Abstract
Purpose of review
The prevalence of heart failure with preserved ejection fraction (HFpEF) is rising and in some places, it is already the most prevalent form of heart failure. The usual treatments of HF do not improve mortality or outcomes in HFpEF, suggesting a distinct pathophysiology that remains poorly characterized. The neutrality of clinical trial results is also attributable to the heterogeneity of patient profiles, and by poor characterization offered by classical echocardiography parameters. Emerging imaging modalities may overcome this problem. We therefore aimed to summarize recent advances offered by cardiovascular imaging in disease characterization, and the implication of findings to new phenotype-specific treatment options.
Recent findings
Novel cardiovascular imaging techniques such as LV global longitudinal strain, left atrial strain, tissue characterization by magnetic resonance T1 time, as well as incorporation of systolic and diastolic stress testing offer greatly improved characterization, diagnosis, and stratification of disease pathogenesis. These techniques offer insight into identification of HFpEF sub-phenotypes that are resistant to, or responsive to therapies.
Summary
There is a growing body of evidence that novel cardiovascular imaging modalities are able to characterize HFpEF patients with much greater accuracy than current guideline-driven parameters. Whether this information can be synthesized to adequately stratify patients into sub-phenotypes with clearer disease pathogenesis amenable to targeted intervention will be of particular future interest.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Heart failure with preserved ejection fraction (HFpEF) is a complex and heterogeneous condition with a rapidly rising prevalence that already affects up to 1 in 10 elderly individuals, and is becoming the most common form of heart failure (HF) [1]. To date, there remains no identified therapy that can reduce mortality, and this contributes to it being a major public health challenge. The syndrome of HFpEF is inherently heterogeneous, and we lack a single international consensus that can accurately characterize patients with HFpEF among the community of patients with chronic exertional dyspnea.
Imaging and diagnosis of HFpEF
Two main commonly accepted HFpEF phenotypic definition have been derived from American (ACC/AHA/HFSA [2]) and European (ESC) guidelines [3]. Both definitions require clinical diagnosis based on classical patient symptomatology as initially defined by the Framingham criteria [4]; LV ejection fraction (EF) ≥ 50%; combined with exclusion of valvular and non-cardiac causes. In addition, the 2016 ESC criteria offers specific cutoff values for noninvasive diagnosis defined as echocardiographic evidence of cardiac structural and/or functional alterations (LAVI > 34 mL/m2, or LVMI ≥ 115/95 g/m2 for males/females, or mean E/e′ ≥ 13 and mean e′ < 9 cm/s), and elevated natriuretic peptide levels (BNP > 35 pg/mL and/or NT-proBNP > 125 pg/ml). This is an interesting choice, both because obesity is strongly linked to HFpEF and also linked to disproportionally low BNP levels for the ambient hemodynamic status, as well as the fact that many patients with HFpEF are symptomatic and have increased wall stress only with exercise [5]. If such individuals exercise little, then it is likely that BNP will not be elevated. Both of these conditions likely lead guideline-based diagnosis to be specific but insensitive.
In contrast, the ACC/AHA/HFSA guidelines cite disease stages stratified by symptom status, and no absolute requirement for serum biomarker elevation. There are many dyspneic patients in whom a cardiac etiology is suspected despite preserved EF. Although recommendations are made for invasive determination of elevated filling pressures, which remain the gold standard for true HFpEF diagnosis—including when performed during exercise [6]—it is not feasible to perform widespread invasive investigation at the population level. Therefore, guideline-driven diagnosis of HFpEF, for which the presence of diastolic dysfunction and/or structural remodeling is a prerequisite, by and large continues to rely on classical echocardiographic parameters such as estimated LV filling pressures E/e′. As a result, current noninvasive recommendations can be effective in identifying HFpEF in an acute, decompensated setting. However, both ESC and ACC/AHA/HFSA guidelines offer poor noninvasive combined sensitivity and specificity to accurately classify chronically dyspneic HFpEF patients. There is therefore a need to identify novel cardiovascular imaging parameters that can accurately characterize this growing population of individuals with debility and cardiovascular mortality and morbidity.
The search for phenotypic homogeneity
Over recent decades, therapies have been shown to improve outcomes and quality of life in HF with reduced ejection fraction (HFrEF) patients. Neutral results in several large randomized controlled trials have confirmed that the same principles cannot be applied to HFpEF. Based on the lack of significant reduction of cardiovascular death in HFpEF provided by angiotensin receptor blockade with the use of candesartan in the CHARM-Preserved Trial [7], and the absence of significant improvement in patient symptoms, exercise capacity or quality of life with long-term aldosterone receptor blockade using spironolactone in the Aldo-DHF Randomized Controlled Trial [8], it has been concluded that pharmacological inhibition of the renin angiotensin aldosterone system (RAAS) is ineffective. Similarly, spironolactone also failed to significantly reduce the incidence of cardiovascular mortality or hospitalization in HFpEF in the Treatment of Preserved Cardiac Function Heart Failure with an Aldosterone Antagonist (TOPCAT) trial [9]. More recently, a meta-analysis of 18,000 patients in 25 randomized controlled trials confirmed neutrality in all-cause mortality reduction in HFpEF with the use of RAAS blockade [10••]. Although this analysis showed that beta blockade may confer a mortality benefit (RR 0.78, 95% CI 0.65–0.94, p = 0.008), this improvement was strongly influenced by ischemic heart disease [10••]. Moreover, a recent multicenter double-blind study targeting the nitric oxide pathway with isosorbide mononitrate failed to demonstrate significant improvements in HFpEF [11•]. These results highlight that no individual class of medication is known to improve outcomes in HFpEF. Howver, combined treatment may offer some benefit in HFpEF, with improvement in exercise tolerance (exercise capacity graded by treadmill time) demonstrated in a meta-analysis of 183 patients in 6 randomized controlled trials (weighted MD 51.5, 95% CI 27.3–75.7, p < 0.001) [12].
The limitations of current diagnostic tools for HFpEF are a major challenge in modern cardiology. In our aging population, with increasing numbers of comorbidities, the numbers of individuals with HFpEF are increasing, with outcomes comparable to HFrEF [1, 13, 14], but no available therapy. There is an urgent need for the discovery of parameters that can categorize homogeneous sub-phenotypes in these individuals. This step may be an important key to successfully tailoring individual therapies.
Imaging clues to etiology and pathogenesis in HFpEF
Etiology
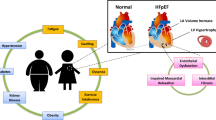
The etiology of HFpEF itself is broad, and remains poorly characterized. HFpEF is multifactorial, risk factors include older age and female sex, as well as an increasing number of comorbidities such as hypertension, obesity and diabetes mellitus [15, 16]. Hypertension is particularly prevalent in HFpEF, affecting up to 60% of individuals [17]. Older age (OR: 1.057, 95% CI 1.015–1.100, p = 0.007) and obesity (OR: 1.096, 95% CI 1.035–1.161, p = 0.002) are key contributors to dyspnea, and demonstrate an independent association with NYHA class [18]. Moreover, right ventricular dysfunction [19], atrial fibrillation [20], and renal dysfunction are also common [21]; and associated with worse outcomes than HFpEF patients without these sequelae [18, 20, 22]. HFpEF is characterized by hypertensive LV remodeling in 60% of patients, whereby the LV wall is thickened but the LV cavity is not dilated, and is accompanied by diastolic dysfunction in nearly 70% of patients, engendering left atrial enlargement in over 65% of individuals as a reflection of increased filling pressures [23].
Pathophysiology
In contrast to HFrEF, the pathophysiology of HFpEF remains incompletely understood. The contributors are such that no single resting parameter can reliably characterize these individuals. Indeed, clinical trials have often relied on poorly defined means to stratify patients, including exclusion of patients with normal natriuretic peptide levels—even though these are present in up to 29% of hemodynamically verified individuals with HFpEF [24]. Therefore, the lack of definitive positive trial outcomes may in fact be engendered by the large heterogeneity of individuals with HFpEF, compounded by the limited specificity and sensitivity of guideline-driven noninvasive measures of diastolic dysfunction [25].
Despite its heterogeneity, common pathophysiologic features of HFpEF are increased myocardial stiffness (contributed to by excess interstitial collagen deposition, cross-linking, and aberrant myocardial cytoskeletal protein modifications such as titin). This is evidenced by upward and leftward shift in end-diastolic pressure-volume relationship, particularly during physiological stress. In addition, prolongation of active myocardial relaxation is a consequence of impaired active muscular inactivation and contraction dyssynchrony manifest by prolongation of the time constant of isovolumetric relaxation (tau) [26, 27]. Novel measures such as untwisting rate, precursor to isovolumic pressure decay, can also noninvasively reflect active myocardial relaxation properties [28•]. Myocardial stiffness and delayed relaxation restrict LV diastolic inflow, which in turn leads to elevated filling pressures. Such elevated pressures are exacerbated by even modest physical exertion in HFpEF, which is a key determinant of the debilitating symptoms of dyspnea and fatigue experienced by these patients [29]. In fact, the extent of the exercise-mediated elevation in filling pressures, invasively determined by pulmonary capillary wedge pressures (PCWP), carries great prognostic importance and is the strongest hemodynamic predictor of outcomes in HFpEF [30].
Nevertheless, multiple other factors synergistically worsen HFpEF symptomatology and add to the complexity of its pathophysiology. In conjunction to decreased ventricular compliance, impaired ventriculo-arterial coupling, arterial stiffness and endothelial dysfunction are often present [31, 32]. These features contribute to heightened sensitivity of the HFpEF phenotype to loading conditions, and greater predisposition to pulmonary edema under small increments of loading. Skeletal muscle dysfunction has also been demonstrated, for example, by increased muscular fat content that is strongly associated with peak VO2 in HFpEF [33]. There is also evidence of impaired oxygen extraction and oxidative metabolism in HFpEF [34]. Coronary microvascular dysfunction is another contributor to disease pathogenesis. Impaired coronary flow reserve in the absence of overt coronary artery disease is independently associated with worse echocardiographic evidence of diastolic dysfunction (e′ and E/e′, p < 0.0001); and associated with worse cardiovascular outcomes (adjusted HR: 2.38, 95% CI 1.21–4.67, p = 0.01) and hospitalizations (HR: 2.47, 95% CI 1.09–5.62, p = 0.03) in HFpEF [35••]. HFpEF patients also display baroreflex and peripheral autonomic dysfunction, with blunted exertional responses showing a 40% lower increase in heart rate [36]. The resulting inadequate chronotropic responses are associated with exercise limitation in HFpEF [37••]. Moreover, atrial mechanics [38], as well as systolic performance are also of increasingly recognized importance in HFpEF, and have direct implications to the non-invasive assessment of disease progression and diagnosis.
Diastolic dysfunction and diagnosis of HFpEF
Implications for imaging—is this necessarily a diastolic disease?
The paradigm of HFpEF as an isolated disease of diastolic function originated as a result of erroneous interpretation of the information that is conveyed by EF measurement. Normal systolic function in HFpEF cannot be established on the basis of preserved EF alone, as EF is highly load dependent (both preload and afterload), and also highly influenced by structural changes with variations in end-diastolic volume greatly altering EF irrespective of contractility [39]. Several pathophysiological features of HFpEF, such as myocardial fibrosis and microvascular dysfunction, can affect both diastolic and systolic function. In fact, there is now clear evidence of significant systolic impairment in HFpEF, such as decreased contractility, which is associated with greater mortality [40]. Importantly, systolic function reserve is particularly affected with HFpEF patients showing significantly lower cardiac output reserve [31, 37••], that is also present in the right heart [41].
“Classical” diagnosis of diastolic dysfunction
The echocardiographic evidence of diastolic dysfunction falls short of providing a unifying characterization in HFpEF. The previous recommendations for diastolic assessment were complex and ambiguous [42]. The current recommendations have been simplified but now recognize a greater proportion of studies as non-diagnostic [3]. Part of the problem is that the markers of diastolic dysfunction (E/A ratio, E/e′, LA volume) are the same as have been used for the last few decades, but the sequence of their use has been revised. Perhaps the diagnostic content of the echocardiogram would be increased by adding newer parameters.
Although diastolic impairment is common, up to 30% of patients show normal resting diastolic function by standard echocardiography [23]. Surrogate markers such as E/e′ are load dependent, and have poor predictive ability to detect true elevation of filling pressures, especially as measures are usually only performed at rest. The American Society of Echocardiography cutoff for mean E/e′ has poor sensitivity in detecting elevated filling pressures, estimated to be as low as 37% [43]. Moreover, diastolic dysfunction is not exclusive to HFpEF, and is often present in patients without HF [44].
Problems with late-stage disease
Multiple pathogenic contributors further complicate the development of late stages of HFpEF. Diastolic dysfunction alone is unlikely to explain the transition to clinically overt symptomatology. There are also significant contributions by impaired systolic and chronotropic responses, particularly during physiological stress, to worsening hemodynamic phenotype [37••]. A number of comorbidities, including coronary artery disease [45], atrial fibrillation [22] and right ventricular dysfunction [18] contribute to impaired contractile reserve and higher mortality in the late stages of HFpEF.
Alternative resting parameters and diagnosis of HFpEF
LV global longitudinal strain
Speckle-tracking analysis is an evolving modality with additive value to standard echocardiography. Acoustic reverberations of the myocardium, or “speckles”, can be computationally identified and tracked over time to measure segmental and global myocardial mechanical properties across the cardiac cycle that extend beyond standard volumetric and velocity measures. LV global longitudinal strain (GLS) is relatively independent of traditional diastolic parameters such as E/e′ and e′ [46]. In contrast to ejection fraction, GLS reflects the performance of longitudinally arranged subendocardial fibers that are affected early in disease pathogenesis, allowing detection of even subtle impairment [47]. Such systolic deficits have been reported in HFpEF [31, 37••], with confirmation of profound impairment in GLS (− 14.6% vs − 20%, p < 0.001) demonstrated in a large analysis where > 2/3 of the 219 HFpEF patients included showed systolic deficit on the basis of GLS [46].
GLS is associated with circulating levels of collagen synthetic biomarkers [48•], and independently associated with natriuretic peptide levels [46]. It shows discriminative diagnostic capacity for HFpEF based on the noninvasive ESC criteria [49••]. Importantly, we have recently demonstrated the diagnostic capacity of GLS, based on hemodynamically verified HFpEF diagnosis at rest and peak exertion using PCWP [50]. GLS impairment is associated with greater risk of cardiovascular death or hospitalization, even following adjustment for clinical and conventional echocardiographic parameters (adjusted HR 2.14, 95% CI 1.26–3.66, p = 0.005) [51••]; thus, GLS assessment of systolic longitudinal function will be of growing importance in HFpEF characterization and phenotype-driven interventions (Table 1).
Left atrial strain
In a similar fashion, speckle-tracking echocardiography applied to the left atrium (LA) has recently yielded promising results. There is a clear and profound impairment in LA mechanics in HFpEF, depicted by reductions in both reservoir and booster functions [63, 64], related to elevated natriuretic peptide levels [58] and to reduced exercise capacity [59]. Given the strong relationship between elevated LA pressures and mortality in HFpEF [30], it is not surprising that impaired LA strain is associated with worse outcome [60•], being independently associated with cardiovascular hospitalization, HF or death even following adjustment for clinical parameters, RV and LV systolic function (B = 1.43, 95% CI 1.05–1.95, p = 0.02) [61•].
Moreover, impaired LA reservoir strain appears to be strongly related to worse hemodynamic profiles of pulmonary artery pressures, pulmonary elastance, cardiac index, and stroke volume index in HFpEF [62]. Importantly, worse LA reservoir strain is also independently associated with higher exercise PCWP following adjustment for indexed LV mass, indexed LA volume, mean E/e′ and exercise systolic blood pressure (B = − 0.66, 95% CI − 0.87 to −0.46, p < 0.001). LA reservoir strain at a cutoff of ≤ 33% also has significant diagnostic ability with a net reclassification improvement of 16% in comparison to current noninvasive guidelines (Fig. 1) [62].
Example of HFpEF reclassification improvement offered by left atrial strain: Global left atrial strain at cutoff ≤ 33% is able to provide significant reclassification improvement in comparison to the 2016 ESC criteria for the noninvasive diagnosis of HFpEF. This example shows false negative (top) and false (positive) patients according to the ESC criteria that were correctly reclassified by reservoir strain.
T1 mapping
In contrast to echocardiography, cardiac magnetic resonance imaging (cMR) has the advantage of providing excellent contrast resolution that allows detailed tissue characterization. Late gadolinium enhancement requires a frame of normal reference that is unavailable in the diffusely diseased heart. However, novel techniques such as T1 mapping, using a variety of methodologies for calculation of T1 times, such as the modified Look-Locker inversion recovery (MOLLI) sequence, have shown promising results in HFpEF phenotyping. The quantification of myocardial fibrosis from myocardial extracellular volume (ECV) underpins one of the pathophysiologic mechanisms of increased stiffening in HFpEF [65]. HFpEF patients demonstrate significantly higher levels of ECV that are independently associated with invasively attained LV stiffness [52•], and associated with cardiac events [53, 54•]. T1 mapping-derived ECV is also able to discriminate between hypertensive heart disease and the diagnosis of HFpEF by the ESC noninvasive criteria [49••]. In the analysis of 117 invasively verified HFpEF patients, ECV correlates with natriuretic peptide levels, aerobic capacity as well as symptomatic status in HFpEF [54•].
Use of the exercise response in HFpEF
Exercise testing is an important and under-used step in the evaluation of patients with dyspnea. First, it provides an objective assessment of exercise capacity—sometimes obesity and lack of physical fitness are the primary drivers of exercise intolerance, rather than myocardial disease. Second, it provides a means of excluding a functional contribution from myocardial ischemia. Testing for myocardial ischemia should be performed in at risk patients as this group of individuals benefit from established phenotype-specific therapy such as revascularization and beta blockade [45]. Finally, it provides a means to unmask impairments that can be subtle and undetected at rest, especially in early disease, but are exacerbated during exercise in HFpEF patients.
Invasive testing using thermodilution at right heart catheterization are able to detect deficits in cardiac index at rest in HFpEF patients [66]. Although such subtle systolic impairments at rest are often not detected with noninvasive imaging, exercise in HFpEF patients have markedly impaired augmentation of stroke volume and cardiac output easily identified by noninvasive measurement [31]. Moreover, a large proportion of HFpEF patients show normal resting filling pressures even during invasive testing, despite significant hemodynamic impairment during exercise [55••]. Thus, the focus of current HFpEF diagnostic guidelines on resting parameters contributes to poor diagnostic sensitivity, as estimated normal filling pressures by E/e′ cannot exclude HFpEF diagnosis even in combination with normal natriuretic peptide levels [66].
Exercise E/e′
Diastolic impairment in HFpEF is exacerbated by physiological stress. This leads to increases in both pulmonary venous and pulmonary artery pressure that is well characterized by invasive testing [55••]. In the presence of tricuspid regurgitation, the latter can also be assessed from the velocity of the regurgitant jet, although high velocity (e.g., > 3.5 m/s) is more meaningful at low rather than high levels of exercise [67].
Assessment of diastolic function via E/e′ measurement during exercise has the ability to increase current noninvasive diagnostic sensitivity due to unmasking of impairments in intermediate risk patients. Using the cutoff value of exercise E/e′ > 14, the sensitivity of hemodynamically verified diagnosis can be increased to 90% [55••]. However, this comes at a cost of decreased specificity, suggesting usefulness of diastolic stress testing in exclusion of intermediate risk HFpEF in patients that do not warrant costly invasive assessment [55••]. Moreover, exercise diastolic impairment also carries prognostic importance in HFpEF. Symptomatic patients with elevated exercise E/e′ show worse longitudinal systolic function reserve, higher levels of the fibrosis biomarker galectin-3, lower exercise capacity and greater incidence of HF hospitalization [37••, 56•]. Elevated exercise E/e′ is also associated with composite endpoint of cardiovascular hospitalization or death independently of natriuretic peptide levels and clinical characteristics (HR 2.69, 95% CI 1.44–5.04, p = 0.002) [57]. However, despite the advantages of noninvasive diastolic stress testing, this test can be limited by image quality. While the feasibility of obtaining diagnostic-quality measurements decreases with increasing levels of exercise, this is not commonly a problem in symptomatic patients (Table 2).
Systolic stress testing: exercise GLS physiological stress testing in HFpEF has the ability of unmasking impairments in cardiac reserve that are well described in HFpEF [31, 37••]. Parameters such as exercise GLS show marked deficits in HFpEF that are exacerbated by exertion [50]. In receiver operator curve analysis, exercise has excellent predictive ability to differentiate symptom status in HFpEF patients (AUC: 0.78) [37••]. Exercise GLS also has diagnostic capacity in cohort where HFpEF is identified on hemodynamically grounds (AUC: 0.67) [50].
There are two disadvantages to the use of GLS for assessing systolic reserve. First, GLS lacks a temporal component, and therefore ignores speed of contraction, which is impaired in myocardial disease. Second, the temporal resolution of speckle-tracking echocardiography is lower than that of tissue Doppler, which may be the preferable modality for assessing responses during tachycardia.
Targeting of management to pathophysiology and phenotype
Recent advances in HFpEF therapy have in part originated from strategies aimed at hemodynamic consequences of disease pathogenesis. A novel interatrial shunt device targeting the pathological rise in exercise PCWP in HFpEF [68•] shows an effective response in reducing filling pressure [69•]. Similarly, the use of type III phosphodiesterase inhibition via intravenous milrinone have also led to reductions in exercise PCWP [70•].
An alternative strategy has been phenotype-specific stratification of HFpEF, which thus far has been largely focused on comorbidities such as obesity, hypertension, coronary artery disease, atrial fibrillation, kidney disease, and skeletal muscle dysfunction [25, 71]; or clinical parameters combined with traditional echocardiography markers [72]. A number of promising imaging modalities have shown significant improvement in the noninvasive characterization of HFpEF. Metabolic indexes of ventricular stiffness have been shown to offer good HFpEF stratification, resulting in identification of a HFpEF biochemical phenotype of high collagen cross-linking that identifies patients resistant to spironolactone therapy with no effect of improving diastolic dysfunction [73••]. Similarly, by selecting HFpEF phenotypes with more severe diastolic impairment evidenced by the presence of exercise-induced E/e′ > 13, the STRUCTURE (SpironolacTone in myocaRdial dysfUnCTion with redUced exeRcisE capacity) trial identified a sub-group of patients responsive to spironolactone [74••], which conferred significant improvement in peak VO2 in comparison to placebo (2.9 ml/min/kg, 95% CI 1.9–3.9 vs. 0.3 ml/min/kg, 95% CI 0.5–1.1; p < 0.001) [74••]. Whether advances in cardiovascular imaging can be similarly applied to further stratify HFpEF patients into homogenous groups with clearer pathophysiological basis of disease, amenable to targeted intervention, will be of particular public health interest.
Conclusion
HFpEF remains a major challenge in modern cardiology, with an incompletely understood pathophysiology, rising prevalence, high morbidity and mortality but without available effective therapy. The lack of response to therapies is contributed by the heterogeneity of patient profiles, and poor characterization by classical parameters. The response to this challenge may be to better define the sub-phenotypes, and imaging may be the best way to accomplish this. Novel cardiovascular imaging modalities offer greatly improved characterization in these patients. Whether this information can be synthesized to adequately stratify patients into sub-phenotypes with clearer disease pathogenesis amenable to targeted intervention will be of particular future interest.
Abbreviations
- HFpEF:
-
Heart failure with preserved ejection fraction
- AHA:
-
American Heart Association
- ACC:
-
American College of Cardiology
- HFSA:
-
Heart Failure Society of America
- ESC:
-
European Society of Cardiology
- LAVI:
-
Left atrial volume index
- LVMI:
-
Left ventricular mass index
- e′:
-
Early diastolic mitral annular velocity
- E/e′:
-
Ratio of early diastolic trans-mitral to mitral annular velocities
- BNP:
-
Brain natriuretic peptide
- NT:
-
ProBNP N-terminal pro-B-type natriuretic peptide
- PCWP:
-
Pulmonary capillary wedge pressures
- GLS:
-
Global longitudinal strain
- ECV:
-
Myocardial extracellular volume
References and Recommended Readings
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Owan TE, Hodge DO, Herges RM, Jacobsen SJ, Roger VL, Redfield MM. Trends in prevalence and outcome of heart failure with preserved ejection fraction. N Engl J Med. 2006;355(3):251–9.
Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Colvin MM, et al. 2017 ACC/AHA/HFSA Focused Update of the 2013 ACCF/AHA Guideline for the management of heart failure: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America. J Card Fail. 2017;23(8):628–51.
Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–200.
McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285(26):1441–6.
Madamanchi C, Alhosaini H, Sumida A, Runge MS. Obesity and natriuretic peptides, BNP and NT-proBNP: mechanisms and diagnostic implications for heart failure. Int J Cardiol. 2014;176(3):611–7.
Maron BA, Cockrill BA, Waxman AB, Systrom DM. The invasive cardiopulmonary exercise test. Circulation. 2013;127(10):1157–64.
Yusuf S, Pfeffer MA, Swedberg K, Granger CB, Held P, McMurray JJ, et al. Effects of candesartan in patients with chronic heart failure and preserved left-ventricular ejection fraction: the CHARM-Preserved Trial. Lancet (London, England). 2003;362(9386):777–81.
Edelmann F, Wachter R, Schmidt AG, Kraigher-Krainer E, Colantonio C, Kamke W, et al. Effect of spironolactone on diastolic function and exercise capacity in patients with heart failure with preserved ejection fraction: the Aldo-DHF randomized controlled trial. JAMA. 2013;309(8):781–91.
Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, et al. Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370(15):1383–92.
•• Zheng SL, Chan FT, Nabeebaccus AA, Shah AM, McDonagh T, Okonko DO, et al. Drug treatment effects on outcomes in heart failure with preserved ejection fraction: a systematic review and meta-analysis. Heart. 2018;104(5):407–15. Large recent meta-analysis evaluating the effect of pharmacological treatment in HFpEF.
• Redfield MM, Anstrom KJ, Levine JA, Koepp GA, Borlaug BA, Chen HH, et al. Isosorbide mononitrate in heart failure with preserved ejection fraction. N Engl J Med. 2015;373(24):2314–24. Recent multicenter double-bind pharmacological crossover study using isosorbide mononitrate in 110 HFpEF patients.
Holland DJ, Kumbhani DJ, Ahmed SH, Marwick TH. Effects of treatment on exercise tolerance, cardiac function, and mortality in heart failure with preserved ejection fraction: a meta-analysis. J Am Coll Cardiol. 2011;57(16):1676–86.
Shah KS, Xu H, Matsouaka RA, Bhatt DL, Heidenreich PA, Hernandez AF, et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J Am Coll Cardiol. 2017;70(20):2476–86.
Bhatia RS, Tu JV, Lee DS, Austin PC, Fang J, Haouzi A, et al. Outcome of heart failure with preserved ejection fraction in a population-based study. N Engl J Med. 2006;355(3):260–9.
Shah AM, Solomon SD. Phenotypic and pathophysiological heterogeneity in heart failure with preserved ejection fraction. Eur Heart J. 2012;33(14):1716–7.
Obokata M, Reddy YNV, Pislaru SV, Melenovsky V, Borlaug BA. Evidence supporting the existence of a distinct obese phenotype of heart failure with preserved ejection fraction. Circulation. 2017;136(1):6–19.
McMurray JJ, Carson PE, Komajda M, McKelvie R, Zile MR, Ptaszynska A, et al. Heart failure with preserved ejection fraction: clinical characteristics of 4133 patients enrolled in the I-PRESERVE trial. Eur J Heart Fail. 2008;10(2):149–56.
Dalos D, Mascherbauer J, Zotter-Tufaro C, Duca F, Kammerlander AA, Aschauer S, et al. Functional status, pulmonary artery pressure, and clinical outcomes in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;68(2):189–99.
Mohammed SF, Hussain I, AbouEzzeddine OF, Takahama H, Kwon SH, Forfia P, et al. Right ventricular function in heart failure with preserved ejection fraction: a community-based study. Circulation. 2014;130(25):2310–20.
Santhanakrishnan R, Wang N, Larson MG, Magnani JW, McManus DD, Lubitz SA, et al. Atrial fibrillation begets heart failure and vice versa: temporal associations and differences in preserved versus reduced ejection fraction. Circulation. 2016;133(5):484–92.
Ter Maaten JM, Damman K, Verhaar MC, Paulus WJ, Duncker DJ, Cheng C, et al. Connecting heart failure with preserved ejection fraction and renal dysfunction: the role of endothelial dysfunction and inflammation. Eur J Heart Fail. 2016;18(6):588–98.
Elshazly MB, Senn T, Wu Y, Lindsay B, Saliba W, Wazni O, Cho L. Impact of atrial fibrillation on exercise capacity and mortality in heart failure with preserved ejection fraction: insights from cardiopulmonary stress testing. J Am Heart Assoc. 2017;6(11).
Zile MR, Gottdiener JS, Hetzel SJ, McMurray JJ, Komajda M, McKelvie R, et al. Prevalence and significance of alterations in cardiac structure and function in patients with heart failure and a preserved ejection fraction. Circulation. 2011;124(23):2491–501.
Anjan VY, Loftus TM, Burke MA, Akhter N, Fonarow GC, Gheorghiade M, et al. Prevalence, clinical phenotype, and outcomes associated with normal B-type natriuretic peptide levels in heart failure with preserved ejection fraction. Am J Cardiol. 2012;110(6):870–6.
Shah SJ, Kitzman DW, Borlaug BA, van Heerebeek L, Zile MR, Kass DA, et al. Phenotype-specific treatment of heart failure with preserved ejection fraction: a multiorgan roadmap. Circulation. 2016;134(1):73–90.
Penicka M, Bartunek J, Trakalova H, Hrabakova H, Maruskova M, Karasek J, et al. Heart failure with preserved ejection fraction in outpatients with unexplained dyspnea: a pressure-volume loop analysis. J Am Coll Cardiol. 2010;55(16):1701–10.
Zile MR, Baicu CF, Gaasch WH. Diastolic heart failure--abnormalities in active relaxation and passive stiffness of the left ventricle. N Engl J Med. 2004;350(19):1953–9.
• Kosmala W, Przewlocka-Kosmala M, Marwick TH. Association of active and passive components of lv diastolic filling with exercise intolerance in heart failure with preserved ejection fraction: mechanistic insights from spironolactone response. JACC Cardiovascular imaging. 2017. Article provides insight into the relation between a novel non-invasive marker of active myocardial relaxation (untwisting rate), and exercise capacity in 194 patients.
Maeder MT, Thompson BR, Brunner-La Rocca HP, Kaye DM. Hemodynamic basis of exercise limitation in patients with heart failure and normal ejection fraction. J Am Coll Cardiol. 2010;56(11):855–63.
Dorfs S, Zeh W, Hochholzer W, Jander N, Kienzle RP, Pieske B, et al. Pulmonary capillary wedge pressure during exercise and long-term mortality in patients with suspected heart failure with preserved ejection fraction. Eur Heart J. 2014;35(44):3103–12.
Borlaug BA, Olson TP, Lam CS, Flood KS, Lerman A, Johnson BD, et al. Global cardiovascular reserve dysfunction in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2010;56(11):845–54.
Akiyama E, Sugiyama S, Matsuzawa Y, Konishi M, Suzuki H, Nozaki T, et al. Incremental prognostic significance of peripheral endothelial dysfunction in patients with heart failure with normal left ventricular ejection fraction. J Am Coll Cardiol. 2012;60(18):1778–86.
Haykowsky MJ, Kouba EJ, Brubaker PH, Nicklas BJ, Eggebeen J, Kitzman DW. Skeletal muscle composition and its relation to exercise intolerance in older patients with heart failure and preserved ejection fraction. Am J Cardiol. 2014;113(7):1211–6.
Dhakal BP, Malhotra R, Murphy RM, Pappagianopoulos PP, Baggish AL, Weiner RB, et al. Mechanisms of exercise intolerance in heart failure with preserved ejection fraction: the role of abnormal peripheral oxygen extraction. Circulation Heart Failure. 2015;8(2):286–94.
•• Taqueti VR, Solomon SD, Shah AM, Desai AS, Groarke JD, Osborne MT, et al. Coronary microvascular dysfunction and future risk of heart failure with preserved ejection fraction. Eur Heart J. 2018;39(10):840–9. Highlights strong link between coronary microvascular dysfunction to worse HFpEF phenotype and worse outcomes.
Borlaug BA, Melenovsky V, Russell SD, Kessler K, Pacak K, Becker LC, et al. Impaired chronotropic and vasodilator reserves limit exercise capacity in patients with heart failure and a preserved ejection fraction. Circulation. 2006;114(20):2138–47.
•• Kosmala W, Rojek A, Przewlocka-Kosmala M, Mysiak A, Karolko B, Marwick TH. Contributions of nondiastolic factors to exercise intolerance in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2016;67(6):659–70. Highlights significant contributions by impaired systolic and chronotropic exercise responses to worsening HFpEF phenotype.
Melenovsky V, Hwang SJ, Redfield MM, Zakeri R, Lin G, Borlaug BA. Left atrial remodeling and function in advanced heart failure with preserved or reduced ejection fraction. Circulation Heart failure. 2015;8(2):295–303.
Konstam MA, Abboud FM. Ejection Fraction: Misunderstood and overrated (changing the paradigm in categorizing heart failure). Circulation. 2017;135(8):717–9.
Borlaug BA, Lam CS, Roger VL, Rodeheffer RJ, Redfield MM. Contractility and ventricular systolic stiffening in hypertensive heart disease insights into the pathogenesis of heart failure with preserved ejection fraction. J Am Coll Cardiol. 2009;54(5):410–8.
Borlaug BA, Kane GC, Melenovsky V, Olson TP. Abnormal right ventricular-pulmonary artery coupling with exercise in heart failure with preserved ejection fraction. Eur Heart J. 2016;37(43):3293–302.
Paulus WJ, Tschope C, Sanderson JE, Rusconi C, Flachskampf FA, Rademakers FE, et al. How to diagnose diastolic heart failure: a consensus statement on the diagnosis of heart failure with normal left ventricular ejection fraction by the Heart Failure and Echocardiography Associations of the European Society of Cardiology. Eur Heart J. 2007;28(20):2539–50.
Sharifov OF, Schiros CG, Aban I, Denney TS, Gupta H. Diagnostic accuracy of tissue Doppler Index E/e′for evaluating left ventricular filling pressure and diastolic dysfunction/heart failure with preserved ejection fraction: a systematic review and meta-analysis. J Am Heart Assoc. 2016;5(1).
Redfield MM, Jacobsen SJ, Burnett JC Jr, Mahoney DW, Bailey KR, Rodeheffer RJ. Burden of systolic and diastolic ventricular dysfunction in the community: appreciating the scope of the heart failure epidemic. JAMA. 2003;289(2):194–202.
Hwang SJ, Melenovsky V, Borlaug BA. Implications of coronary artery disease in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(25 Pt A):2817–27.
Kraigher-Krainer E, Shah AM, Gupta DK, Santos A, Claggett B, Pieske B, et al. Impaired systolic function by strain imaging in heart failure with preserved ejection fraction. J Am Coll Cardiol. 2014;63(5):447–56.
Sengupta PP, Narula J. Reclassifying heart failure: predominantly subendocardial, subepicardial and transmural. Heart Fail Clin. 2008;4(3):379–82.
• De Vore AD, McNulty S, Alenezi F, Ersboll M, Vader JM, Oh JK, et al. Impaired left ventricular global longitudinal strain in patients with heart failure with preserved ejection fraction: insights from the RELAX trial. Eur J Heart Fail. 2017;19(7):893–900. Provides associations of global longitudinal strain in 187 patients to biomarkers of collagen synthesis.
•• Mordi IR, Singh S, Rudd A, Srinivasan J, Frenneaux M, Tzemos N, et al. Comprehensive echocardiographic and cardiac magnetic resonance evaluation differentiates among heart failure with preserved ejection fraction patients, hypertensive patients, and healthy control subjects. JACC Cardiovascular Imaging. 2018;11(4):577–85. Study highlights the utility of novel cardiac magnetic resonance imaging in the diagnosis of HFpEF.
Nanayakkara S, Telles F, Evans S, Patel HC, Vizi D, William J, Marwick TH, Kaye D. Association of rest and exercise left ventricular strain with exercise haemodynamics in patients with heart failure with preserved ejection fraction. Heart, Lung and Circulation. 2018;27:S78–S79.
•• Shah AM, Claggett B, Sweitzer NK, Shah SJ, Anand IS, Liu L, et al. Prognostic importance of impaired systolic function in heart failure with preserved ejection fraction and the impact of spironolactone. Circulation. 2015;132(5):402–14. Highlights that left ventricular global longitudinal strain is an important determinant of outcomes in HFpEF.
• Rommel KP, von Roeder M, Latuscynski K, Oberueck C, Blazek S, Fengler K, et al. Extracellular volume fraction for characterization of patients with heart failure and preserved ejection fraction. J Am Coll Cardiol. 2016;67(15):1815–25. Cardiac magnetic resonance study showing that T1 mapping derived myocardial extracellular volume predicts invasively measured LV stiffness in HFpEF.
Mascherbauer J, Marzluf BA, Tufaro C, Pfaffenberger S, Graf A, Wexberg P, et al. Cardiac magnetic resonance postcontrast T1 time is associated with outcome in patients with heart failure and preserved ejection fraction. Circulation Cardiovascular Imaging. 2013;6(6):1056–65.
• Duca F, Kammerlander AA, Zotter-Tufaro C, Aschauer S, Schwaiger ML, Marzluf BA, Bonderman D, Mascherbauer J. Interstitial Fibrosis, Functional Status, and Outcomes in Heart Failure With Preserved Ejection Fraction: Insights From a Prospective Cardiac Magnetic Resonance Imaging Study. Circulation Cardiovascular imaging. 2016;9(12). T1 mapping study utilizing the modified Look-Locker inversion recovery (MOLLI) sequence showing shorter eventfree survival in patients with higher myocardial extracellular volume.
•• Obokata M, Kane GC, Reddy YN, Olson TP, Melenovsky V, Borlaug BA. Role of Diastolic Stress Testing in the Evaluation for Heart Failure With Preserved Ejection Fraction: A Simultaneous Invasive-Echocardiographic Study. Circulation. 2017;135(9):825–38. Highlights the usefulness of exercise stress testing in the diagnosis of HFpEF
• Kosmala W, Przewlocka-Kosmala M, Rojek A, Marwick TH. Comparison of the Diastolic Stress Test With a Combined Resting Echocardiography and Biomarker Approach to Patients With Exertional Dyspnea: Diagnostic and Prognostic Implications. JACC Cardiovascular imaging. 2018. Study of 171 patients showing that patients with abnormal diastolic response to exercise are at higher risk of cardiovascular hospitalization or death.
Kosmala W, Przewlocka-Kosmala M, Rojek A, Mysiak A, Dabrowski A, Marwick TH. Association of Abnormal Left Ventricular Functional Reserve With Outcome in Heart Failure With Preserved Ejection Fraction. JACC Cardiovascular imaging. 2017.
Abid L, Charfeddine S, Kammoun S. Relationship of left atrial global peak systolic strain with left ventricular diastolic dysfunction and brain natriuretic peptide level in end-stage renal disease patients with preserved left ventricular ejection fraction. J Echocardiogr. 2016;14(2):71–8.
Kusunose K, Motoki H, Popovic ZB, Thomas JD, Klein AL, Marwick TH. Independent association of left atrial function with exercise capacity in patients with preserved ejection fraction. Heart. 2012;98(17):1311–7.
• Santos AB, Roca GQ, Claggett B, Sweitzer NK, Shah SJ, Anand IS, et al. Prognostic relevance of left atrial dysfunction in heart failure with preserved ejection fraction. Circulation Heart Failure. 2016;9(4):e002763. Provides cues to the prognostic value of left atrial strain in HFpEF
• Freed BH, Daruwalla V, Cheng JY, Aguilar FG, Beussink L, Choi A, Klein DA, Dixon D, Baldridge A, Rasmussen-Torvik LJ, Maganti K, Shah SJ. Prognostic utility and clinical significance of cardiac mechanics in heart failure with preserved ejection fraction: importance of left atrial strain. Circulation Cardiovascular imaging. 2016;9(3). Shows with confidence that abnormal left atrial strain in HFpEF is associated to poorer outcomes in HFpEF, revealing a relationship of greater magnitude than left or right ventricular measures.
Telles F, Nanayakkara S, Evans S, Vizi D, William J, Marwick T, Kaye D. Impaired left atrial strain predict abnormal haemodynamics in heart failure with preserved ejection fraction. Heart, Lung and Circulation. 2018;27:S55.
Santos AB, Kraigher-Krainer E, Gupta DK, Claggett B, Zile MR, Pieske B, et al. Impaired left atrial function in heart failure with preserved ejection fraction. Eur J Heart Fail. 2014;16(10):1096–103.
Obokata M, Negishi K, Kurosawa K, Arima H, Tateno R, Ui G, et al. Incremental diagnostic value of la strain with leg lifts in heart failure with preserved ejection fraction. JACC Cardiovascular Imaging. 2013;6(7):749–58.
Martos R, Baugh J, Ledwidge M, O’Loughlin C, Conlon C, Patle A, et al. Diastolic heart failure: evidence of increased myocardial collagen turnover linked to diastolic dysfunction. Circulation. 2007;115(7):888–95.
Borlaug BA, Nishimura RA, Sorajja P, Lam CS, Redfield MM. Exercise hemodynamics enhance diagnosis of early heart failure with preserved ejection fraction. Circulation Heart Failure. 2010;3(5):588–95.
Nagueh SF, Smiseth OA, Appleton CP, Byrd BF 3rd, Dokainish H, Edvardsen T, et al. Recommendations for the Evaluation of Left Ventricular Diastolic Function by Echocardiography: An Update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2016;17(12):1321–60.
• Hasenfuss G, Hayward C, Burkhoff D, Silvestry FE, McKenzie S, Gustafsson F, et al. A transcatheter intracardiac shunt device for heart failure with preserved ejection fraction (REDUCE LAP-HF): a multicentre, open-label, single-arm, phase 1 trial. Lancet (London, England). 2016;387(10025):1298–304. Novel therapeutic approach in HFpEF targeting elevated left atrial pressure with an interatrial shunt device.
• Feldman T, Mauri L, Kahwash R, Litwin S, Ricciardi MJ, van der Harst P, et al. Transcatheter Interatrial Shunt Device for the Treatment of Heart Failure With Preserved Ejection Fraction (REDUCE LAP-HF I [Reduce Elevated Left Atrial Pressure in Patients With Heart Failure]): A Phase 2, Randomized, Sham-Controlled Trial. Circulation. 2018;137(4):364–75. 20.Early results of novel interatrial shunt device showing significant reduction in exercise pulmonary capillary wedge pressure.
• Kaye DM, Nanayakkara S, Vizi D, Byrne M, Mariani JA. Effects of Milrinone on Rest and Exercise Hemodynamics in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;67(21):2554–6. Trial showing that potential of intravenous milrinone for therapeutics in HFpEF with significant reductions in exercise pulmonary capillary wedge pressure.
Kitzman DW, Shah SJ. The HFpEF Obesity Phenotype: The Elephant in the Room. J Am Coll Cardiol. 2016;68(2):200–3.
Shah SJ, Katz DH, Selvaraj S, Burke MA, Yancy CW, Gheorghiade M, et al. Phenomapping for novel classification of heart failure with preserved ejection fraction. Circulation. 2015;131(3):269–79.
•• Ravassa S, Trippel T, Bach D, Bachran D, Gonzalez A, Lopez B, Wachter R, Hasenfuss G, Delles C, Dominiczak AF, Pieske B, Diez J, Edelmann F. Biomarker-based phenotyping of myocardial fibrosis identifies patients with heart failure with preserved ejection fraction resistant to the beneficial effects of spironolactone: results from the Aldo-DHF trial. European journal of heart failure. 2018. Provides insight into a subgroup of HFpEF patients that is resistant to spironolactone therapy.
•• Kosmala W, Rojek A, Przewlocka-Kosmala M, Wright L, Mysiak A, Marwick TH. Effect of Aldosterone Antagonism on Exercise Tolerance in Heart Failure With Preserved Ejection Fraction. J Am Coll Cardiol. 2016;68(17):1823–34. Provides insight into a subgroup of HFpEF patients that is responsive to spironolactone therapy.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Telles, F., Marwick, T.H. Imaging and Management of Heart Failure and Preserved Ejection Fraction. Curr Treat Options Cardio Med 20, 90 (2018). https://doi.org/10.1007/s11936-018-0689-9
Published:
DOI: https://doi.org/10.1007/s11936-018-0689-9