Abstract
Tricuspid valve (TV) disease commonly occurs in combination with left-sided heart disease. Despite the growing enthusiasm for developing novel minimally invasive therapies for the mitral or aortic valve, the TV disease formerly received less attention and was frequently left untreated. Strong evidence has increased the awareness of the impact of severe TV regurgitation on patient survival, functional capacity, and surgical risk. Preoperative TV accurate description is challenging because, unlike left-sided valves, a complete visualization of tricuspid annulus and all three leaflets in one view is routinely impossible by two-dimensional transthoracic or transesophageal echocardiography. Three-dimensional echocardiography (3DE) with its unrestricted imaging capabilities has sparked significant research interest for a better understanding and improved treatment of valvular heart disease. This review summarizes the current status of 3DE for the assessment of TV morphology and function, with its clinical applications and current limitations, as well as its potential implications for designing TV repair techniques.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Despite having been commonly referred to as the “forgotten valve” in comparison with the left-sided valves, the tricuspid valve (TV) is recently gaining more attention. Accumulating evidence highlights the important implications of functional tricuspid regurgitation on patient survival and exercise capacity [1]; thus, TV repair is being increasingly applied at the time of mitral valve surgery. However, the unsatisfactory results of the current strategies for TV repair suggest an incomplete understanding of the underlying mechanisms of functional tricuspid regurgitation.
Despite the anatomic complexity of the right ventricle and the three-dimensional configuration of TV apparatus, two-dimensional echocardiography (2DE) and Doppler imaging are the standard methods to diagnose TV pathology and quantify its severity. However, several limitations inherent to 2DE modality frequently preclude an accurate characterization of TV anatomy and function:
-
1.
Only two of the three leaflets are routinely imaged in a single 2D view; consequently, a great amount of controversy and uncertainty exists in their precise identification (i.e., septal, anterior or posterior leaflet) from each view, depending on the variable angulations and rotations of the transducer relative to the leaflet orientation;
-
2.
The maximal dimensions and spatial configuration of the oval, saddle-shaped tricuspid annulus cannot be precisely and reproducibly quantified by a single linear measure (usually performed as the mediolateral diameter in apical four-chamber view);
-
3.
TV leaflet commissures, coaptation orifice, and/or valve area planimetry are generally impossible to assess by 2DE, either from transthoracic or transesophageal approach, because an en face view of the entire valve (usually obtainable for mitral or aortic, but not for TV) is required for these purposes;
-
4.
Doppler methods are less valid for the TV than for the mitral valve pathology (eg, geometric assumptions about regurgitant orifice shape, pressure half-time method to calculate valve area).
In contrast, three-dimensional echocardiography (3DE) is especially suited for the assessment of cardiac valves and its clinical acceptance for the management of valve pathology has broadened significantly in the recent years. 3DE has unique capabilities to display en face the nonplanar TV leaflets and annulus, the subvalvular apparatus, and their spatial relationships with the surrounding structures [2•, 3]. The complementary use of 3D color Doppler flow adds information about valve integrity and enables the quantitation of TV regurgitation. Preliminary evidence suggests that transthoracic 3DE could probably become the reference technique to noninvasively assess TV morphology and function in daily practice (Table 1).
The following section briefly reviews the normal anatomy and function of TV, with particular focus on 3D echo imaging capabilities, to subsequently introduce its potentialities in various TV pathologies.
Anatomy of the Tricuspid Valve Complex
The TV has the largest orifice and it is the most apically located among the four cardiac valves. Its role is to prevent backflow during systole from the right ventricle into the right atrium, while permitting the systemic venous blood from the two venae cavae to advance to the right ventricle during diastole.
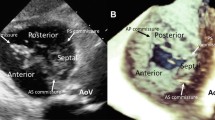
The TV complex is composed of the three leaflets (septal, anterior, and posterior) and supporting annulus, the subvalvular apparatus (chordae and papillary muscles), as well as the right atrial (RA) and right ventricular (RV) geometry and function. The anterior and posterior TV leaflets arise from the annulus along the RV free wall (Fig. 1a). The anterior leaflet is the largest in size, followed by the posterior and septal leaflet, respectively. The septal leaflet arises from the tricuspid annulus at the interventricular septal level. There are two formally named papillary muscles: anterior and posterior. The chordae tendineae tether the anterior and posterior leaflets to the anterior papillary muscle and the posterior, and the septal leaflets to the posterior papillary muscle. There is no formal septal papillary muscle, rather the interventricular septum anchors chordae to the anterior and septal leaflets.
Three-dimensional transthoracic echocardiographic images of the tricuspid valve in various clinical settings. Volumetric tricuspid valve renderings are obtained from cropping, rotating, and thresholding a full-volume data set containing the tricuspid valve, acquired from an apical approach. a, Normal aspect of the tricuspid valve in a healthy subject as seen from the right ventricle, showing all three leaflets en face, the anterior leaflet being larger in comparison with the others, and the oval, normally sized annulus. b, Tricuspid valve visualization from the ventricular aspect in a patient with severe pulmonary arterial hypertension and functional tricuspid regurgitation, demonstrating thickened and tethered leaflets, increased size and sphericity of the tricuspid annulus with flattening of the interventricular septum. c, Carcinoid heart disease, showing the characteristic appearance of the tricuspid valve involvement with thickened fibrotic leaflets immobilized in semi-opened position and a large central regurgitant orifice (ventricular view). d, Right atrial view of a posterior leaflet flail and anterior leaflet prolapse with sizeable regurgitant orifice (arrow) and dilated annulus, resulting in severe organic tricuspid regurgitation. e, Demonstration of pacemaker lead (arrow) interference with septal leaflet coaptation, coexisting with a posterior leaflet rupture and flail (visualized as a lack of leaflet tissue from this ventricular view), causing a massive tricuspid regurgitation with large regurgitant orifice area. f, Mild rheumatic tricuspid stenosis seen en face from the ventricular aspect of the tricuspid valve, with thickened leaflets, fused commissures, and an area of 2.5 cm2 measured by planimetry. AML—anterior mitral leaflet; Ao—aorta; ATL—anterior tricuspid leaflet; PTL—posterior tricuspid leaflet; STL—septal tricuspid leaflet
The normal tricuspid annulus is oval in shape, its size is dynamic and can change markedly with loading conditions. The average normal annular diameter is 21 mm/m2 [4]. During the cardiac cycle, there is normally a 19% reduction in annular circumference with atrial systole [5]. It has been shown that the tricuspid annulus actually has a more complex, three-dimensional saddle-like shape that progressively becomes more circular and flatter with the worsening of functional tricuspid regurgitation [5].
Tricuspid Valve Diseases
TV diseases are generally classified as primary (or intrinsic) valve pathology, and secondary (or functional) valve dysfunction. TV diseases may cause a pure or predominant valve stenosis, a pure or predominant valve regurgitation, or a mixed lesion. Tricuspid regurgitation is only rarely caused by structural abnormalities of the valve apparatus. Functional tricuspid regurgitation (Fig. 1b) is far more frequent than the organic one, and it is caused by dysfunction and/or distortion of the subvalvular apparatus, tricuspid annular dilatation, or a combination of these factors that generally are the consequences of left heart diseases resulting in RV hypertension, dilatation, and dysfunction. The development of functional tricuspid regurgitation starts a vicious circle in which the regurgitation leads to further RV dilation, which increases tricuspid annulus dilation, leaflet tethering, and, as a result, more tricuspid regurgitation.
3DE is able to provide important additional information on leaflet morphology, tricuspid annulus size, as well as on RV and left ventricular volumes, shape, and systolic function, which enables one to reliably identify the mechanism of the TV disease and to assess its severity [2•, 3].
Primary Tricuspid Valve Disease
Congenital
Rawlins et al. [6] reported the added value of the third dimension and of an improved image quality to delineate the anatomy of atrioventricular valves, by using intraoperative epicardial 3DE in eight patients. Seliem et al. [7] studied 41 patients with atrioventricular valve abnormalities (10 with TV disease, and 5 with a common atrioventricular valve) and found that 3DE imaging was helpful in delineating the morphology of the valve leaflets and their chordal attachments, the subchordal apparatus, the mechanism and origin of regurgitation, and the geometry of the regurgitant jet. In 26 TVs, Takahashi et al. [8•] found that, in comparison with 2DE, 3DE improved the detection of leaflet abnormalities, particularly the prolapse of the anterior and posterior leaflets, and commissural pathology.
Among the congenital abnormalities of the TV, 3DE plays a particular role in delineating the anatomy and functional consequences of Ebstein’s anomaly. Ebstein’s anomaly is a congenital defect of the TV in which the origins of the septal or posterior leaflets, or both, are displaced downward into the right ventricle of more than 8 mm/m2, resulting in the atrialization of the RV inflow. A redundant, sail-like anterior leaflet with several fenestrations is generally present. There is a wide spectrum of the severity of TV involvement and the outcome of patients with Ebstein’s anomaly is mainly dependent on its severity [9]. Although 2DE can show the characteristic displacement of the septal leaflet and the redundant and elongated anterior leaflet, the complex anatomy of the disease and the mechanisms of valve regurgitation are very difficult to assess by conventional tomographic views.
In adult patients with Ebstein’s anomaly, it has been reported that 3DE was particularly useful in delineating the chordal attachment of the three leaflets of the TV [10, 11]. This was accomplished by multiple systematic cropping and sectioning of the 3DE data sets, enabling the visualization of the characteristic “bubble-like” appearance of the leaflets, resulting from the bulging of the nontethered leaflet areas. In addition, an en face view of the valve is easily obtainable with 3DE, to measure the leaflet surface areas and to visualize the regions of ineffective leaflet coaptation. The ability to measure the surface and the free leaflet margin by 3DE is particularly noteworthy in view of the current repair techniques that involve the construction of a monocuspid TV using the tissue of the large anterior leaflet [9]. Moreover, 3DE can be useful in evaluating the size of the functional right ventricle, and in estimating the severity of tricuspid regurgitation by measuring the vena contracta area on cross-sectional planes placed at the narrowest region of the 3D color Doppler jet.
Carcinoid Heart Disease
The TV is the most frequently affected valve in carcinoid heart disease [12••]. The valvular involvement consists of leaflet thickening with excessive fibrosis and markedly restricted motion. The fibrotic leaflets move in a stiff “board-like” fashion rather than the normal undulating motion [12••] and their restricted opening leads to the RV inflow obstruction. The TV leaflets are usually retracted and held partially open during both systole and diastole, thus resulting in a combined tricuspid stenosis and regurgitation, the latter being predominant (Fig. 1c).
3DE allows an en face view of the valve from either atrial or ventricular side, as well as a detailed assessment of subvalvular apparatus [12••]. Because individual leaflet involvement can be highly variable and associated with various extents of subvalvular apparatus thickening and fibrosis, 3DE is particularly valuable in assessing patients with carcinoid disease, due to its ability to visualize simultaneously all three TV leaflets and their chordal attachments from unique perspectives.
Pacemaker-Related Tricuspid Regurgitation
It is well known that a sizable number of patients with a permanent pacemaker or an implantable cardioverter-defibrillator may show significant tricuspid regurgitation and that the leads of such devices may be the primary cause of symptomatic tricuspid regurgitation [13]. However, the diagnosis of lead-induced tricuspid regurgitation may be challenging using conventional 2DE, because of the difficulties in identifying the anatomical relationship between the lead and the tricuspid leaflet. Lin et al. [13] reported that the tricuspid regurgitation induced by device leads was diagnosed using 2DE in only five of 41 patients (12%) who underwent surgery for pacemaker-related tricuspid regurgitation. The en face view of the TV obtained by 3DE allows one to precisely identify the route of the lead across the right heart cavities, of its position at the TV level, and its spatial relationship with the individual leaflets (Fig. 1e). Seo et al. [14••] reported that in almost all patients with mild to moderate tricuspid regurgitation, the pacemaker lead position was in between the tricuspid leaflets, particularly between the posterior and the anterior or the septal leaflet. This may explain the poor performance of 2DE for an accurate diagnosis in this clinical scenario, because the TV commissures cannot be visualized by 2DE, and the posterior leaflet can be usually seen in the parasternal RV inflow view only (especially challenging in the not so rare case of a poor parasternal acoustic window). The main mechanism of severe pacemaker lead-induced tricuspid regurgitation was the interference with the effective TV leaflet coaptation (7/12 patients), the posterior leaflet being obstructed in four patients, and the septal leaflet in three. Importantly, the study by Seo et al. [14••] confirmed the findings of Schnabel et al. [15], who reported an easier visualization of the TV leaflets in patients with RV dysfunction than in healthy subjects.
Traumatic Tricuspid Regurgitation
Traumatic tricuspid regurgitation is a rare cardiovascular complication that may occur as a consequence of blunt chest trauma, with disruption of chordal structures, or from internal (usually iatrogenic) trauma from a pacemaker lead, a stiff guidewire, a bioptome during RV endomyocardial biopsy, or during radiofrequency ablation for treatment of arrhythmias when devices can perforate TV leaflets or damage subvalvular chordal apparatus. 3DE can accurately delineate the anatomy of the TV and identify the flail leaflet(s) and the involvement of the subvalvular apparatus (ruptured chordae and/or papillary muscle), and may help in the decision making and in planning the surgical correction [16, 17].
Tricuspid Stenosis
Nowadays, tricuspid stenosis is rather uncommon in adult patients. In nearly all cases it is due to rheumatic disease in association with rheumatic mitral and/or aortic valve involvement. Carcinoid heart disease can lead to some degree of tricuspid stenosis as well. Valvulopathy associated with Fen-Phen and methysergide is also characterized by thickened fibrotic and hypomobile tricuspid leaflets, with various degrees of valve stenosis and regurgitation.
2DE images show thickening and shortening of the TV leaflets, which may exhibit some doming in diastole. Doppler recordings of trans-tricuspid flow velocity allow the calculation of mean gradient and of the valve area by pressure half-time method, as described for the mitral valve [18]. However, unlike what is routinely done for assessing mitral stenosis severity, neither transthoracic nor transesophageal 2DE can provide en face views of the stenotic orifice and the fused commissures of the TV. Using 3DE, the stenotic orifice of the TV can be clearly visualized from the ventricular side and planimetered (Fig. 1f) [19].
Infective Endocarditis of the Tricuspid Valve
Vegetations or masses of the TV apparatus can be nicely characterized as shape and size more readily and accurate with 3DE. 3DE can also depict leaflet perforations/prolapse and color Doppler supports the diagnosis with information on the mechanism and the severity of tricuspid regurgitation. 3DE is particularly useful in patients with prosthetic devices (e.g., pacemaker or intracardiac defibrillator leads, or TV prostheses) to assess the precise location of the vegetations and their relationship with the prosthetic structures [20].
It is a fact that 2DE has a good sensitivity to detect pathologic masses and to assess their mobility, due to its high temporal and spatial resolution. However, its 2D nature and limited views induce the inherent risk of missing the diagnosis when the vegetations develop outside the standard 2D views of the TV, as well as uncertainties and errors regarding their true size and precise insertion. Vegetation size is an important predictor for embolic events and for response to treatment [21]. The measurements of the maximum diameter(s) by 2DE are routinely used to quantify the vegetation size. However, most vegetations are irregularly shaped and highly mobile, making it difficult to accurately image them in one 2D view or select the largest diameter. The selection of a diameter that is not truly the largest may lead to the underestimation of the vegetation size and to the misinterpretation of patient prognosis. 3DE images the entire volume of the vegetation mass, allowing for accurate measurements from multiple planes, properly aligned to delineate the true largest dimensions [22].
Functional Tricuspid Regurgitation
Tricuspid annular dilatation and leaflet tethering seem to be the underlying mechanisms of functional TV regurgitation. In addition, the extent of TV annulus dilatation may be a more reliable indicator of TV pathology than the degree of regurgitation itself [23].
Using 3DE, two independent groups [5, 24] were able to demonstrate that, similar to the mitral annulus, the normal TV annulus is saddle-shaped, with the highest points located in an anterior-posterior orientation and the lowest points in a mediolateral orientation. They also elucidated the mechanism of functional tricuspid regurgitation by showing that, with the progression of valvular insufficiency, the tricuspid annulus not only dilates, but also becomes flatter and more circular. In addition, they showed that the increase of the mediolateral distance was greater than that of the anterior-posterior distance with worsening of TV regurgitation. This may result from the dilation of the tricuspid annulus preferentially along its free wall. This latter finding has important clinical implications for planning surgical annuloplasty techniques.
Matsunaga and Duran [25] demonstrated that preoperative tricuspid annular dilation was associated with the development of late postoperative tricuspid regurgitation after the repair of ischemic mitral regurgitation. Dreyfus et al. [26] reported that the long-term outcome of the patients improved when the decision to perform tricuspid annuloplasty was based on the extent of tricuspid annular dilation rather than on the degree of tricuspid regurgitation at the time of surgery. Reference measures for TV repair include tricuspid annulus size greater than 2.1 cm/m² and tricuspid annulus fractional shortening less than 25% [27]. However, both Matsunaga and Duran [25] and Dreyfus et al. [26] used 2DE for surgery decision making and this may have led to some inaccuracies in measuring the true annular size.
Anwar et al. [28] demonstrated that the tricuspid annulus shape is not circular, but oval, with a minor and a major diameter, both in normally sized and in dilated annulus. In addition, they showed that the currently used tricuspid annulus diameters measured with 2DE (both measured in apical four-chamber view and in parasternal short-axis view) systematically underestimated the actual tricuspid annulus size. As a consequence, 65% of patients with normal tricuspid annulus diameter at 2DE showed grade 1–2 tricuspid regurgitation compared with 30% of patients with normal tricuspid annulus size at 3DE [28]. Conversely, calculation of tricuspid annulus fractional shortening yielded the same results using 2DE and 3DE, because there is a comparable extent of underestimation of the tricuspid annulus diameter by 2DE in both diastole and in systole.
In addition to tricuspid annulus shape, size, and function, 3DE enables the assessment of TV leaflet geometry in functional tricuspid regurgitation. Sukmawan et al. [29] reported that, in patients with pulmonary hypertension (i.e., RV to RA gradient ≥30 mm Hg), the tricuspid regurgitation coexists with the tethering of tricuspid leaflets into the right ventricle. The measured TV tenting volume was linearly correlated with RV volume and with TV regurgitant jet area. Min et al. [30••] evaluated the TV apparatus using 3DE to predict residual tricuspid regurgitation after surgical annuloplasty. Tenting volume and anterior-posterior tricuspid annulus diameter before surgery emerged as independent preoperative predictors of short-term residual tricuspid regurgitation. In addition, the tenting angle between tricuspid annulus plane and septal leaflet was the most powerful predictor of successful operative correction of the regurgitation. The authors also assessed the geometric changes occurring after surgery and found that annuloplasty led not only to the reduction of annulus size, but also to the worsening of leaflet tenting due to the inward displacement of the tricuspid annulus.
Finally, 3DE can help in estimating the severity of TV regurgitation using Doppler color flow. Velayudhan et al. [31] reported a good feasibility for obtaining the vena contracta area of the tricuspid regurgitant jet from cropping the 3DE color Doppler data set by imaging planes exactly parallel to the TV orifice. The authors also found a poor correlation between the vena contracta area obtained by 3DE and its width measured by 2DE, supporting the concept that, similar to mitral regurgitation, the vena contracta of the tricuspid regurgitant jet has a complex geometry. However, they found reasonable correlations between the vena contracta area measured with 3DE and the conventional estimates of tricuspid regurgitation severity by 2DE color Doppler (i.e., regurgitant jet area and its ratio with RA area), and proposed new criteria for estimating tricuspid regurgitation severity based on vena contracta area: less than 0.5 cm2 for mild; 0.5 to 0.75 cm2 for moderate; and greater than 0.75 cm2 for severe.
3D Transesophageal Echocardiography
Three-dimensional transesophageal (3D TEE) assessment of the native TV is more challenging than of the mitral valve. TV can be optimally visualized from a single mid-esophageal four-chamber view using the zoom mode only in 11% of patients, largely because this valve is anteriorly located, at farther distance from the TEE transducer, is associated with a less favorable angle of insonation, and has thinner leaflets compared with the mitral valve [32]. Whether 3D TEE will improve the accuracy in the assessment of valvular dysfunction similar to what has been described for the mitral valve still needs to be established.
Prosthetic valves in the tricuspid position are less reliably imaged using 3D TEE than in the mitral or aortic position [33]. The oval shape of the tricuspid annuloplasty rings can be displayed by 3D TEE using both zoom and full-volume modes [34••]. Even though the qualitative and quantitative assessment of the TV annulus by 3DE is feasible, correlates well with cardiac magnetic resonance data [35], and may significantly improve our understanding of functional tricuspid regurgitation pathophysiology [5], the assessment of the TV annulus by 3D TEE for intraoperative surgical decision making has never been investigated. Similarly, no studies have described so far the use of 3D TEE in the assessment of tricuspid stenosis or to describe TV leaflet morphology in patients with organic TV regurgitation.
Present Limitations and Future Perspectives
Despite all the data supporting the use of 3DE to assess TV morphology and function, especially in patients who are candidates to cardiac surgery for left-sided diseases, this technique has not been integrated yet into the clinical routine. Some reasons pertain to the 3D technique, whereas others are related to its application for TV study. For an effective application of the 3DE, echocardiographers need a specific education and a dedicated training (e.g., 3D volume acquisition, navigation within the data set and proper use of cropping, slicing and thresholding to obtain the desired images, accurate interpretation of pathology and discrimination from technical limitations, such as dropouts, artifacts, or too low gain). The 3D equipment and software used for quantitation are rather expensive, and the addition of 3DE acquisition and analysis after performing the routine echo study may adversely impact on the work flow in the echo laboratory.
In addition, there are specific issues related to the application of 3DE for TV study. At the moment, there is no evidence that 3D assessment of TV anatomy and function may improve surgical results. However, clinical research is active in this field and results are expected soon. Another reason currently limiting the implementation of 3DE in the clinical routine is the lack of standardized measures and specific software to be used to quantitate the tricuspid annulus and leaflets, as it has been developed for the mitral and aortic valve. However, the recent growing interest to understand the TV pathology both from echocardiographers and surgeons will most probably fuel the development and the implementation of such tools.
Conclusions
3DE complements the conventional 2D and Doppler echocardiographic assessment of the TV with detailed information on leaflet morphology and spatial geometry, mechanism and severity of tricuspid regurgitation, more accurate determination of TV area, and will probably become the preferred adjunct for standard ultrasound imaging in patients with TV pathology. At the moment, there is no solid data supporting the use of 3DE to select patients to be addressed for the surgical repair of functional tricuspid regurgitation. However, as for mitral regurgitation, an improved assessment of valve anatomy and understanding of the pathophysiologic mechanisms underlying the TV regurgitation could lead to the development of more effective techniques for TV repair.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Mascherbauer J, Maurer G. The forgotten valve: lessons to be learned in tricuspid regurgitation. Eur Heart J. 2010;31:2841–3.
• Badano LP, Agricola E, Perez de Isla L, Gianfagna P, Zamorano JL. Evaluation of the tricuspid valve morphology and function by transthoracic real-time three-dimensional echocardiography. Eur J Echocardiogr. 2009;10:477–84. This review provides a practical guide to the assessment of the TV by transthoracic 3D and summarizes its main strengths over the conventional echocardiography for an accurate diagnosis of TV anatomy and function in various clinical settings.
Muraru D, Badano LP. Assessment of tricuspid valve morphology and function. In: Badano LP, Lang RM, Zamorano JL, editors. Textbook of real-time three dimensional echocardiography. London: Springler Verlag; 2011. p. 173–82.
Bruce CJ, Connolly HM. Right-sided valve diseases deserves a little more respect. Circulation. 2009;119:2726–34.
Fukuda S, Saracino G, Matsumara Y, et al. Three-dimensional geometry of the tricuspid annulus in healthy subjects and in patients with functional tricuspid regurgitation: a real-time, 3-dimensional echocardiographic study. Circulation. 2006;114:I-492–8.
Rawlins DB, Austin C, Simpson JM. Live three-dimensional paediatric intraoperative epicardial echocardiography as a guide to surgical repair of atrioventricular valves. Cardiol Young. 2006;16:34–9.
Seliem MA, Fedec A, Szwast A, et al. Atrioventricular valve morphology and dynamics in congenital heart disease as imaged with real-time 3-dimensional matrix-array echocardiography: comparison with 2-dimensional imaging and surgical findings. J Am Soc Echocardiogr. 2007;20:869–76.
• Takahashi K, Mackie AS, Rebeyka IM et al. Two-dimensional versus transthoracic real-time three-dimensional echocardiography in the evaluation of the mechanisms and sites of atrioventricular valve regurgitation in a congenital heart disease population. J Am Soc Echocardiogr. 2010;23:726–34. The authors of this paper report the complementary role of transthoracic 3DE for the assessment of congenital abnormalities of the TV, using surgical findings as reference.
Attenhofer CH, Connolly HM, Dearani JA, Edwards WD, Danielson GK. Ebstein’s anomaly. Circulation. 2007;115:277–85.
Patel V, Nanda NC, Rajdev S, et al. Live/real time three-dimensional transthoracic echocardiographic assessment of Ebstein’s anomaly. Echocardiography. 2005;22:847–54.
Bharucha T, Anderson RH, Lim ZS, Vettukattil JJ. Multiplanar review of three-dimensional echocardiography gives new insights into the morphology of Ebstein’s malformation. Cardiol Young. 2010;20:49–53.
•• Bhattacharyya S, Toumpanakis C, Burke M, Taylor AM, Caplins ME, Davar J. Features of carcinoid heart disease identified by 2- and 3-dimensional echocardiography and cardiac MRI. Circ Cardiovasc Imaging. 2010;3:103–11. This paper details the characteristic features of the carcinoid heart disease, including the spectrum of TV involvement, in a large cohort of patient by various imaging modalities encompassing 2D and 3DE, magnetic resonance, and positron emission tomography.
Lin G, Nishimura RA, Connolly HM, Dearani JA, Sundt 3rd TM, Hayes DL. Severe symptomatic tricuspid valve regurgitation due to permanent pacemaker or implantable cardioverter-defibrillator leads. J Am Coll Cardiol. 2005;45:1672–5.
•• Seo Y, Ishizu T, Nakajima H, Sekiguchi Y, Watanabe S, Aonuma K. Clinical utility of 3-dimensional echocardiography in the evaluation of tricuspid regurgitation caused by pace-maker leads. Circ J 2008;72:1465–70. This study reports the superiority of 3DE over the 2D approach for accurately diagnosing pacemaker-induced tricuspid regurgitation by its ability to identify the spatial relationship between the lead and valve leaflets, particularly the posterior one.
Schnabel R, Khaw AV, von Bardeleben RS, et al. Assessment of the tricuspid valve morphology by transthoracic real-time-3D-echocardiography. Echocardiography. 2005;22:15–23.
Nishimura K, Okajama H, Inoue K, et al. Visualization of traumatic tricuspid insufficiency by three-dimensional echocardiography. Circ J. 2010;55:143–6.
Kamiya C, Ohara T, Nakatani S, et al. Traumatic tricuspid regurgitation caused by myocardial laceration: a three dimensional echocardiographic study. J Am Soc Echocardiogr. 2010;903:903.e1–3.
Pearlman AS. Role of echocardiography in the diagnosis and evaluation of severity of mitral and tricuspid stenosis. Circulation. 1991;84(Suppl):I 193–7.
Faletra F, La Marchesina U, Bragato R, De Chiara F. hree dimensional transthoracic echocardiography images of tricuspid stenosis. Heart. 2005;91:499.
Naqvi TZ, Rafie R, Ghalichi M. Real-time 3D TEE for the diagnosis of right-sided endocarditis in patients with prosthetic devices. J Am Coll Cardiol Img. 2011;3:325–7.
Habib G, Badano L, Tribouilloy C, et al. Recommendations for the practice of echocardiography in infective endocarditis. Eur J Echocardiogr. 2010;11:202–19.
Asch FP, Bieganski PM, Panza JA, Weissman NJ. Real-time 3-dimensional echocardiography evaluation of intracardiac masses. Echocardiography. 2006;23:218–24.
Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127–32.
Ton-Nu TT, Levine RA, Handschumacher MD, et al. Geometric determinants of functional tricuspid regurgitation:insights from 3-dimensional echocardiography. Circulation. 2006;114:143–9.
Matsunaga A, Duran CM. Progression of TR after repaired functional ischemic mitral regurgitation. Circulation. 2005;112(suppl):I-453–7.
Dreyfus GD, Corbi PJ, Chan KM, Bahrami T. Secondary tricuspid regurgitation or dilatation: which should be the criteria for surgical repair? Ann Thorac Surg. 2005;79:127–32.
Colombo T, Russo C, Cilibert GR, et al. Tricuspid regurgitation secondary to mitral valve disease: tricuspid annulus function as guide to tricuspid valve repair. Cardiovasc Surg. 2001;9:369–77.
Anwar AM, Geleijnse MI, ten Cate FJ, Meijboom FJ. Assessment of tricuspid valve annulus size, shape and function using real-time three-dimensional echocardiography. Interact Cardiovasc Thorac Surg. 2006;5:683–7.
Sukmawan R, Watanabe N, Ogasawara Y, et al. Geometric changes of tricuspid valve tenting in tricuspid regurgitation secondary to pulmonary hypertension quantified by novel system with transthoracic real-time 3-dimensional echocardiography. J Am Soc Echocardiogr. 2007;20:470–6.
•• Min SY, Song JM, Kim MK et al. Geometric changes after tricuspid annuloplasty and predictors of residual tricuspid regurgitation: a real-time three-dimensional echocardiography study. Eur Heart J. 2010;31:2871–80. This nice study used 3DE to describe the geometric changes of tricuspid apparatus after annuloplasty. They reported that preoperative tenting volume, as a measure including both annulus size and leaflet tethering, and anteroposterior annulus diameter independently predict the surgical outcome.
Velayudhan DE, Brown TM, Nanda NC, et al. Quantification of tricuspid regurgitation by live three-dimensional transthoracic echocardiographic measurements of vena contracta area. Echocardiography. 2006;23:793–800.
Sugeng L, Shernan SK, Salgo IS, et al. Live 3-dimensional transesophageal echocardiography initial experience using the fully-sampled matrix array probe. J Am Coll Cardiol. 2008;52:446–9.
Sugeng L, Shernan SK, Weinert L, et al. Realtime three-dimensional transesophageal echocardiography in valve disease: comparison with surgical findings and evaluation of prosthetic valves. J Am Soc Echocardiogr. 2008;21:1347–54.
•• Vegas A, Meineri M. Three-dimensional transesophageal echocardiography is a major advance for intraoperative clinical management of patients undergoing cardiac surgery: a core review. Anesth Analg. 2010;1548–73. This nice review presents the key features of the 3DE and its emerging clinical applications, including for the TV pathology, with a special focus on the utility of transesophageal examination in the operating room.
Anwar AM, Soliman OI, Nemes A, van Geuns RJ, Geleijnse MI, ten Cate FJ. Value of assessment of tricuspid annulus: real-time three-dimensional echocardiography and magnetic resonance imaging. Int J Cardiovasc Imaging. 2007;23:701–5.
Disclosure
Conflicts of interest: D. Muraru: has received a research grant from GE Healthcare; L.P. Badano: has received research grants and is on the speakers bureau for GE Healthcare; C. Sarais: none; E. Soldà: none; S. Iliceto: none.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muraru, D., Badano, L.P., Sarais, C. et al. Evaluation of Tricuspid Valve Morphology and Function by Transthoracic Three-Dimensional Echocardiography. Curr Cardiol Rep 13, 242–249 (2011). https://doi.org/10.1007/s11886-011-0176-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11886-011-0176-3