Opinion statement
Pericardial diseases have changed their epidemiology in the past few years. With the aging population and decreasing incidence of communicable diseases, the causes of pericardial diseases have significantly changed from infectious and malignant to postradiation and cardiac surgery causes. Despite that, pericardial diseases remain difficult to diagnose. The accurate and timely diagnosis of these diseases is essential to avoid the late sequela of pericardial constriction and pericardial cirrhosis. Echocardiography remains the first test of choice for the assessment of patients with suspected pericardial diseases. Most patients with acute pericarditis have a self-limiting course and do not need further imaging. However, in the era of multimodality imaging, other modalities, namely, computed tomography (CT) and magnetic resonance imaging (CMR), are often utilized in complex cases. These two modalities provide a wide-open view of the pericardium and adjacent structures. They have high resolution to assess pericardial calcification, a hallmark of many diseases especially tuberculous constrictive pericarditis. CMR is also unique in its ability to assess pericardial late gadolinium enhancement (LGE) and edema. These have been recently suggested to be very important in the progression from acute pericarditis to constrictive pericarditis. In addition, they provide prognostic value to assess which patients are at high risk of developing heart failure and resource utilization. Thus, in the current era, patients with suspected complex pericardial diseases will need a multimodality approach rather than a single modality approach.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pericardial diseases are relatively common worldwide and present an important cause of morbidity and mortality among patients with heart failure symptoms [1]. However, these diseases are often underdiagnosed [2]. This is partially due to uncommon presentations of pericardial pathologies ranging from acute inflammatory diseases to chronic constrictive pericarditis, pericardial masses, cysts, and diverticula as well as congenital absence of the pericardium [3].
The diagnosis of pericardial diseases may cause considerable diagnostic dilemmas especially when the symptoms are non-specific, the physical signs are equivocal and the initial tests of choice are non-diagnostic [4]. In these clinical situations, multimodality imaging has a clear valuable role in establishing the accurate diagnosis and guiding the appropriate management of these patients [4, 5]. However, the appropriate choice of imaging tests requires familiarity with the key imaging modalities needed for each patient to avoid unnecessary testing, undesirable side effects, false-positive results, and inappropriate utilization of resources [3]. Moreover, early diagnosis and prompt treatment increase the likelihood of complete resolution of hemodynamic complications of pericardial diseases. Therefore, the aim of this article is to review the potential role of different imaging modalities in the diagnosis and management of different pericardial disorders and its impact on guiding therapy.
Normal pericardial anatomy
The pericardium consists of two distinct layers that surround the heart. The fibrous pericardium composes the outer sac, encircling the heart and extending up to the proximal portions of the great vessels [6]. The serous pericardium is a very thin layer of tissue that has two layers: the visceral layer, adjacent to the myocardium, and a parietal layer, which is in contact with the inner surface of the fibrous pericardium. Pericardial fat can be found on the surface of the fibrous pericardium, within the pericardial space, or on the epicardial surface between the heart and visceral pericardial layer [6]. The normal thickness of the fibrous and parietal pericardium upon pathological inspection is 0.5–1 mm, but due to partial volume averaging, cardiac motion, and chemical shift artifact, the normal pericardium measures up to 4 mm by cardiac magnetic resonance imaging (CMR) or computed tomography (CT). Table 1 summarizes the differences between echocardiography, CT, and CMR in the assessment of pericardial diseases.
Acute pericardial disease
Acute pericarditis (AP) is a common clinical syndrome encountered in clinical practice. AP is characterized by infiltration of pericardium by inflammatory cells due to primary or secondary pericardial process [7]. It usually lasts less than 3 months, after which time it can be referred to as chronic pericarditis [8]. The common causes of AP include viral and bacterial infection (especially tuberculosis), renal failure, connective tissue diseases, neoplastic invasion of the pericardium, acute myocardial infarction(MI) (25% in post MI patients), aortic dissection, invasive procedures, and trauma [8]. In vast majority of patients, the etiology is thought to be idiopathic or viral. Tuberculous pericarditis is a major cause of pericarditis in developing countries and also in immunocompromised patients.
The diagnosis of acute pericarditis can be easily made, based on the history of chest pain, the presence of a pericardial rub, and the typical changes on electrocardiogram and elevated inflammatory markers [8]. Imaging may not be needed most of the times. In patients with significant clinical symptoms, echocardiography is an essential imaging test for all patients with acute pericarditis and it should be done within 24 h of presentation. The common abnormal findings on echocardiography include increased pericardial brightness, pericardial thickening, `pericardial effusion, and abnormal septal bounce, suggesting an early constriction. Although a pericardial effusion may be absent in a majority of patients with acute pericarditis (up to 60%), it is useful to confirm the diagnosis when a pericardial effusion is found [9, 10]. CT and CMR should be considered in patients with poor prognostic features: high fever > 38.8 °C, atypical presentations, suspicion of constriction, associated chest trauma or concomitant chest and lung diseases [11], failure to respond to therapy, and patients with inconclusive echocardiographic [12]. The 2015 ESC guidelines state that CT and/or CMR are recommended as second-level testing for diagnostic work-up in pericarditis (class I, level of evidence C) [13].
Pericardial effusion
The physiologic amount of fluid in the pericardial space is usually less than 50 ml. The acute and rapid accumulation of fluid in the pericardium as in trauma, ruptured myocardium, aortic dissection, coronary intervention, intracardiac devices implantation, anti-coagulation, and thrombolytic therapy can cause compression of the cardiac chambers, restrict diastolic filling, and cause tamponade and cardiogenic shock [10], whereas larger volumes are needed to cause hemodynamic compromise in subacute and chronic accumulations of pericardial fluid in conditions such as neoplastic involvement of the pericardium, infectious disease (tuberculosis and viral infection), connective tissue diseases, hypothyroidism, and renal failure [14]. Large pleural effusions alone or in association with a small pericardial effusion can also lead to tamponade physiology [10, 15].
Echocardiography remains the test of choice for the assessment of pericardial effusions and their hemodynamic effects [16]. It has the advantage of being a quick, cost-effective, and readily available technique at the patient’s bedside [16]. Pericardial effusion appears as an echo-free space between the two layers of the pericardium and its size can be estimated by echocardiography. However, the volume of pericardial effusion does not necessarily correlate with the clinical symptoms. The qualitative assessment of fluid characteristics by echocardiographic is usually difficult. Nevertheless, echogenic densities seen in complex exudates or hemopericardium with fibrin strands and clots can be easily recognized by echocardiography [17]. This is especially important in patients with associated aortic dissection. A left pleural effusion is distinguished from pericardial fluid as pericardial fluid is anterior, while the pleural effusion is posterior to the descending aorta. Compared to pericardial fluid, the epicardial fat is brighter than the myocardium and moving in concert with the heart. However, this distinction may not be easy and other modalities like CT is needed to make this distinction [12]. CMR could also provide assessment of the pericardial fluid characteristics [6, 18, 19] and help in differentiating exudates from transudates (Table 2).
In addition, the 2015 ESC guidelines for the diagnosis and management of pericardial diseases recommend that CT or CMR should be considered in suspected cases of loculated pericardial effusion, pericardial thickening and masses, as well as associated chest abnormalities [13] (class IIa, level of evidence C).
Cardiac tamponade
Echocardiography remains the main test in the assessment of patients with suspected tamponade, especially in hemodynamically unstable patients [17]. The most important echocardiographic findings include diastolic collapse of right heart chambers, septal bounce, dilated inferior vena cava (IVC), abnormalities of the hepatic vein Doppler, and variability of Doppler flow velocity of mitral and tricuspid valves with respiratory cycle. The right atrial collapse is usually observed during early systole before ventricular collapse when the intracavity pressure is lower and the atrial indentation of the thin-free wall is seen. It is important to note that a brief collapse of the right atrium may occur even in moderate size pericardial effusion in the absence of tamponade because of its thin-walled structure. In addition, the right chamber collapse could be delayed or absent in patients with high baseline right ventricular (RV) pressure such as right ventricular hypertrophy, pulmonary hypertension, positive pressure ventilation, severe LV failure, or some congenital cardiac conditions with increased RV diastolic pressures. In addition, the dilatation of the IVC > 20 mm (IVC plethora), although not very specific, is a very sensitive sign of cardiac tamponade (92%).
Thus, one should keep in mind that there is a large variability in specificity and sensitivity of some echo findings [10, 20]. Of all echocardiographic signs, the absence of collapse of any of the cardiac chambers is the sign with a higher and more consistent negative predictive value to rule out cardiac tamponade. Thus, final decision and management of pericardial effusion should always be made following a detailed physical examination and a proper clinical assessment.
Constrictive pericarditis
Constrictive pericarditis (CP) is characterized by non-compliant pericardium due to focal or global scarring with or without calcification resulting in high impedance to diastolic filling causing elevated systemic venous pressures and right heart failure [21]. The causes of CP are numerous: tuberculous pericarditis is the most common cause of CP in developing countries while idiopathic, postcardiac surgery cases are common in developed countries (1%, 0.2–0.4%, respectively). Less frequent etiologies include connective tissue diseases, malignancies, and radiation.
The diagnosis of CP requires high index of clinical suspicion and often requires confirmation by other imaging modalities including CT, CMR, or even invasive assessment. Echocardiography is the first imaging test in the evaluation of CP [22]. 2D and Doppler echocardiography findings that may suggest CP include increased pericardial thickness with or without calcification, moderate biatrial enlargement, abnormal ventricular septal motion (septal bounce), restrictive mitral and tricuspid inflow velocities with respiratory variation, preserved or increased medial mitral annulus early diastolic (e´) velocity, dilatation of the inferior vena cava (IVC) with < 50% collapsibility with respiration, and increased hepatic vein flow reversal with expiration [23].
However, echocardiography may not be enough to confirm the diagnosis of CP, despite echo-contrast use [24••, 25, 26]. Other modalities may be utilized including CT or CMR. The current ESC guidelines suggest that CT and/or CMR are indicated as second-level imaging techniques (after echocardiography and chest X-ray) to assess calcifications (CT), pericardial thickness, and degree and extension of pericardial involvement [13, 27]. CT is a highly accurate method of evaluating pericardial thickness and thus plays an important role in the diagnosis and management of CP [28]. The normal pericardium is identified as 1 to 2 mm, whereas in CP, the parietal pericardium is usually 4 to 20 mm thick. However, 28% of 143 surgically confirmed cases had normal pericardial thickness on CT, and 18% had normal thickness on histopathologic examination [2, 29]. CT is also an important tool to identify and quantify the extent of pericardial calcification. About 50% of constriction cases show some degree of calcification [27]. Other findings on CT that may suggest CP include a narrowing and tubular deformation of the right or left ventricle, normal or small ventricular size, biatrial enlargement, and straightening of the interventricular septum. Signs of impaired diastolic filling of the RV include dilatation of the IVC, hepatic veins, and right atrium; hepatosplenomegaly; ascites; and pleural effusions. It is important to recognize that thickened pericardium by CT can be found in other conditions, including the early postoperative period, uremia, rheumatic heart disease, sarcoidosis, acute pericarditis with pericardial edema (in the absence of constriction), or as a consequence of radiation therapy [30]. Increased thickness of the pericardium per se does not constitute proof of constriction, but it is a helpful sign if the clinical signs and symptoms of constriction are present. One should also note that CP could be transient too [31].
Given the above, the 2015 ESC guidelines for the diagnosis and management of pericardial diseases provide useful recommendations for the diagnosis and management of pericardial disease which is largely based on expert consensus [13]. CT is particularly important to improve diagnostic confidence when other imaging studies are inconclusive for visualization and diagnosis of pericardial disease. It is a quick test which is often done in few minutes. While it involves exposure to ionizing radiation, routine low dose cardiac CTs are done nowadays [32], [33]. In addition, incidental findings on CT may point to the cause of the CP and have prognostic value [34].
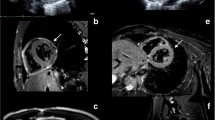
Unlike cardiac CT, CMR does not involve exposure to ionizing radiation. Gated CMR provides direct visualization of the normal pericardium, which is composed of fibrous tissue and has a low CMR signal intensity [12]. CMR is advocated by some as the diagnostic procedure of choice for the detection of certain pericardial diseases, including CP [35]. Comprehensive CMR examinations include bright blood cine images, dark blood edema imaging, tagged cine images, and late gadolinium enhancement (LGE) inversion recovery images [36]. One should note that many of these patients have reduced fitness and advanced heart failure symptoms and could not tolerate the entire long exam; thus, a customized approach is needed per patient [37]. These sequences provide the ability to assess atrial and ventricular size and function, diastolic restraint, diastolic septal bounce, conical deformity of the ventricles or tubular shape of the RV, evidence of increased systemic venous pressure, pericardial thickness, and myocardial fibrosis and inflammation, as well as pericardial inflammation and edema which is seen as pericardial LGE (Fig. 1) [38].
Characteristic CMR features in patients with CP include increased pericardial tethering, pericardial thickening/calcification, tubular/conical deformity of a right ventricle, abnormal diastolic septal motion, and diastolic restraint of the ventricles. CMR better differentiates small effusions from pericardial thickening [39,40,41]. CMR of the pericardium commonly demonstrates LGE in some patients with CP; however, this is not a universal finding [42, 43]. Some patients with CP do not have LGE. Patients with CP and pericardial LGE have greater fibroblast proliferation, chronic inflammation, neovascularization, and pericardial thickening compared with those without LGE [44]. Pericardial LGE might also be a predictor of reversibility of CP after treatment with anti-inflammatory agents [45, 46]. In a study of 29 CP patients received anti-inflammatory medications after CMR, 14/29 patients had resolution of CP, whereas 15/29 patients had persistent CP after 13 months of follow-up [45]. The best predictor of the resolution of CP is the presence of delayed enhancement. In addition, it has an additional prognostic value in predicting clinical improvement and clinical remission [47••, 48••].
In addition, CMR myocardial tagging sequences can demonstrate pericardial-myocardial adherence. Fibrotic pericardial adhesions are present when tag deformation is absent [49, 50]. A recent study demonstrated that real-time phase-contrast CMR acquired over 10 s of unrestricted breathing and without ECG gating could demonstrate the characteristic hemodynamic changes of constrictive physiology. Respiratory variation in transmitral valve flow velocities exceeding 25% was seen in all patients with CP, and a greater variation of 45% was seen across the tricuspid valve in patients with CP. Although theoretically appealing, this sequence is not easy to use in practice, because respiration changes the position of the imaging slice with regard to the mitral and tricuspid valves, potentially influencing inflow velocities or patterns. Calculation of the maximal septal shift is easily achieved providing useful information with regard to ventricular coupling.
Pericardial masses, cysts, and diverticula
Tumors of the pericardium are uncommon and divided into primary (benign and malignant) and secondary (metastatic). Benign tumors can be found in both the parietal pericardium and epicardium as discrete pedunculated or sessile masses and may grow to sizable lesions before they produce compression of cardiac chambers or displacement of mediastinal structures. Even when benign, primary pericardial neoplasms may cause significant cardiovascular complications due to mass effect and hemodynamic effects. The most common benign lesions are pericardial cysts and lipomas, followed by teratoma, fibroma, and hemangioma and lymphangioma. Mesothelioma is the most common primary malignancy of pericardium. Other primary malignant neoplasms include sarcoma (angiosarcoma, liposarcoma, undifferentiated) and lymphoma. Secondary tumors from either local invasion or metastases are by far more common in the pericardium and most frequently occur from lymphoma, melanoma, as well as lung and breast carcinoma. Pericardial tumors generally do not invade into the myocardium except melanoma, which classically involves the myocardium as well.
Although echocardiography is an appropriate initial study for evaluation of cardiac tumors, or metastatic involvement and hemodynamic consequences of pericardial involvement, it is limited by being operator-dependent, bone and lung interference, narrow sector window and poor visualization in obesity, chronic obstructive pulmonary disease, and postcardiac surgery patients. Hence, cross-sectional imaging, either CT or CMR, is required for further tissue characterization and involvement of adjacent structures. CT provides a good assessment of the mass, its vascularity, and involvement of adjacent tissues. CMR is the test of choice for tissue characterization and assessment of the lesion.
Pericardial cysts and diverticula
Similarly, pericardial cystic lesions are rare and comprise 7% of the mediastinal masses and 33% of mediastinal cysts [51]. Pericardial cyst and diverticulum share similar developmental origin and may appear as an incidental finding in chest X-ray in an asymptomatic patient. Pericardial cysts are probably a remnant of a diverticulum whose communication to the pericardial cavity has been obliterated [15, 51, 52].
At chest X-ray, a pericardial cyst appears as a well-defined, smooth, anterior mediastinal mass. On echocardiography, pericardial cyst appears as a well-circumscribed, homogeneous echolucent space adjacent to the cardiac border. The presence of echo-free space indicates its separation from the cardiac chambers [52]. The common site of cyst is adjacent to the right atrium and frequently compressing the atrium. The lack of flow by color or PW Doppler supports the diagnosis of a cyst.
Pericardial diverticula appear similar on echocardiography to cysts. The distinction between pericardial cysts and diverticulum is difficult as both lesions have similar radiological findings and is based on identifying a defect in the pericardial lining and the presence of communication between pericardium and the cystic cavity in the case of a diverticulum.
Conclusion
Multimodality imaging is very helpful in the diagnosis of pericardial disorders. While echocardiography is the initial test to make these diagnoses, CT and CMR provide accurate measurement of the pericardial thickness. In addition, CMR is the test of choice to diagnose constriction, guide therapy, and provide prognostic information.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Yusuf SW, Hassan SA, Mouhayar E, Negi SI, Banchs J, O’Gara PT. Pericardial disease: a clinical review. Expert Rev Cardiovasc Ther. 2016;14:525–39.
Maisch B, Seferovic PM, Ristic AD, Erbel R, Rienmuller R, Adler Y, et al. Task Force on the D and Management of Pricardial Diseases of the European Society of C. Guidelines on the diagnosis and management of pericardial diseases executive summary: the task force on the diagnosis and management of pericardial diseases of the European society of cardiology. Eur Heart J. 2004;25:587–610.
Kireyev D, Hung J. Pericardial disease. Card Imaging Clin Pract: Springer; 2016: 247.
Troughton RW, Asher CR, Klein AL. Pericarditis. Lancet. 2004;363:717–27.
Hoit BD. Imaging the pericardium. Cardiol Clin. 1990;8:587–600.
Al-Mallah M, Kwong RY. Assessing pericardial disease by CMR. Cardiovasc Magn Reson Imaging. Humana Press;2008: 467.
Spodick DH. Acute pericarditis: current concepts and practice. JAMA. 2003;289:1150–3.
LeWinter MM. Acute pericarditis. N Engl J Med. 2014;371:2410–6.
Bogaert J, Cruz I, Voigt J-U, Sinnaeve P, Imazio M. Value of pericardial effusion as imaging biomarker in acute pericarditis, do we need to focus on more appropriate ones? Int J Cardiol. 2015;191:284–5.
Sagrista-Sauleda J, Merce AS, Soler-Soler J. Diagnosis and management of pericardial effusion. World J Cardiol. 2011;3:135–43.
Morris PB, Ference BA, Jahangir E, Feldman DN, Ryan JJ, Bahrami H, et al. Cardiovascular effects of exposure to cigarette smoke and electronic cigarettes. J Am Coll Cardiol. 2015;66:1378–91.
Wang ZJ, Reddy GP, Gotway MB, Yeh BM, Hetts SW, Higgins CB. CT and MR imaging of pericardial disease. Radiographics. 2003;23:S167–80.
Adler Y, Charron P, Imazio M, Badano L, Barón-Esquivias G, Bogaert J, et al. 2015 ESC Guidelines for the diagnosis and management of pericardial diseases: the Task Force for the Diagnosis and Management of Pericardial Diseases of the European Society of Cardiology (ESC) Endorsed by: The European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J. 2015;36:2921–64.
Rehman KA, Betancor J, Xu B, Kumar A, Rivas CG, Sato K, et al. Uremic pericarditis, pericardial effusion, and constrictive pericarditis in end-stage renal disease: insights and pathophysiology. Clin Cardiol. 2017; https://doi.org/10.1002/clc.22770.
Verhaert D, Gabriel RS, Johnston D, Lytle BW, Desai MY, Klein AL. The role of multimodality imaging in the management of pericardial disease. Circ Cardiovasc Imaging. 2010;3:333–43.
Ristić AD, Imazio M, Adler Y, Anastasakis A, Badano LP, Brucato A, et al. Triage strategy for urgent management of cardiac tamponade: a position statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur Heart J. 2014;35:2279–84.
Klein AL, Abbara S, Agler DA, Appleton CP, Asher CR, Hoit B, et al. American Society of Echocardiography clinical recommendations for multimodality cardiovascular imaging of patients with pericardial disease: endorsed by the Society for Cardiovascular Magnetic Resonance and Society of Cardiovascular Computed Tomography. J Am Soc Echocardiogr. 2013;26:965–1012 e15.
Al-Mallah M, Kwong RY. Clinical application of cardiac CMR. Rev Cardiovasc Med. 2009;10:134–41.
Al-Mallah MH. Introduction to the special issue: myocardial imaging in heart failure. Heart Fail Rev. 2017;22:381–3.
Palmer WC, Kurklinsky A, Lane G, Ussavarungsi K, Blackshear JL. Cardiac tamponade due to low-volume effusive constrictive pericarditis in a patient with uncontrolled type II autoimmune polyglandular syndrome. Acute Card Care. 2014;16:23–7.
Porta-Sánchez A, Sagristà-Sauleda J, Ferreira-González I, Torrents-Fernández A, Roca-Luque I, García-Dorado D. Constrictive pericarditis: etiologic spectrum, patterns of clinical presentation, prognostic factors, and long-term follow-up. Rev Esp Cardiol (Engl Ed). 2015;68:1092–100.
Welch TD, Ling LH, Espinosa RE, Anavekar NS, Wiste HJ, Lahr BD, et al. Echocardiographic diagnosis of constrictive pericarditis: Mayo Clinic criteria. Circ Cardiovasc Imaging. 2014;7:526–34.
Klein AL, Cohen GI, Pietrolungo JF, White RD, Bailey A, Pearce GL, et al. Differentiation of constrictive pericarditis from restrictive cardiomyopathy by Doppler transesophageal echocardiographic measurements of respiratory variations in pulmonary venous flow. J Am Coll Cardiol. 1993;22:1935–43.
•• Alraies MC, Aljaroudi W, Yarmohammadi H, Yingchoncharoen T, Schuster A, Senapati A, et al. Usefulness of cardiac magnetic resonance-guided management in patients with recurrent pericarditis. Am J Cardiol. 2015;115:542–7. The aim of this study was to assess the utility of CMR in the management of RP compared with standard therapy. A total of 507 consecutive patients with RP after the first attack, all of whom were treated with colchicine and nonsteroidal anti-inflammatory drugs as first-line therapy, were retrospectively evaluated. CMR-guided therapy modulates the management of RP. This approach decreased pericarditis recurrence and exposure to steroids.
Alraies MC, Klein AL. Q: Should we still use electrocardiography to diagnose pericardial disease? Cleve Clin J Med. 2013;80:97–100.
Khawaja OA, Shaikh KA, Al-Mallah MH. Meta-analysis of adverse cardiovascular events associated with echocardiographic contrast agents. Am J Cardiol. 2010;106:742–7.
Al-Mallah MH, Aljizeeri A, Villines TC, Srichai MB, Alsaileek A. Cardiac computed tomography in current cardiology guidelines. J Cardiovasc Comput Tomogr. 2015;9:514–23.
Ling LH, Oh JK, Schaff HV, Danielson GK, Mahoney DW, Seward JB, et al. Constrictive pericarditis in the modern era evolving clinical spectrum and impact on outcome after pericardiectomy. Circulation. 1999;100:1380–6.
Talreja DR, Edwards WD, Danielson GK, Schaff HV, Tajik AJ, Tazelaar HD, et al. Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation. 2003;108:1852–7.
Sechtem U, Tscholakoff D, Higgins CB. MRI of the abnormal pericardium. Am J Roentgenol. 1986;147:245–52.
Gentry J, Klein AL, Jellis CL. Transient constrictive pericarditis: current diagnostic and therapeutic strategies. Curr Cardiol Rep. 2016;18:41.
Chinnaiyan KM, Bilolikar AN, Walsh E, Wood D, DePetris A, Gentry R, et al. CT dose reduction using prospectively triggered or fast-pitch spiral technique employed in cardiothoracic imaging (the CT dose study). J Cardiovasc Comput Tomogr. 2014;8:205–14.
Al-Mallah MH, Aljizeeri A, Alharthi M, Alsaileek A. Routine low-radiation-dose coronary computed tomography angiography. Eur Heart J Suppl. 2014;16:B12–6.
Qureshi WT, Alirhayim Z, Khalid F, Al-Mallah MH. Prognostic value of extracardiac incidental findings on attenuation correction cardiac computed tomography. J Nucl Cardiol. 2016;23:1266–74.
Aljizeeri A, Sulaiman A, Alhulaimi N, Alsaileek A, Al-Mallah MH. Cardiac magnetic resonance imaging in heart failure: where the alphabet begins! Heart Fail Rev. 2017;22:385–99.
Al-Mallah MH, Shareef MN. The role of cardiac magnetic resonance imaging in the assessment of non-ischemic cardiomyopathy. Heart Fail Rev. 2011;16:369–80.
Al-Mallah MH, Keteyian SJ, Brawner CA, Whelton S, Blaha MJ. Rationale and design of the Henry Ford Exercise Testing Project (the FIT project). Clin Cardiol. 2014;37:456–61.
Frank H, Globits S. Magnetic resonance imaging evaluation of myocardial and pericardial disease. J Magn Reson Imaging. 1999;10:617–26.
Young PM, Glockner JF, Williamson EE, Morris MF, Araoz PA, Julsrud PR, et al. MR imaging findings in 76 consecutive surgically proven cases of pericardial disease with CT and pathologic correlation. Int J Cardiovasc Imaging. 2012;28:1099–109.
Taylor AM, Dymarkowski S, Verbeken EK, Bogaert J. Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: initial results. Eur Radiol. 2006;16:569–74.
Pinamonti B, Habjan S, De Luca A, Proclemer A, Morea G, Abate E, et al. Work-up and management of constrictive pericarditis: a critical review. G Ital Cardiol. 2016;17:197–207.
Glower DD. Sticking points in magnetic resonance diagnosis of constrictive pericarditis. J Thorac Cardiovasc Surg. 2016;151:1356–7.
Mullen L, Chew PG, Frost F, Ahmed A, Khand A. Hyperenhancement of the pericardium on cardiac magnetic resonance imaging: a marker of acute inflammation and neovascularization or a chronic fibrotic state? Cardiology. 2016;133:239–41.
Rienmüller R, Gröll R, Lipton MJ. CT and MR imaging of pericardial disease. Radiol Clin. 2004;42:587–601.
Feng D, Glockner J, Kim K, Martinez M, Syed IS, Araoz P, et al. Cardiac magnetic resonance imaging pericardial late gadolinium enhancement and elevated inflammatory markers can predict the reversibility of constrictive pericarditis after antiinflammatory medical therapy: a pilot study. Circulation. 2011;124:1830–7.
Zurick AO, Bolen MA, Kwon DH, Tan CD, Popovic ZB, Rajeswaran J, et al. Pericardial delayed hyperenhancement with CMR imaging in patients with constrictive pericarditis undergoing surgical pericardiectomy: a case series with histopathological correlation. J Am Coll Cardiol Img. 2011;4:1180–91.
•• Kumar A, Sato K, Yzeiraj E, Betancor J, Lin L, Tamarappoo BK, et al. Quantitative pericardial delayed hyperenhancement informs clinical course in recurrent pericarditis. JACC Cardiovasc Imaging. 2017; https://doi.org/10.1016/j.jcmg.2016.10.020. This is a retrospective cohort study of 159 patients with RP who underwent DHE imaging and had a follow-up period of more than 6 months. Pericardial inflammation was quantified on short-axis DHE sequences by contouring the pericardium, selecting normal septal myocardium as a reference region, and then quantifying the pericardial signal that was > 6 SD above the reference. Quantitative assessment of pericardial DHE was associated with clinical outcomes among patients with RP and provided incremental information regarding the clinical course of patients with RP.
•• Cremer PC, Tariq MU, Karwa A, Alraies MC, Benatti R, Schuster A, et al. Quantitative assessment of pericardial delayed hyperenhancement predicts clinical improvement in patients with constrictive pericarditis treated with anti-inflammatory therapy. Circ Cardiovasc Imaging. 2015;8:e003125. This study included 41 consecutive patients with constrictive pericarditis who had a cardiovascular magnetic resonance study with DHE prior to the initiation of anti-inflammatory medications. In patients with constrictive pericarditis treated with anti-inflammatory therapy, a quantitative assessment of pericardial DHE can provide incremental information to predict clinical improvement when added to clinical factors and Westergren sedimentation rates.
Thavendiranathan P, Verhaert D, Walls MC, Bender JA, Rajagopalan S, Chung Y-C, et al. Simultaneous right and left heart real-time, free-breathing CMR flow quantification identifies constrictive physiology. J Am Coll Cardiol Img. 2012;5:15–24.
Oh JK, Chang S-A, Choe Y-H, Young PM. CMR imaging for diastolic hemodynamic assessment: fantasy or reality? J Am Coll Cardiol Img. 2012;5:25–7.
Patel J, Park C, Michaels J, Rosen S, Kort S. Pericardial cyst: case reports and a literature review. Echocardiography. 2004;21:269–72.
Cangemi V, Volpino P, Gauldi G, Polettini E. Pericardial cysts of the mediastinum. J Cardiovasc Surg. 1999;40:909.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
The authors declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Imaging
Rights and permissions
About this article
Cite this article
Al-Mallah, M.H., Almasoudi, F., Ebid, M. et al. Multimodality Imaging of Pericardial Diseases. Curr Treat Options Cardio Med 19, 89 (2017). https://doi.org/10.1007/s11936-017-0590-y
Published:
DOI: https://doi.org/10.1007/s11936-017-0590-y