Abstract
To describe findings of patients with surgically confirmed pericardial disease on state of the art MR sequences. Retrospective review was performed for patients who underwent pericardiectomy and preoperative MR over a 5 year period ending in 2009. Patients’ records were reviewed to confirm the diagnosis of chronic recurrent pericarditis, constrictive pericarditis, or pericardial tumor. MR imaging findings of pericardial thickness, IVC diameter, presence or absence of pericardial or pleural effusion, pericardial edema, pericardial enhancement, and septal “bounce” were recorded. Patients with constriction had a larger IVC diameter (3.1 ± 0.4 cm) than patients with recurrent pain and no constriction (2.0 ± 0.4 cm). Mean pericardial thickness for the 16 patients with chronic recurrent pericarditis but no evidence of constriction was 4.8 ± 2.9 mm. Mean pericardial thickness for patients with constriction was 9.2 ± 7.0 cm with calcification, and 4.6 ± 2.1 cm without calcification. 94% of patients with chronic recurrent pericarditis had gadolinium enhancement of the pericardium, while 76% of patients with constriction had pericardial enhancement. Septal “bounce” was present in 19% of chronic recurrent pericarditis cases and 86% of constriction cases. 5 patients had a pericardial neoplasm, 1 of which was not identified preoperatively. State of the art MR techniques can identify significant and distinct findings in patients with chronic recurrent pericarditis, constrictive pericarditis, and pericardial tumors.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Pericardial disease can be difficult to diagnose, and often requires a multimodality imaging approach. Magnetic resonance (MR) imaging of pericardial disease was first reported over 25 years ago [1]. However, correlation of MR imaging findings to pathology [1–7] has been limited to small samples of patients. In addition, the largest studies correlating MR imaging features with clinical and pathologic findings were performed using what is today considered low field strength MR technology [1, 3, 4, 6] and relied on conventional spin echo sequences which are rarely employed today.
More recent reports have focused additional use of other MR techniques for assessment of pericardial disease, including use of contrast enhancement [5, 7–9], fluid sensitive imaging [10], and abnormal diastolic septal motion [11]. These reports of imaging findings in different forms of pericardial disease, however, are limited by small cohort sizes.
Most patients in our active pericardial disease clinic undergo MR imaging. Using 1.5 Tesla scanners with modern coils and some of the imaging approaches detailed below, we feel that MR provides valuable insight into the disease processes affecting these patients. In addition, many patients with chronic recurrent pericarditis, constrictive pericarditis, or pericardial tumors undergo radical or partial pericardiectomy procedures at our institution. With these considerations in mind, the current retrospective study was designed to correlate MR imaging findings with clinical and pathologic features in a relatively large number of patients from a single institution who had subsequently undergone pericardiectomy. We acknowledge that there is inherent bias in our findings because the results only represent findings in the subset of patients with disease sufficiently severe to warrant referral to and surgical intervention in a tertiary center. Thus, we cannot provide meaningful sensitivity and specificity numbers for MR to detect pericarditis or constriction in a specific patient with this approach. However, we feel that presenting findings in a sizable cohort of patients with significant confirmed disease and correlating the findings with clinical and pathologic features represents an important contribution to understanding MR findings in patients with pericardial disease.
Methods and materials
This retrospective study had institutional IRB approval. All subjects had signed permission for their medical records to be used for research purposes pursuant to Minnesota state law. The radiology records system was searched for all MR examinations of the heart or chest with a clinical indication including “pericardium”, “pericardial”, “pericarditis”, or “constrictive”, over a 5 year period from January 1, 2005, to December 31, 2009. This resulted in 494 individual exams, which were cross-referenced to determine whether the patients had undergone pericardial surgery.
This resulted in 76 subjects with preoperative MR and subsequent pericardiectomy. The patients’ medical records, including operative findings, pathologic findings, echocardiographic findings, catheterization results, and preoperative and postoperative clinic visits, were comprehensively reviewed by a single author (PMY) to confirm the presence of chronic recurrent pericarditis, constrictive pericarditis, or pericardial tumor. No patients were excluded from the analysis based on this comprehensive review.
All of the examinations were performed using an 8 element phased array cardiac coil on a 1.5T scanner (Signa, GE Healthcare, Wauskesha, WI, USA). Imaging protocols varied somewhat over the 5 years and between patients, but generally involved short and long axis balanced steady-state free precession (bSSFP) sequences, axial or short axis double inversion recovery fast spin echo (DIRFSE) sequences (TR 1-2RR, TE 15–46, ETL 32), dynamic myocardial perfusion imaging, and inversion recovery gradient echo (IR-GRE) sequences beginning 5–10 min after IV contrast administration, with the inversion time set to null myocardial signal. Other sequences often or sometimes employed included triple inversion recovery fast spin echo (TR 2RR, TE 46–85), axial gated bSSFP, fat-suppressed ungated bSSFP, and free breathing short axis bSSFP for assessment of free-breathing changes in septal motion.
The exams were reviewed on in-house imaging review software (QREADS) by a single reader (PMY) to confirm findings on the official clinical interpretations. The presence or absence of pericardial delayed enhancement was recorded, and any delayed enhancement was classified as diffuse or segmental and smooth or nodular. Maximum pericardial thickness was measured on delayed IR-GRE imaging (if performed) or on double inversion recovery fast spin echo (DIRFSE) sequences if delayed enhancement was not performed. Delayed enhancement sequences were chosen because it was easier to avoid including pericardial fluid in the measurement on these sequences than on DIRFSE [7, 8], and because they were more routinely and consistently prescribed than DIRFSE. In most patients, we run the IR-GRE sequences in both the short axis and axial planes for pericardial imaging. The measurement was obtained in the thickest section of apposed pericardium which was not artificially thickened because of through-plane volume averaging. In patients who also had a preoperative chest CT, the presence or absence of pericardial calcification was also recorded. If the thickest portion of pericardium was calcified on CT, the calcification was included in the measurement.
Also recorded were presence or absence pleural effusion, pleural enhancement, and pericardial effusion. IVC diameter was measured in shortest dimension on axial imaging (usually DIRFSE) on the highest image below the confluence with the right atrium.
Finally, the subjective presence or absence of abnormal diastolic septal motion (flattening or inversion) on breath-held bSSFP short axis and 4 chamber images was noted as described in the clinical dictations.
In 8 cases in which the interpretation of the primary study reader regarding presence or absence of delayed enhancement of the myocardium or pericardium or of abnormal septal motion differed from the official clinical interpretation, a tie-breaker reader (EEW) analyzed the images, blinded to the clinical dictation and first reader’s interpretation, and his interpretation was considered final. The tie-breaker reader was aware that the study was regarding MR findings in pericardial disease but was not aware that each patient had undergone subsequent pericardiectomy.
Results
Clinical categories
The total number of patients operated was 76, with characteristics described in Table 1. The indications for surgery were constrictive pericarditis in 53 patients, chronic relapsing pericarditis in 16, overlapping symptoms of constrictive and chronic recurrent pericarditis in 2, and known pericardial malignancy in 5 (Table 2). In the 2 patients with a mixed picture of constriction and chronic relapsing pericarditis, constriction was the dominant symptom leading to operation in both patients; these 2 patients are included in both groups for purposes of analysis unless noted otherwise. In addition to the 52 patients with constriction listed above and the 5 patients with known tumors, 1 additional patient was operated for constriction and found by the pathologist to have malignant mesothelioma as the primary underlying process. This patient was therefore counted as part of the malignancy group even though he presented with symptoms of constriction.
Etiologies
Chronic recurrent pericarditis was idiopathic in 12 of the 18 patients (including the 2 with overlapping symptoms). For the remaining 6 patients, there was strong evidence for a viral cause in 2, autoimmune disorder in 2, eosinophilic pericarditis in 1, and iatrogenic pericarditis following an epicardial electrophysiologic radiofrequency ablation for frequent symptomatic premature ventricular contractions in 1 (Table 3).
Pericardial constriction was iatrogenic in 19 of the 54 patients (52 with isolated constriction, not including the patient with mesothelioma, plus the 2 with overlapping constriction and chronic recurrent pericarditis). The iatrogenic causes included heart surgery in 13 and mediastinal irradiation in 6. Etiology was idiopathic in 18 patients. Other clinically suspected causes included post-viral in 10 patients, autoimmune in 3 patients, post-traumatic in 2 patients, and exposure to Histoplasma or asbestos in 1 patient each.
Pathologic features
There were notable differences in pathologic findings between patients with chronic recurrent pericarditis and those with constriction. Pericardial histology from 18 patients with chronic pain revealed not only thickening and inflammation but also edema in 7 patients, granulation tissue in 7, organizing fibrinous exudates in 7, neovascularization in 3, and mesothelial cell proliferation in 2. An additional 2 patients were simply described as having “features consistent with chronic pericarditis”.
All patients operated for isolated constriction (not including the patients with overlapping chronic recurrent pericarditis) showed fibrous or fibrocalcific thickening and varying degrees of chronic lymphoplasmacytic inflammation in the excised parietal pericardium. Six patients also showed organizing fibrinohemorrhagic exudates, indicative of an ongoing inflammatory process. No pathology report mentioned neovascularization, edema, granulation tissue, or mesothelial cell proliferation.
MR imaging features
IVC diameter
Patients with constriction had a larger IVC diameter (3.1 ± 0.4 cm) than patients with recurrent pain and no constriction (2.0 ± 0.4 cm; Fig. 1).
Calcification
10 of 18 patients with chronic relapsing pericarditis had a preoperative chest CT. Only 1/10 patients had calcifications evident. These were quite small and confirmed on histologic examination. This was not one of the 2 patients with overlapping symptoms of constriction. None of the other 17 patients had calcification noted on histology (Fig. 2).
a Axial noncontrast CT image in a 37 year old female with viral chronic relapsing pericarditis demonstrates small pericardial effusion anteriorly (arrow) without calcification. b Axial contrast enhanced CT image obtained 12 months after 12a demonstrates pericardial thickening, fluid, and enhancement (arrow), again without calcification. c Axial post-contrast IR-GRE MR sequence 12 months after 12b demonstrates resolution of the effusion, but persistent pericardial enhancement (arrow)
51 of 54 patients with constriction had a preoperative chest CT. 22/51 (43%) had calcifications evident on CT. None of the clinically suspected etiologies was more likely to have associated calcifications than another (Fig. 3).
Pleural and pericardial effusion
4 of 18 patients (22%) with chronic relapsing pericarditis had a pleural effusion, although 2 of these 4 had primary symptoms of constriction which was secondarily associated with recurrent pain. 3 of 18 patients with pain had visible pericardial fluid; 2 of these 3 patients were the 2 patients with pleural effusion but no evidence of constriction (Fig. 1).
37 of 54 patients (69%) with constriction had pleural fluid present (Figs. 1, 4, 6); 12 of these patients had pericardial fluid as well (“effusive-constrictive pericarditis”). 3 patients had pericardial fluid visible but no pleural effusion. 7 of the patients with pleural effusion had adjacent pleural enhancement; 1 patient had pleural delayed enhancement without adjacent pleural effusion.
Pericardial thickness
The mean pericardial thickness for the 16 patients with chronic recurrent pericarditis but no evidence of constriction was 4.8 ± 2.9 mm.
Mean pericardial thickness for patients with constriction (with or without symptoms of chronic recurrent pain) was 9.2 ± 7.0 mm for patients with calcification, and 4.6 ± 2.1 cm for those without calcification (Fig. 4).
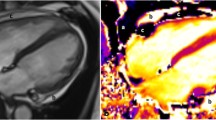
a Axial contrast-enhanced CT scan in a 65 year old patient with constriction demonstrating normal thickness pericardium (arrows). b Horizontal long axis (4 chamber) post contrast fat-suppressed inversion recovery gradient echo MR sequence (IR-GRE) performed 2 weeks after the CT scan demonstrates smooth circumferential delayed enhancement of the normal thickness pericardium (arrowheads)
Edema-sensitive imaging
8 of 18 patients with chronic recurrent pericarditis had a fluid-sensitive triple inversion recovery fast spin echo (TIRFSE) sequence performed as part of their examination. 5 of 8 patients had markedly increased signal in the pericardial tissue (not due to effusion; Fig. 5). In each of these cases, the pathologic report specifically commented on significant edema (4 patients) and chronic nongranulomatous inflammation. 2 further patients had mild to moderately increased pericardial signal on TIRFSE, and the pathology in these cases reported mild nongranulomatous inflammation. One of these 2 cases actually was primarily operated for constriction as the dominant symptom (but also had symptoms of recurrent pain), and the pathology report also indicated focal calcification and marked fibrotic thickening. Only one patient with a dominant symptom of pain had no evidence of signal abnormality on TIRFSE; this patient’s pathology demonstrated marked fibrosis and no comment of edema. The patient did not experience improvement of his baseline pain postoperatively.
a Axial triple inversion recovery fast spin echo (TIRFSE) MR image in a 44 year old male with idiopathic chronic relapsing pericarditis demonstrates T2 hyperintensity, suggesting pericardial edema (arrows). Pleural fluid is also present. b Post contrast fat-suppressed IR-GRE MR sequence demonstrates extensive pericardial thickening and enhancement (arrows), pericardial effusion, and enhancing pericardial synechia. Pleural fluid is also present
Among patients operated for constriction, 34/54 had a TIRFSE sequence performed. Of these, only 1/34 had marked pericardial signal abnormality in the pericardium. Histology on this patient demonstrated evidence of prior hemorrhage and diffuse organizing pericarditis. 2 had moderate signal abnormality; both had pathology reports which were identical to the other patients, describing noncalcific fibrous thickening and nongranulomatous lymphoplasmacytic infiltrates. 11/34 had mild pericardial signal abnormality and 13/34 had no abnormal pericardial signal. 7/34 had mildly elevated signal in the epicardial or mediastinal fat adjacent to the pericardium but not in the pericardium itself (Fig. 6).
a Short axis TIRFSE MR sequence in a 39 year old male with autoimmune constrictive pericarditis demonstrates edema-like signal (arrow) in the epipericardial fat adjacent to pericardium demonstrating no abnormal signal (arrowheads). b Post-contrast IR-GRE MR sequence in a patient with constriction demonstrates faint increased delayed enhancement of the visceral (arrowheads) and parietal (arrows) pericardium, both of which appear normal in thickness
Pericardial delayed enhancement
17/18 patients with chronic pain symptoms had post contrast delayed enhancement imaging as part of their MR examination, and 16/17 patients (94%) had evidence of pericardial delayed enhancement. In 11/16 patients (69%) the delayed enhancement was diffuse, while in 5/16 it was segmental (31%). In 12/16 (75%) patients it was characterized as smooth, while in 4/16 (25%) cases it was nodular (Fig. 5).
3 patients with constrictive symptoms did not receive IV gadolinium contrast because of renal insufficiency. 39 of the 51 patients (76%) with constrictive symptoms and post-contrast delayed enhancement imaging did demonstrate some degree of pericardial delayed enhancement; it was usually much less intense than seen in the patients with pain and became more prominent with longer delays from injection (Fig. 6). An additional 4 patients had prominent delayed enhancement of the adjacent mediastinal or epicardial fat which did not involve the pericardium per se. In 18/39 cases (46%) the delayed enhancement was segmental, and in 21/39 (54%) it was diffuse. In 32/39 patients (82%) the pericardial enhancement was smooth, while in 7/39 (18%) it was nodular. The presence of diffuse/segmental or smooth/nodular enhancement was not particularly associated with any suspected etiology.
Repeat imaging after trial of medical therapy
2 patients with chronic recurrent pericarditis had a trial of medical therapy and pre- and post-therapy contrast-enhanced MR exam. In both cases there was no response to medical therapy; in one case the extent and severity of delayed enhancement was unchanged and in the other it was increased on the followup study.
6 patients with constrictive symptoms had a trial of medical therapy and were imaged with contrast-enhanced MR a second time after medical therapy but prior to surgery. None of these patients had pericardial calcifications; their average pericardial thickness on the initial scan was 5.3 mm. All 6 patients had partial but inadequate clinical response or responded well but were unable to be weaned from high dose steroids without recurrence of symptoms. All these patients demonstrated decreased but persistent pericardial enhancement following medical treatment.
Abnormal septal motion
Of the 16 patients with chronic recurrent pericarditis and no clinical evidence of constriction, 3 were found to have abnormal diastolic septal motion. All 3 had echocardiography performed within 1 day of MR; in 1 case echo findings were positive for constrictive physiology while in 2 echo was negative for constriction. The other 13 patients without abnormal septal motion on MR also had echocardiography within 1 day (10 patients), 2 days (2 patients) or 4 days (1 patient); none had evidence of constriction.
48 of 56 patients (86%) with constrictive symptoms had abnormal diastolic septal motion on breath-held bSSFP sequences. Of the 8 patients without abnormal septal motion, the most recent echocardiograms had been considered negative for constriction in 2 (one performed on the same day, the other 6 days prior), and equivocal in 2 (both 5 days before MR). The other 4 patients had echocardiographic evidence of constriction but normal diastolic septal motion on MR performed on the same day (2 patients) or day prior to (2 patients) the echocardiogram. In one of these 4 patients, invasive hemodynamic measurements were obtained because of the discrepancy, and the findings were considered equivocal for the diagnosis of constriction.
In the 48 constriction patients with abnormal diastolic septal motion on MR, all had a recent (range −14 to +60 days, all but 4 within 4 days) echocardiogram. In 39 patients, the echocardiogram was also suggestive of constriction. However, 7/48 (15%) had an echocardiogram which was inconclusive or technically limited, and in 2 the findings were considered negative for constriction. In 4/7 cases considered inconclusive and 1 case considered negative by echocardiogram, invasive hemodynamics were obtained and considered diagnostic of constrictive physiology in all cases.
Neoplasms
5 patients were operated for known or suspected neoplasm involving the pericardial space. The tumors represented cardiac angiosarcoma, AV groove paraganglioma, primary pericardial synovial sarcoma, recurrent pleiomorphic rhabdomyosarcoma, and invasive thymoma. In 4 of 5, TIRFSE sequences were performed and in all cases showed high signal corresponding to the bulk of the tumor. In 4 of 5 cases, postcontrast imaging was performed and showed nodular enhancement. None of the patients with known tumors had evidence of constriction on MR or echocardiography (Fig. 7). A 6th patient was operated for symptoms of constriction but was found to have malignant pericardial mesothelioma as the underlying process on histology. This patient had diffuse nodular enhancement on delayed post-contrast imaging; a TIRFSE was not performed (Fig. 8).
a Axial TIRFSE in a 42 year old female with recurrent pleiomorphic rhabdomyosarcoma in the pericardium demonstrates diffuse mild pericardial thickening and high signal (arrowheads) with focal masslike areas (arrows). b Short axis post-contrast IR-GRE MR sequence demonstrates diffuse pericardial thickening and enhancement (arrowheads), as well as invasion of the lateral wall of the left ventricle by one of the confluent pericardial masses (arrow)
Short axis post-contrast IR-GRE sequence in a 76 year old male with constriction who was found to have malignant mesothelioma at histology. The MR demonstrates diffuse nodular delayed enhancement; the findings were not prospectively or retrospectively distinguishable from other etiologies of pericardial constriction
Discussion
The results suggest that MR findings can illustrate some different features between the underlying pathologic process and physiologic effects of recurrent relapsing pericarditis, pericardial constriction, and pericardial tumors. Although this study is observational rather than quantitative in nature, a number of features seen on MR showed correlation with pathologic findings.
The presence of significant edema-like signal in pericardial tissue on TIRFSE images correlated in several cases with pathologic findings of edema, neovascularization, and/or granulation tissue. Prolonged pericardial T2 may therefore suggest edema, neovascularization, and/or granulation tissue in the pericardium in patients with chronic pain. Nearly all of the patients with constriction had little or no edema-like signal in the pericardium. A previous case report has documented high T2 signal in the pericardium in acute pericarditis which also suggests that this may be a useful marker for pericardial edema or granulation tissue [10]. Although we have no control group for comparison and the study numbers are small, the presence of edema-like signal in the pericardium despite appropriate medical therapy might also indicate a higher likelihood of necessity for surgical treatment to relieve pericardial pain.
Pericardial calcifications, pleural effusions, and increased IVC diameter were much more common in patients with constrictive physiology than chronic recurrent pericarditis. However, patients with chronic recurrent pericarditis had a similar pericardial thickness to those with non-calcified constrictive pericarditis. The average pericardial thicknesses of 4.6 and 4.8 mm respectively are similar to an average histologic measurement of 4 mm obtained by review of a large cohort at our institution [12]. An upper normal value cutoff for diagnosing thickened pericardium on MR has traditionally been 4 mm [1, 4, 6]. However, the inherent resolution of the images in these studies was 7 × 1.7 × 1.7 mm, which can be exceeded with modern techniques. In addition, recent work has shown that pericardial constriction can occur even in patients with histologically normal pericardial thickness [13]. This has been presumed secondary to epicardial or epipericardial constriction. Interestingly, 7/34 patients with constriction had edema-like signal in the epicardial or mediastinal fat, but not in the pericardium itself, and an additional 4 patients had enhancement in the epi-pericardial fat. These findings might indicate a higher likelihood of constriction in the presence of a normal thickness pericardium. We do not use a strict cut-off value for pericardial thickness in diagnosing pericardial disease.
Previous work has suggested that pericardial delayed enhancement can indicate inflammation [5]. While pericardial delayed enhancement was seen in 94% of patients with chronic recurrent pericarditis, it was also seen in 76% of patients with constriction. The extent and intensity of signal abnormality was generally much less severe for the patients with constriction than for the patients with chronic recurrent pericarditis. This suggests that some component of delayed pericardial enhancement in these patients could be due to progressive extracellular gadolinium concentration in fibrotic tissues, particularly since it frequently became evident with long delays from the injection of contrast (sometimes 15–20 min). However, in the 6 patients with constriction who had a trial of medical therapy followed by repeat imaging, all patients had some clinical response in addition to decreased enhancement on post-contrast imaging, suggesting that at least some of the delayed enhancement reflected active inflammation. Patients with either chronic recurrent pericarditis and constrictive pericarditis were both more likely to have diffuse than segmental enhancement (69 and 54% respectively) and smooth rather than nodular enhancement (75 and 82% respectively), suggesting that these features may not allow much inference about either the cause or resulting clinical symptoms.
Abnormal diastolic septal motion was present in only 3/16 patients with chronic recurrent pericarditis; one of these also had an echocardiogram which showed evidence of constriction. On the other hand, diastolic septal motion abnormality was present in 89% of patients with constriction, a result similar to the sensitivity of 81% reported by Giorgi et al. [11]. In most of these cases there was agreement of MR and echocardiography for the diagnosis of constriction. However, in 15% of cases the echocardiogram was considered technically limited or inconclusive. This suggests a particular role for MR in confirming the presence of constriction in patients with technically limited or equivocal echocardiographic exams. Previous reports have suggested that a combined multimodality approach involving both morphologic assessment of the pericardium and hemodynamic parameters may be necessary to avoid missing subtle or complex cases of pericardial disease—which may not manifest classic findings on all modalities [14, 15].
Summary
Although our review has some limitations because of referral bias and some variability in MR protocols, it does allow us to suggest a correlation of certain imaging findings with pathologic features on MR, and to characterize some features which distinguish patients with chronic recurrent pericarditis from those with constriction and pericardial tumors.
Significant edema-like pericardial signal on fat-nulled spin density or T2-weighted images (separate from pericardial fluid) suggests edema, neovascularization, and congestion in the pericardium related to active inflammation. Pericardial calcifications, pleural effusions, and dilated inferior vena cava suggest constrictive physiology.
Abnormal diastolic septal motion suggests constrictive physiology. In an appreciable minority of cases, MR was particularly useful in making the diagnosis of constriction because of technically limited echocardiographic examinations. Pericardial thickness was similar in both the constriction and chronic pain groups; however there was variability between individual patients.
Pericardial delayed enhancement was present in the majority of patients with constriction as well as those with recurrent pericarditis; a decrease in pericardial enhancement in all constriction patients who underwent a trial medical therapy suggests that at least some of the delayed enhancement was due to active inflammation rather than fibrosis. The morphology of the pericardial delayed enhancement is not helpful in distinguishing chronic recurrent pericarditis from constrictive pericarditis.
Because of small sample size and the lack of a control group, we cannot draw conclusions about the ability of edema-sensitive imaging or pericardial enhancement to predict the likelihood of improvement with aggressive medical therapy. However, the findings described here suggest the potential for these findings to serve as a biomarker for inflammation. Further investigation to evaluate the use of MR in determining likelihood of response to medical therapy may be worthwhile.
Pericardial tumors were generally focal and masslike, and demonstrated high signal on fluid sensitive sequences as well as gadolinium enhancement. However, in at least 1 case, the MR findings were indistinguishable from constrictive pericarditis. Consequently, although rare, tumors should be considered in the differential list of causes of pericardial constriction.
MR proved particularly helpful in evaluating a subset of 15% of patients in whom echocardiographic evaluation was equivocal, technically limited, or negative for constriction. Because diagnosis of pericardial disease can be challenging, MR may be particularly useful for evaluating patients who are not well imaged with echo, or who have a negative echocardiogram but strong clinical suspicion for constrictive physiology.
References
Stark DD, Higgins CB, Lanzer P et al (1984) Magnetic resonance imaging of the pericardium: normal and pathologic findings. Radiology 150:469–474
Taylor AM, Dymarkowski S, Verbeken EK (2006) Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: initial results. Eur Radiol 16:569–574
Sechtem U, Tscholakoff D, Higgins CB (1986) MRI of the normal pericardium. AJR 147:239–244
Sechtem U, Tscholakoff D, Higgins CB (1986) MRI of the abnormal pericardium. AJR 147:245–252
Taylor AM, Dymarkowski S, Verbeken EK, Bogaert J (2006) Detection of pericardial inflammation with late-enhancement cardiac magnetic resonance imaging: initial results. Eur Radiol 16:569–574
Masui T, Finck S, Higgins CB (1992) Constrictive pericarditis and restrictive cardiomyopathy: evaluation with MR imaging. Radiology 182:369–373
Klein C, Graf K, Fleck E, Nagel E (2003) Acute fibrinous pericarditis assessed with magnetic resonance imaging. Circulation 107:e82
Watanabe A, Hara Y, Hamada M et al (1998) A case of effusive-constrictive pericarditis: an efficacy of Gd-DTPA enhanced magnetic resonance imaging to detect a pericardial thickening. Magn Resonan Imaging 16:347–350
Matsuoka H, Hamada M, Honda T et al (1994) Evaluation of acute myocarditis and pericarditis by Gd-DTPA enhanced magnetic resonance imaging. Eur Heart J 15:283–284
Sa MI, Kiesewetter CH, Jagathesan R, Prasad SK (2009) Acute pericarditis assessed with magnetic resonance imaging: a new approach. Circulation 119:3183–3186
Giorgi B, Mollet NRA, Dymarkoswki S, Rademakers FA, Bogaert J (2003) Assessment of ventricular septal motion in patients clinically suspected of constrictive pericarditis, using magnetic resonance imaging. Radiology 228:417–424
Oh KY, Shimizu M, Edwards WD, Tazelaar HD, Danielson GK (2001) Surgical pathology of the parietal pericardium: a study of 344 cases (1993–1999). Cardiovasc Pathol 10(4):157–168
Talreja DR, Edwards WD, Danielson GK, Schaff HV, Tajik AJ, Tazelaar HD, Breen JF, Oh JK (2003) Constrictive pericarditis in 26 patients with histologically normal pericardial thickness. Circulation 108(15):1852–1857
Mueller C, Globits S, Glogar D, Klepetko W, Knoflach P (1991) Constrictive pericarditis without significant hemodynamic changes as a cause of oedema formation due to protein-losing enteropathy. Eur Heart J 12:1140–1143
Nishimura RA (2001) Constrictive pericarditis in the modern era: a diagnostic dilemma. Heart 86:619–623
Conflict of interest
None.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Young, P.M., Glockner, J.F., Williamson, E.E. et al. MR imaging findings in 76 consecutive surgically proven cases of pericardial disease with CT and pathologic correlation. Int J Cardiovasc Imaging 28, 1099–1109 (2012). https://doi.org/10.1007/s10554-011-9916-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10554-011-9916-0