Opinion statement
Ebstein anomaly is a developmental abnormality of the tricuspid valve and right ventricle that results in tricuspid regurgitation and right heart enlargement. Because of the variation in clinical severity and associated findings, patients require a detailed, well-tailored evaluation. For these reasons, management of adults with Ebstein anomaly should take place in a center with expertise in adult congenital heart disease. In many patients, the decision regarding if and when to perform surgery remains controversial, largely because of a lack of published data demonstrating improved postoperative symptoms and survival compared to the natural history of the disease. Because standard two-dimensional echocardiography and cardiovascular magnetic resonance imaging planes do not provide the necessary data to preoperatively manage patients, comprehensive echocardiography and cardiovascular magnetic resonance imaging protocols by experts trained in congenital heart disease are essential in the preoperative management of patients with Ebstein anomaly. As patients may be unaware of their exercise limitations, and for prognostic value, serial cardiopulmonary exercise stress testing is very useful in the evaluation of Ebstein anomaly patients. Surgical tricuspid valve repair historically has not been highly successful because of the marked distortion of tricuspid valve leaflets and right ventricular pathology. Over the last several years, reports of newer surgical techniques to repair the valve, with concurrent advances in arrhythmia management of patients hold promise for improved long term outcomes of patients with Ebstein anomaly. However, because Ebstein anomaly is rare and tricuspid valve repair remains technically challenging, the newer valve repair techniques have not yet gained widespread acceptance throughout the adult congenital heart disease community.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In 1866, Dr. Wilhelm Ebstein published the case of a 19-year-old man who died of cyanotic heart disease secondary to a malformation of the tricuspid valve, which ultimately became known as Ebstein anomaly (EA) [1]. Since that time, advances have been made in the diagnosis and management of this unique congenital heart defect.
Epidemiology and genetics
The incidence of Ebstein anomaly is ~1 per 200,000 live births [2, 3]. Although most cases of Ebstein anomaly are sporadic, familial cases have been well described in the literature [4, 5]. Furthermore, mutations in several genes encoding sarcomeric proteins have been identified in association with Ebstein anomaly including cardiac myosin-binding protein C (MYBPC3), α-cardiac actin (ACTC1), cardiac troponin T (TNNT2), α-tropomyosin (TPM1), and cardiac troponin I (TNNI3)[6]. The genetic association with sarcomeric proteins lends credence to the assertion that Ebstein anomaly is a disease of the myocardium as well as valve tissue [7]. More recently, a mutation in the gene encoding β-myosin heavy chain (MYH7) has been described in several families with Ebstein anomaly and left ventricular noncompaction, a well-known association [6, 8]. Given its multifactorial inheritance, the morphology and presentation of this disease is variable and unique to each patient.
Anatomy
Ebstein anomaly is a congenital heart defect characterized by three major pathologic abnormalities of the tricuspid valve that result in tricuspid regurgitation: (1) adhesion of the posterior and septal leaflets to the underlying myocardium with rightward and anterior displacement of the functional annulus (by echocardiography, an exaggerated displacement of the septal leaflet into the ventricular cavity in the apical four-chamber view), (2) redundancy and fenestrations of the anterior tricuspid valve leaflet, and (3) dilatation of the anatomic tricuspid valve annulus at the true atrioventricular junction [9, 10]. The pathologic basis is a failure of ‘delamination’ of the leaflet tissue from the right ventricular myocardium, a process that involves the valve tissue, annulus, and subvalvular apparatus. The anterior leaflet retains a normal attachment to the native tricuspid annulus. The direction of functional annulus displacement has been previously described as ‘downward’ and ‘apical,’ but is more appropriately characterized as ‘rightward and anterior,’ as there is a rotational displacement toward the right ventricular outflow tract [10]. The anterior leaflet is classically large and irregularly shaped, with short chordal attachments or direct myocardial insertion [9]. There are a host of associated abnormalities, resulting in a broad spectrum of pathologic, echocardiographic, and clinical features (Table 1).
Clinical presentation and physical examination
There is a wide variation in clinical presentation from the fetus to the adult with Ebstein anomaly. The timing of presentation depends on the severity of tricuspid valve leaflet displacement, the quality of prenatal ultrasound screening, and the clinical severity of the disease. Generally speaking, more severe disease results in earlier presentation. Adults will present either with symptomatic arrhythmias, or with evidence of right- or left-sided heart failure. Occasionally, the initial presentation may also be that of sudden cardiac death, which has been attributed to atrial fibrillation with accelerated conduction through an accessory pathway, or from ventricular arrhythmias. On physical examination, patients may have a right ventricular lift and a murmur of tricuspid regurgitation. The first heart sound is widely split, and the tricuspid component is loud and delayed because of closure of the large anterior leaflet. Patients with severe right heart failure may have jugular venous distention. However, the V wave of tricuspid regurgitation seldom appears in the jugular pulse because of the damping effect of the large atrium and the thin walled atrialized right ventricle, even in Ebstein patients with severe tricuspid regurgitation. Forty seven percent of adult patients with Ebstein anomaly have clinical cyanosis because of a combination of increased right atrial pressure from tricuspid regurgitation and a right-to-left atrial level shunt [11].
Electrocardiography
The electrocardiogram (ECG) is usually abnormal in patients with Ebstein anomaly. P waves are often quite tall and peaked (Himalayan P waves). A qR pattern can be seen in lead V1 to V4. There can be some intraventricular conduction delay with a widened QRS with or without a right bundle branch pattern. Pre-excitation, in the form of Wolff-Parkinson-White syndrome and manifest pre-excitation, may be present in 18 %‒44 % of the cases with usually a right sided bypass tract in the right posterior or right posteroseptal region. A fractionated QRS, defined as a conduction delay presumably caused by myocardial scar, is associated with increased atrialized right ventricular volume, lower right ventricular ejection fraction, lower oxygen consumption, lower oxygen saturation, and an increased risk of arrhythmic events in Ebstein anomaly [12••, 13]. Pseudo normalization of the right bundle branch block through a slow accessory pathway can also be seen. Multiple accessory pathways may be present [3, 14–18].
Chest radiography
The chest x-ray can be nearly normal in mild cases. Right atrial enlargement with a globular cardiac contour can be seen severe cases. Cardiomegaly on chest x-ray has been shown to be an independent risk factor for adverse outcome [11].
Echocardiography
Transthoracic echocardiography is the modality of choice for the diagnosis and morphologic evaluation of Ebstein anomaly. The goals of echocardiography are (1) to identify the classic morphologic features of Ebstein anomaly as well as nonessential, but commonly associated features (see Table 1); (2) to evaluate the cardiac physiology including severity of tricuspid regurgitation, ventricular function, and presence or absence of right-to-left atrial level shunting; and (3) to exclude other causes of tricuspid regurgitation (for example dysplastic tricuspid valve, tricuspid valve prolapse, right ventricular dysplasia, endocarditis, and annular dilatation) [19]. The anatomic assessment that is particularly well suited to echocardiography is the evaluation for leaflet dysplasia, including position of valve leaflets, thickening, tethering, and fenestrations [20]. Misunderstanding about tricuspid valve morphology based on two-dimensional (2D) echocardiography is common, partly because only two of the three leaflets are typically seen in standard long axis views resulting in difficulty correctly identifying each leaflet. In addition, tricuspid valve leaflet commissures, coaptation orifice and the en face view of the valve are often difficult to see by 2D imaging [21]. Because the valve is not seen in its entirety in any standard 2D imaging plane, 2D sweeps are essential to define valve morphology in the parasternal long axis, short axis, and apical views. Good quality 3D echocardiography can add useful information about valve morphology in patients with good imaging windows. In particular, the degree of rotation of the effective tricuspid valve annulus toward the right ventricular outflow tract is well seen by 3D imaging [22]. Paradoxical septal motion, typically seen at the base of the heart, may be related to pressure differences between atrialized right ventricle and adjacent left ventricle, and/or conduction abnormalities. Transesophageal echocardiography is useful for preoperative definition of tricuspid valve anatomy and the atrial septum in patients with poor transthoracic acoustic windows, and also intraoperatively during surgical repair. A proposed transthoracic echocardiography imaging protocol is show in Table 2.
Cardiovascular magnetic resonance imaging
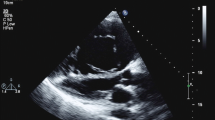
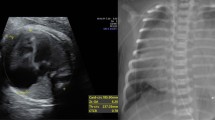
Cardiovascular magnetic resonance (CMR) imaging is an important emerging modality in the pre- and postoperative evaluation of patients with Ebstein anomaly (Fig. 1). CMR adds morphologic and physiologic information that cannot be obtained by echocardiography alone, including evaluation of valve morphology in patients with difficult acoustic windows, accurate evaluation of right atrial and right ventricular volumes, quantification of tricuspid regurgitation fraction, and shunt quantification in patients with an atrial septal defect. The imaging protocol should address the same areas of interest as outlined in the transthoracic echocardiography imaging protocol (Table 2), with additional data on right atrial and ventricular volumes, right ventricular ejection fraction, tricuspid regurgitation fraction, and shunt quantification. CMR imaging also allows for assessment of left ventricular size and function, left ventricular noncompaction, and regions of fibrosis on myocardial delayed enhancement imaging. Chest computerized tomography is an alternative to CMR imaging but has a more limited application because of lower temporal resolution and inability to quantify flow volume.
Echocardiogram (A-F) and cardiovascular magnetic resonance (G-L) images of Ebstein anomaly. (A) Apical four-chamber view tilted posteriorly, demonstrates severe tethering of the posterior leaflet of the tricuspid valve (arrow) with atrialization of the right ventricle; (B) apical four-chamber view at the midventricular level demonstrates severe tethering of the septal leaflet (arrow); and (C) apical four-chamber view tilted anteriorly demonstrates an en face view of the tricuspid valve, which is oriented toward the right ventricular outflow tract (*); (D) parasternal long axis view tilted toward the right ventricle demonstrates severe tethering of the posterior leaflet (arrow); (E) parasternal short axis view demonstrates atrialization of the right ventricle (*) with rotation of the tricuspid valve orifice toward the right ventricular outflow tract; (F) subxiphoid color Doppler imaging of the atrial septum demonstrates bidirectional flow through a patent foramen ovale; (G) CMR of a normal heart in the four-chamber view demonstrates normal attachment of the septal leaflet of the tricuspid valve to the annulus (arrow); (H) normal heart in the short axis view at the base demonstrates the normal formation and orientation of the septal (white arrow) and posterior (black arrow) tricuspid valve leaflets at the base of the heart with inflow directed toward the apex of the heart; (I) CMR of a heart with Ebstein anomaly demonstrates tethering of the septal leaflet (arrow), and (J) an elongated anterior leaflet (arrow) with atrialization of the right ventricle (asterisk) at the basal and mid-ventricular level; (K) preoperative CMR images of a patient with Ebstein anomaly after dehiscence of a previous right ventricular plication (*); (L) postoperative CMR images after Cone reconstruction of the tricuspid valve, resulting in a bileaflet valve (black arrows) and tissue thickening along the inferior wall of the right ventricle from a repeat plication (white arrows).
Exercise stress testing
Patients with congenital heart disease often self-limit and are, therefore, unaware of their exercise limitations. Exercise stress testing offers a reliable tool for assessment of functional capacity. Deterioration in exercise capacity has important prognostic implications [23]. Peak oxygen consumption is significantly reduced even in relatively asymptomatic patients with Ebstein anomaly [24]. Peak VO2 has been found to be the strongest exercise marker of outcome in this population. A predicted peak VO2 < 60 % is associated with a higher risk of death, non-elective hospitalization and surgical repair [25]. A progressive decline in biventricular and tricuspid valve function, and chronotropic insufficiency are thought to contribute to the time-related decline in exercise function [26]. Right-to-left shunting across a patent foramen ovale or an atrial septal defect can be exacerbated during exercise. The cyanosis contributes to exercise intolerance through a reduction in arterial oxygen content and increase in physiological dead space [25]. This can manifest as inefficient ventilation, an elevated VE/VCO2, as well as a decrease in peak VO2 [24, 25].
Medical management
Medical management of Ebstein anomaly includes noninvasive imaging, evaluation and treatment of occult and symptomatic arrhythmias, and assessment for cyanosis and right heart failure. Patients with progressive right ventricular enlargement and dysfunction, and progressive tricuspid regurgitation may develop signs of right heart failure with peripheral edema and cyanosis. Diuretics may result in reduction of peripheral edema but will not affect the fatigue and dyspnea related to low left sided cardiac output. Anticoagulation with warfarin is recommended for patients with Ebstein anomaly with a history of paradoxical embolus or atrial fibrillation [27]. Newer oral anticoagulants such as rivaroxaban, dabigatran, and apixaban may be considered as alternatives to warfarin, though these have not been directly studied in the Ebstein population [28]. Antibiotic prophylaxis before dental procedures that involve manipulation of gingival tissue or the periapical region of the teeth or perforation of the oral mucosa is reasonable in cyanotic patients with Ebstein anomaly and postoperative patients with a prosthetic cardiac valve. Endocarditis prophylaxis is usually unnecessary in acyanotic, unoperated patients.
Ultimately, all patients with Ebstein anomaly should have a periodic evaluation in a center with expertise in ACHD management [27]. The timing of follow-up at an expert ACHD center depends on the clinical status of the patient. For low risk patients who are asymptomatic and acyanotic with mild tricuspid regurgitation, we suggest follow-up every 2–3 years. For higher risk patients, we suggest follow-up every 6‒12 months. The Seattle Heart Failure Model can be used by general cardiologists to identify Ebstein patients at highest risk for adverse outcome, who should be referred to tertiary care centers [29•].
Physical activity
Exercise recommendations vary based on the severity of the disease. Even mild cases may be associated with arrhythmias; and there is an increased risk of sudden death with exercise in severe cases. The exercise recommendations according to the Task Force I report on CHD include the following: (1) athletes with mild Ebstein anomaly, without cyanosis, and with a nearly normal heart size and no evidence of arrhythmia can participate in all sports; (2) athletes with tricuspid regurgitation of moderate severity can participate in low intensity competitive sports if there is no evidence of arrhythmia on ambulatory ECG monitoring; and (3) athletes with severe Ebstein anomaly are precluded from all sports participation. However, after surgical repair, low intensity competitive sports can be permitted if tricuspid regurgitation is absent or mild, heart size on chest radiograph is not substantially increased and arrhythmia is not present on ambulatory ECG monitoring and exercise test [30].
Arrhythmia management
Supraventricular tachycardia secondary to accessory pathways, primary atrial tachycardia and atrioventricular node reentrant tachycardia are often seen in Ebstein anomaly. A high percentage of patients have other arrhythmia substrates including atrial flutter, atriofascicular fibers, and ventricular tachycardia [31]. Management of the tachyarrhythmias can be challenging but effective control is important for optimal patient outcome. Current data suggests that all Ebstein anomaly patients with symptomatic arrhythmias and those scheduled for surgical repair should undergo pre-operative electrophysiology study regardless of the presence of symptoms [16, 32, 33]. Localization of accessory pathways is often challenging due to a massively enlarged right heart, displaced tricuspid annulus, and distortion of anatomic landmarks, and because over 50 % of patients have multiple accessory pathways [16, 31]. The success rate for catheter ablation is lower in Ebstein anomaly (≤81 %) patients compared with patients with structurally normal hearts (≥95 %) [14, 15, 34]. Rarely, right coronary artery stenosis has been seen following catheter ablation as reported by Bertram et al in two case reports [35]. If catheter ablation is unsuccessful or deemed inappropriate, surgical interruption can be performed. For any patients with history of atrial flutter or fibrillation a Maze procedure can be incorporated into the surgery.
Cardiac catheterization
Adults with Ebstein anomaly have varying degrees of right-to-left shunting across an atrial level defect. Percutaneous closure of atrial septal defects in Ebstein anomaly is indicated in patients with paradoxical embolus (defined as stroke or transient ischemic attack, brain abscess, or myocardial infarction) [36]. Although there is paucity of data, percutaneous closure may reduce cyanosis and improve functional capacity in selected cases [37]. Cardiac catheterization for hemodynamic assessment only is rarely needed. Preoperative coronary artery angiography should be considered if there is a suspicion of coronary artery disease (CAD), in men ≥35 years, in premenopausal women ≥35 years who have CAD risk factors, and postmenopausal women [27].
Surgery
Surgeons with training and expertise in congenital heart disease should be chosen to operate on patients with Ebstein anomaly [27]. The goals of surgery are to improve functional status and reduce the risk of sudden death in patients with Ebstein anomaly. In neonates, surgery should be avoided if possible to allow time for pulmonary vascular resistance to decline and right ventricular output to improve, potentially obviating the need for early surgery. The American College of Cardiology/American Heart Association 2008 guidelines for surgical intervention include (1) symptoms or deteriorating exercise capacity; (2) cyanosis (oxygen saturation less than 90 %); (3) paradoxical embolism; (4) progressive cardiomegaly on chest x-ray; and (5) progressive right ventricular dilation or reduction in right ventricular systolic function [27]. Nevertheless, the decision about whether to perform surgery in individual patients with Ebstein anomaly is often debated because of unpredictable surgical outcomes. Preoperative decision making and planning should involve a team of cardiologists, cardiac surgeons and electrophysiologists when appropriate.
Surgical techniques are tailored to each patient with the goal of improving the severity of tricuspid regurgitation, reducing the arrhythmia burden, closing any inter- atrial communications, and pacemaker placement when necessary. When possible, tricuspid valve repair is the preferred surgical method. Techniques for tricuspid valve repair have undergone an evolution over the last 30 years, and generally involve reducing the annulus size with or without an annuloplasty ring, repositioning tricuspid valve leaflets to allow better coaptation, reducing the size of the right atrium and atrialized right ventricle, and closing inter atrial communications. Although technically challenging with a steep learning curve, there have been promising results of the cone operation from various institutions worldwide [33, 38, 39, 40••]. The cone operation is a repair technique that aims to create a ‘cone’ shaped reconstructed leaflet that coapts as a bicuspid valve rather than a monocusp valve as was traditionally performed (Fig. 1) [38].
Surgical options include tricuspid valve repair (cone vs. monocusp repair), bioprosthetic valve replacement, and bidirectional cavopulmonary anastomosis. When possible, valve repair is the preferred surgical method. Factors that favor valve repair include younger age at operation, the absence of massive right ventricular or annular dilatation, the presence of a septal leaflet, and no history of surgical leaflet delamination during a previous procedure [39]. Even though the cone operation is preferable to a monocusp technique, patients with highly muscularized anterior leaflets or completely absent septal leaflets may not be candidates for the cone operation. Many factors in support of valve repair can be determined by preoperative assessment, including a detailed echocardiographic evaluation. However, because the degree of anterior leaflet muscularization and presence of septal leaflet tissue may be difficult to assess by preoperative echocardiography, the final decision regarding surgical technique is made in the operating room. Tricuspid valve replacement is recommended for older patients (>60 years old) and those with pulmonary hypertension [39, 41••]. Rarely, patients with severely enlarged right ventricles, severe right ventricular dysfunction, or low cardiac output syndrome may require bidirectional cavopulmonary anastomosis or the Fontan operation [42]. Risk factors for postoperative death include lack of postoperative improvement, older age at surgery, preoperative left ventricular ejection fraction <50 %, diabetes mellitus, and preoperative history of heart failure [41••].
Prognosis
The prognosis of Ebstein anomaly varies from one patient to another, depending on the morphology of the heart, burden of arrhythmias, and resultant clinical sequelae. In an adult cohort of 72 unoperated patients over the age of 25, survival was 89 % at 1 year, 76 % at 10 year, and 41 % at 20 years of follow-up. Risk factors for cardiac-related adverse outcome include earlier age at diagnosis, increasing degree of cardiomegaly on chest radiography, male gender, increasing severity of tricuspid valve leaflet displacement, increasing tricuspid regurgitation severity, New York Heart Association functional class III or IV, and reduced exercise capacity [11, 25]. In contrast, of a cohort of 81 adult patients aged 50–79 years with Ebstein anomaly who underwent surgical intervention, 20-year survival was 65 % vs 74 % for age- and sex-matched controls. Although postoperative outcomes have improved over time, a lack of published data persists on whether surgical management of adult patients with Ebstein anomaly improves the natural history and mortality of the disease.
Pregnancy
With physiologic changes of pregnancy including increased blood volume and increased blood flow, pregnant women with Ebstein anomaly are at risk for right heart failure, arrhythmias and occasionally sudden death [43, 44]. Following a review of 127 pregnancies in women with Ebstein anomaly, the risk of heart failure was 3.1 % with a 4 % risk of arrhythmias [44]. Thus women with Ebstein anomaly should undergo prepregnancy counseling with a multidisciplinary team with expertise in adult congenital heart disease [27]. Although most women with Ebstein anomaly can have a successful pregnancy with proper care, there is an increased risk of low birth weight and fetal loss if significant cyanosis is present. Asymptomatic women with New York Heart Association class I and no history of arrhythmias or WPW may plan for pregnancy without prior intervention. For patients with a history of arrhythmia, catheter ablation should be considered prior to pregnancy [45]. Surgical tricuspid valve repair or replacement with closure of atrial septal defect should be considered prior to pregnancy in higher risk patients, including those with exercise intolerance, cyanosis, significant tricuspid regurgitation or cardiomegaly on chest radiography [45]. Mechanical tricuspid valve replacement prior to pregnancy is not recommended because of the risk of bleeding during pregnancy [45]. The risk of congenital heart disease in offspring is ~6 % [27]. Therefore, all women with Ebstein anomaly should be offered fetal echocardiography screening in the 18th‒22nd week of pregnancy.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of outstanding importance
van Son JA, Konstantinov IE, Zimmermann V. Wilhelm Ebstein and Ebstein's malformation. Eur J Cardio-Thorac Surg: Off J Eur Assoc Cardio-Thorac Surg. 2001;20:1082–5.
Hoffman JI, Kaplan S. The incidence of congenital heart disease. J Am Coll Cardiol. 2002;39:1890–900.
Attenhofer Jost CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein's anomaly. Circulation. 2007;115:277–85.
Balaji S, Dennis NR, Keeton BR. Familial Ebstein's anomaly: a report of six cases in two generations associated with mild skeletal abnormalities. Br Heart J. 1991;66:26–8.
Rosenmann A, Arad I, Simcha A, Schaap T. Familial Ebstein's anomaly. J Med Genet. 1976;13:532–5.
Vermeer AM, van Engelen K, Postma AV, Baars MJ, Christiaans I, De Haij S, et al. Ebstein anomaly associated with left ventricular noncompaction: an autosomal dominant condition that can be caused by mutations in MYH7. Am J Med Genet C: Semin Med Genet. 2013;163C:178–84.
Anderson KR, Lie JT. The right ventricular myocardium in Ebstein's anomaly: a morphometric histopathologic study. Mayo Clin Proc Mayo Clin. 1979;54:181–4.
Bettinelli AL, Mulder TJ, Funke BH, Lafferty KA, Longo SA, Niyazov DM. Familial ebstein anomaly, left ventricular hypertrabeculation, and ventricular septal defect associated with a MYH7 mutation. Am J Med Genet A. 2013;161A:3187–90.
Barbara DW, Edwards WD, Connolly HM, Dearani JA. Surgical pathology of 104 tricuspid valves (2000–2005) with classic right-sided Ebstein's malformation. Cardiovasc pathol: Off J Soc Cardiovasc Pathol. 2008;17:166–71.
Martinez RM, O'Leary PW, Anderson RH. Anatomy and echocardiography of the normal and abnormal tricuspid valve. Cardiol Young. 2006;16 Suppl 3:4–11.
Attie F, Rosas M, Rijlaarsdam M, Buendia A, Zabal C, Kuri J, et al. The adult patient with Ebstein anomaly. Outcome in 72 unoperated patients. Medicine. 2000;79:27–36.
Park SJ, Chung S, On YK, Kim JS, Yang JH, Jun TG, et al. Fragmented QRS complex in adult patients with Ebstein anomaly and its association with arrhythmic risk and the severity of the anomaly. Circ Arrhythm Electrophysiol. 2013;6:1148–55.The first paper to describe an association between fragmented QRS morphology on electrocardiogram with an increased risk of arrhythmic events in patients with Ebstein anomaly.
Egidy Assenza G, Valente AM, Geva T, Graham D, Pluchinotta FR, Sanders SP, et al. QRS duration and QRS fractionation on surface electrocardiogram are markers of right ventricular dysfunction and atrialization in patients with Ebstein anomaly. Eur Heart J. 2013;34:191–200.
Chetaille P, Walsh EP, Triedman JK. Outcomes of radiofrequency catheter ablation of atrioventricular reciprocating tachycardia in patients with congenital heart disease. Heart rhythm : Off J Heart Rhythm Soc. 2004;1:168–73.
Hebe J. Ebstein's anomaly in adults. Arrhythmias: diagnosis and therapeutic approach. Thorac Cardiovasc Surg. 2000;48:214–9.
Khositseth A, Danielson GK, Dearani JA, Munger TM, Porter CJ. Supraventricular tachyarrhythmias in Ebstein anomaly: management and outcome. J Thorac Cardiovasc Surg. 2004;128:826–33.
Oh JK, Holmes Jr DR, Hayes DL, Porter CB, Danielson GK. Cardiac arrhythmias in patients with surgical repair of Ebstein's anomaly. J Am Coll Cardiol. 1985;6:1351–7.
Reich JD, Auld D, Hulse E, Sullivan K, Campbell R. The Pediatric Radiofrequency Ablation Registry's experience with Ebstein's anomaly. Pediatric Electrophysiology Society. J Cardiovasc Electrophysiol. 1998;9:1370–7.
Ammash NM, Warnes CA, Connolly HM, Danielson GK, Seward JB. Mimics of Ebstein's anomaly. Am Heart J. 1997;134:508–13.
Oechslin E, Buchholz S, Jenni R. Ebstein's anomaly in adults: Doppler-echocardiographic evaluation. Thorac Cardiovasc Surg. 2000;48:209–13.
Muraru D, Badano LP, Sarais C, Solda E, Iliceto S. Evaluation of tricuspid valve morphology and function by transthoracic three-dimensional echocardiography. Curr Cardiol Rep. 2011;13:242–9.
Bharucha T, Anderson RH, Lim ZS, Vettukattil JJ. Multiplanar review of three-dimensional echocardiography gives new insights into the morphology of Ebstein's malformation. Cardiol Young. 2010;20:49–53.
Diller GP, Dimopoulos K, Okonko D, Li W, Babu-Narayan SV, Broberg CS, et al. Exercise intolerance in adult congenital heart disease: comparative severity, correlates, and prognostic implication. Circulation. 2005;112:828–35.
Trojnarska O, Szyszka A, Gwizdala A, Siniawski A, Oko-Sarnowska Z, Chmara E, et al. Adults with Ebstein's anomaly–Cardiopulmonary exercise testing and BNP levels exercise capacity and BNP in adults with Ebstein's anomaly. Int J Cardiol. 2006;111:92–7.
Radojevic J, Inuzuka R, Alonso-Gonzalez R, Borgia F, Giannakoulas G, Prapa M, et al. Peak oxygen uptake correlates with disease severity and predicts outcome in adult patients with Ebstein's anomaly of the tricuspid valve. Int J Cardiol. 2013;163:305–8.
Kipps AK, Graham DA, Lewis E, Marx GR, Banka P, Rhodes J. Natural history of exercise function in patients with Ebstein anomaly: A serial study. Am Heart J. 2012;163:486–91.
Warnes CA, Williams RG, Bashore TM, Child JS, Connolly HM, Dearani JA, et al. ACC/AHA 2008 guidelines for the management of adults with congenital heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Develop Guidelines on the Management of Adults With Congenital Heart Disease). Developed in Collaboration With the American Society of Echocardiography, Heart Rhythm Society, International Society for Adult Congenital Heart Disease, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2008;52:e143–263.
Baker WL, Phung OJ. Systematic review and adjusted indirect comparison meta-analysis of oral anticoagulants in atrial fibrillation. Circ Cardiovasc Qual Outcomes. 2012;5:711–9.
Stefanescu A, Macklin EA, Lin E, Dudzinski DM, Johnson J, Kennedy KF, et al. Usefulness of the Seattle heart failure model to identify adults with congenital heart disease at high risk of poor outcome. Am J Cardiol. 2014. 113;865–70.First paper to use a previously validated prediction model for adults patients with heart failure in patients with congenital heart disease. Use of this model can help general cardiologists determine when their patients should be referred to adult congenital heart disease experts.
Graham Jr TP, Bricker JT, James FW, Strong WB. 26th Bethesda conference: recommendations for determining eligibility for competition in athletes with cardiovascular abnormalities. Task Force 1: congenital heart disease. J Am Coll Cardiol. 1994;24:867–73.
Sherwin ED, Triedman JK, Walsh EP. Update on interventional electrophysiology in congenital heart disease: evolving solutions for complex hearts. Circ Arrhythmia Electrophysiol. 2013;6:1032–40.
Huang CJ, Chiu IS, Lin FY, Chen WJ, Lin JL, Lo HM, et al. Role of electrophysiological studies and arrhythmia intervention in repairing Ebstein's anomaly. Thorac Cardiovasc Surg. 2000;48:347–50.
Vogel M, Marx GR, Tworetzky W, Cecchin F, Graham D, Mayer JE, et al. Ebstein's malformation of the tricuspid valve: short-term outcomes of the "cone procedure" versus conventional surgery. Congenit Heart Dis. 2012;7:50–8.
Cappato R, Schluter M, Weiss C, Antz M, Koschyk DH, Hofmann T, et al. Radiofrequency current catheter ablation of accessory atrioventricular pathways in Ebstein's anomaly. Circulation. 1996;94:376–83.
Bertram H, Bokenkamp R, Peuster M, Hausdorf G, Paul T. Coronary artery stenosis after radiofrequency catheter ablation of accessory atrioventricular pathways in children with Ebstein's malformation. Circulation. 2001;103:538–43.
Attenhofer Jost CH, Connolly HM, Scott CG, Burkhart HM, Ammash NM, Dearani JA. Increased risk of possible paradoxical embolic events in adults with ebstein anomaly and severe tricuspid regurgitation. Congenit Heart Dis. 2014;9:30–7.
Jategaonkar SR, Scholtz W, Horstkotte D, Kececioglu D, Haas NA. Interventional closure of atrial septal defects in adult patients with Ebstein's anomaly. Congenit Heart Dis. 2011;6:374–81.
da Silva JP, Baumgratz JF, da Fonseca L, Franchi SM, Lopes LM, Tavares GM, et al. The cone reconstruction of the tricuspid valve in Ebstein's anomaly. The operation: early and midterm results. J Thorac Cardiovasc Surg. 2007;133:215–23.
Dearani JA, Said SM, Burkhart HM, Pike RB, O'Leary PW, Cetta F. Strategies for tricuspid re-repair in Ebstein malformation using the cone technique. Ann Thorac Surg. 2013;96:202–8. discussion 8–10.
Silva JP, Silva Lda F, Moreira LF, Lopez LM, Franchi SM, Lianza AC, et al. Cone reconstruction in Ebstein's anomaly repair: early and long-term results. Arq Bras Cardiol. 2011;97:199–208.Long term follow-up of 52 children and adults who underwent cone operation for repair of Ebstein anomaly, demonstrating clinical improvement in the majority of patients
Attenhofer Jost CH, Connolly HM, Scott CG, Burkhart HM, Warnes CA, Dearani JA. Outcome of cardiac surgery in patients 50 years of age or older with Ebstein anomaly: survival and functional improvement. J Am Coll Cardiol. 2012;59:2101–6.Long-term follow-up of 89 adults with Ebstein anomaly who underwent a wide range of surgeries. Patients had good survival, although lower than expected
Dearani JA, Said SM, O'Leary PW, Burkhart HM, Barnes RD, Cetta F. Anatomic repair of Ebstein's malformation: lessons learned with cone reconstruction. Ann Thorac Surg. 2013;95:220–6. discussion 6–8.
Zhao W, Liu H, Feng R, Lin J. Pregnancy outcomes in women with Ebstein's anomaly. Arch Gynecol Obstet. 2012;286:881–8.
Drenthen W, Pieper PG, Roos-Hesselink JW, van Lottum WA, Voors AA, Mulder BJ, et al. Outcome of pregnancy in women with congenital heart disease: a literature review. J Am Coll Cardiol. 2007;49:2303–11.
Katsuragi S, Kamiya C, Yamanaka K, Neki R, Miyoshi T, Iwanaga N, et al. Risk factors for maternal and fetal outcome in pregnancy complicated by Ebstein anomaly. Am J Obstet Gynecol. 2013;209:452 e1–6.
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Puneeta Arya and Dr. Rebecca Beroukhim each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Adult Congenital Heart Disease
Rights and permissions
About this article
Cite this article
Arya, P., Beroukhim, R. Ebstein Anomaly: Assessment, Management, and Timing of Intervention. Curr Treat Options Cardio Med 16, 338 (2014). https://doi.org/10.1007/s11936-014-0338-x
Published:
DOI: https://doi.org/10.1007/s11936-014-0338-x