac magnetic resonance imaging. J Clin Oncol. 2012;30:2876–84.
van Royen N, Jaffe CC, Krumholz HM, et al. Comparison and reproducibility of visual echocardiographic and quantitative radionuclide left ventricular ejection fractions. Am J Cardiol. 1996;77:843–50.
Nousiainen T, Vanninen E, Jantunen E, et al. Comparison of echocardiography and radionuclide ventriculography in the follow-up of left ventricular systolic function in adult lymphoma patients during doxorubicin therapy. J Intern Med. 2001;249:297–303.
Corapcioglu F, Sarper N, Berk F, Sahin T, Zengin E, Demir H. Evaluation of anthracycline-induced early left ventricular dysfunction in children with cancer: a comparative study with echocardiography and multigated radionuclide angiography. Pediatr Hematol Oncol. 2006;23:71–80.
Walker J, Bhullar N, Fallah-Rad N, et al. Role of three-dimensional echocardiography in breast cancer: comparison with two-dimensional echocardiography, multiple-gated acquisition scans, and cardiac magnetic resonance imaging. J Clin Oncol. 2010;28:3429–36.
Daher IN, Banchs J, Yusuf SW, Mouhayar E, Durand JB, Gladish G. Impact of cardiac computed tomographic angiography findings on planning of cancer therapy in patients with concomitant structural heart disease. Cardiol Res Pract. 2011;2011:268058.
Kupeli S, Hazirolan T, Varan A, et al. Evaluation of coronary artery disease by computed tomography angiography in patients treated for childhood Hodgkin’s lymphoma. J Clin Oncol. 2010;28:1025–30.
Andersen R, Wethal T, Gunther A, et al. Relation of coronary artery calcium score to premature coronary artery disease in survivors >15 years of Hodgkin’s lymphoma. Am J Cardiol. 2010;105:149–52.
Reinders JG, Heijmen BJ, Olofsen-van Acht MJ, van Putten WL, Levendag PC. Ischemic heart disease after mantlefield irradiation for Hodgkin’s disease in long-term follow-up. Radiother Oncol. 1999;51:35–42.
Mast ME, Heijenbrok MW, Petoukhova AL, Scholten AN, Schreur JH, Struikmans H. Preradiotherapy calcium scores of the coronary arteries in a cohort of women with early-stage breast cancer: a comparison with a cohort of healthy women. Int J Radiat Oncol Biol Phys. 2012;83:853–8.
Roziakova L, Bojtarova E, Mistrik M, et al. Serial measurements of cardiac biomarkers in patients after allogeneic hematopoietic stem cell transplantation. J Exp Clin Cancer Res. 2012;31:13.
Fallah-Rad N, Walker JR, Wassef A, et al. The utility of cardiac biomarkers, tissue velocity and strain imaging, and cardiac magnetic resonance imaging in predicting early left ventricular dysfunction in patients with human epidermal growth factor receptor II-positive breast cancer treated with adjuvant trastuzumab therapy. J Am Coll Cardiol. 2011;57:2263–70.
Kang Y, Xu X, Cheng L, et al. Two-dimensional speckle tracking echocardiography combined with high-sensitive cardiac troponin T in early detection and prediction of cardiotoxicity during epirubicine-based chemotherapy. Eur J Heart Fail. 2014;16:300–8.
Ramjaun A, AlDuhaiby E, Ahmed S, et al. Echocardiographic detection of cardiac dysfunction in childhood cancer survivors: how long is screening required? Pediatr Blood Cancer. 2015;62:2197–203.
Children’s Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent and young adult cancers, version 3.0. Arcadia: Children’s Oncology Group; 2008.
Armenian SH, Hudson MM, Mulder RL, et al. Recommendations for cardiomyopathy surveillance for survivors of childhood cancer: a report from the International Late Effects of Childhood Cancer Guideline Harmonization Group. Lancet Oncol. 2015;16:e123–36.
Carver JR, Szalda D, Ky B. Asymptomatic cardiac toxicity in long-term cancer survivors: defining the population and recommendations for surveillance. Semin Oncol. 2013;40:229–38. This article summarizes screening strategies for cancer patients as well as surveillance strategies for long-term cancer survivors.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Vinisha Garg declares that she has no conflict of interest.
Gabriel Vorobiof has received compensation from Lantheus Medical Imaging, Edwards Lifesciences, and Philips Medical for service as a consultant.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Cardio-oncology
Electronic Supplementary Material
Below is the link to the electronic supplementary material.
Video 1
Contrast enhanced 2D echocardiography. Images shown in the apical four-chamber view without contrast (a) and with contrast (b) in a patient with Hodgkin lymphoma with radiation-induced cardiotoxicity with ejection fraction of 48 %. (MP4 485 kb)
Rights and permissions
About this article
Cite this article
Garg, V., Vorobiof, G. Echocardiography and Alternative Cardiac Imaging Strategies for Long-Term Cardiotoxicity Surveillance of Cancer Survivors Treated with Chemotherapy and/or Radiation Exposure. Curr Oncol Rep 18, 52 (2016). https://doi.org/10.1007/s11912-016-0532-y
Published:
DOI: https://doi.org/10.1007/s11912-016-0532-y