Opinion statement
For some time, posterior circulation stroke has been neglected in diagnostic and therapeutic studies for various reasons, such as minor incidence compared to anterior circulation stroke or anatomical and vascular characteristics. This changed at least partly when the New England Medical Center (NEMC) Posterior Circulation Registry was initiated, and now the number of publications concerning posterior circulation stroke is continuously increasing. Whether the differences outweigh the similarities between posterior and anterior circulation stroke remains open to debate, but both are the subject of intensive investigations. In this article, we review the most recent literature on different MRI techniques, such as diffusion-weighted and diffusion tensor imaging (DWI and DTI), perfusion-weighted imaging (PWI), vascular imaging, and susceptibility weighted imaging (SWI), in posterior circulation stroke and discuss their diagnostic and prognostic impact as well as general implications for acute treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
For various reasons, the medical literature dealing with the different aspects of magnetic resonance imaging (MRI) of acute ischemic stroke in the posterior circulation is sparse. First, acute ischemic stroke in the posterior circulation accounts for only approximately 15% of all ischemic strokes. Second, the vertebrobasilar arterial system is unique in that two large vessels, the vertebral arteries (VA), join together to form one larger artery, the basilar artery (BA). Another striking difference between the anterior and posterior circulation is the large number of congenital vessel variations regarding arrangement and size (eg, hypoplasia of one of the VA or a fetal origin of one of the posterior cerebral arteries [PCA]). Third, compared to the anatomic structures supplied by the anterior circulation (neocortex, basal ganglia), there is a substantial variety of anatomic structures supplied by branches of the posterior circulation comprising neocortex (occipital lobe), allocortex (hippocampus), basal ganglia (thalamus), brainstem (mesencephalon, pons, medulla oblongata), and cerebellum. Fourth, for years, stroke neurologists tended to manage patients with acute ischemic stroke in the posterior circulation differently from patients with acute ischemic stroke in the anterior circulation, and a systematic investigation was not undertaken before the New England Medical Center (NEMC) Posterior Circulation Registry was initiated [1, 2]. The large clinical trials investigating acute therapy with recombinant tissue plasminogen activator (rtPA) in acute ischemic stroke focused on anterior circulation stroke or even excluded patients with posterior circulation stroke from participation [3–5]. The same holds true for clinical trials examining MRI-guided thrombolysis based on the perfusion-weighted imaging (PWI)/diffusion-weighted imaging (DWI) mismatch concept [6, 7]. Consequently, data on potential benefits and risks of thrombolysis in posterior circulation stroke is sparse. In this article, we review the most recent literature concerning different aspects of MRI of acute ischemic stroke in the posterior circulation.
Diffusion-weighted and diffusion tensor imaging
-
DWI was introduced and established as a routine imaging procedure in acute ischemic stroke in the late 1990s, and since then a large number of studies covering numerous different angles of ischemic stroke have been published. DWI is exquisitely sensitive and able to demonstrate even minute size acute ischemic lesions [8]. The lack of mobility of water protons in ischemic tissue lights up strongly against the dark background of healthy tissue on DWI, which provides a very high contrast between lesion and background. Despite the large number of publications on anterior circulation stroke, only a subset of articles has looked into posterior circulation ischemia. Nevertheless, there are still noteworthy novel and interesting findings on special aspects of acute ischemic stroke in the posterior circulation that have been reported during the past few years.
-
Lee et al. [9] examined PCA stroke in detail and demonstrated different infarction patterns on DWI depending on the underlying etiology. Patterns were categorized into superficial, deep, and superficial plus deep infarctions. In the study population, infarctions in the posterior cerebral artery (PCA) territory were most frequently found in the parieto-occipital area, followed by the ventrolateral thalamus, the mid-temporal area, the anterolateral mesencephalon, the lateral geniculate body, the anterior thalamus, the anterior temporal area, and the dorsomedial thalamus. Large vessel disease was most frequent, followed by cardioembolism, small vessel disease, undetermined cause, and other etiologies. Small vessel disease was the most common cause of deep infarctions whereas cardioembolism caused most cases with superficial infarction [9].
-
Bilateral thalamic infarction, a rare form of acute ischemic stroke in the posterior circulation, is caused in most cases by blockage of an unpaired paramedian artery (the artery of Percheron) arising from the PCA and supplying both thalami. In a recent article, Lazzaro et al. [10] characterized the imaging spectrum of bilateral thalamic infarction on DWI on the basis of a large cases series and reported four distinct paramedian thalamic infarction patterns: with and without midbrain infarction, with infarction of the anterior thalamus and midbrain, and with infarction of the anterior thalamus without midbrain affection. These findings are consistent with the knowledge from autopsy studies that the tuberothalamic artery is absent in about 30% of patients, and the anterior territory of the thalamus is supplied by the paramedian arteries in these patients.
-
Another study focused on infarction of the hippocampus as a subtype of PCA stroke and its clinical, neuropsychological, and radiologic features. On DWI, four patterns of hippocampal stroke were identified: affection of the complete, the lateral, or dorsal hippocampus, and circumscribed small lesions in the lateral hippocampus (Fig. 1b), corresponding to the vascular anatomy of the hippocampus. Furthermore, ischemic lesions in the posterior circulation were found in all patients, and clinical symptoms caused by these lesions outside the hippocampus were the common leading clinical signs. In contrast to this, obvious memory deficits were found in only one sixth of patients, but thorough neuropsychological examination revealed deficits of verbal episodic long-term memory in left hippocampal stroke and of nonverbal episodic long-term memory in right hippocampal stroke [11••].
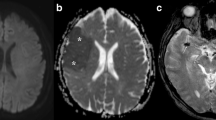
Fig. 1 Vessels of the posterior circulation: 1) posterior communicating artery, 2) posterior cerebral artery, 3) superior cerebellar artery, 4) pontine branches of the basilar artery, 5) basilar artery, 6) anterior inferior cerebellar artery, 7) posterior inferior cerebellar artery, and 8) vertebral artery. Examples of MRI in the posterior circulation: a Paramedian thalamic artery (red arrow) arising from the P1-segment of the left posterior cerebral artery on MR angiography at 3.0 Tesla. b Subtypes of hippocampal stroke: complete, lateral, dorsal, and small circumscribed ischemic lesion. c Diffusion tensor tractography in a patient with left pontine infarction (red arrow) affecting the pyramidal tract. d Hypoperfusion (red arrow) in acute pontine infarction on dynamic susceptibility contrast perfusion MRI.
-
Kim and Han [12] studied clinical presentation, MRI, and angiographic findings of patients with medial medullary infarctions and correlated DWI lesions with clinical findings and clinical outcomes. Ischemic infarctions were located most frequently in the rostral medulla, whereas ventro-dorsal lesion patterns included ventral, ventral plus middle, and ventral plus middle plus dorsal infarction. Clinical symptoms consisted of hemiparesis, hemihypesthesia, and dizziness, each of these significantly related to involvement of ventral, middle, and dorsal portions of the medulla oblongata. Small vessel disease was most frequent, followed by large vessel disease [12].
-
Diffusion tensor imaging (DTI) is a noninvasive technique for investigating cerebral tissue structure providing information about the location, orientation, and anisotropy of the brain’s white matter tracts. Furthermore, DTI can be used to track neural fiber bundles along their whole length within the white matter with diffusion tensor tractography (DTT).
-
Liang et al. [13] used DTI to investigate secondary anterograde and retrograde degeneration in the pyramidal tract following pontine stroke and its potential possible influence on clinical outcome. Fourteen patients with pontine stroke underwent MRI including DTI at three time points after onset of symptoms (week 1, 4, and 12) and were compared to healthy control subjects matched for age and gender. DTI-derived parameters such as fractional anisotropy (FA) and mean diffusivity (MD) were correlated with the neurologic deficit as assessed with the National Institute of Health Stroke Scale (NIHSS). In the ipsilateral medulla oblongata and the proximal portion of the pyramidal tract, FA values decreased progressively during the study period. As one would expect, the measured NIHSS scores decreased over time but the absolute values of percent reduction of the NIHSS scores correlated negatively with the absolute values of percent reduction of FA value at the ipsilateral medulla and the proximal portion of pyramidal tract, indicating that progressive anterograde and retrograde degeneration in the pyramidal tract may hinder clinical recovery after pontine infarction [13].
-
Another study group also analyzed Wallerian degeneration of the pyramidal tract after pontine stroke using DTI at admission and at follow-up [14]. FA as well as axial and radial diffusivity were acquired in the volume of the ipsilateral and contralateral pyramidal tracts distally to the ischemic lesion. Wallerian degeneration was detected at the earliest 1 to 3 days after onset of symptoms. Interestingly, only a subset of patients developed Wallerian degeneration, detected as significant decrease in FA values over time in the distal pyramidal tract. A correlation between the initial FA value and the clinical outcome as well as the degree of the initial clinical deficit and the extent of Wallerian degeneration of the pyramidal tract over time could not be demonstrated [14].
-
Jang et al. [15] investigated whether the integrity of the corticospinal tract classified by DTT may be useful as predictor of motor outcome after pontine infarction (see Fig. 1c for an example of DTT in pontine stroke). DTT of the pyramidal tract was achieved after 5 to 30 days and patients were classified into two groups depending on whether integrity of the corticospinal tract was preserved or not. Patients with preserved structural integrity showed a better motor function than patients with interruption of the corticospinal tract at 6-month follow-up. Consequently, the authors concluded that DTT in the early stage after pontine infarction might have predictive value for the motor outcome in patients with pontine infarction [15].
Perfusion-weighted imaging
-
PWI is of particular importance for acute ischemic stroke imaging as it may be used not only to show areas of altered perfusion (hypoperfusion/hyperperfusion) in the human brain but also to draw conclusions from these perfusion patterns regarding persistent vessel pathology and to demonstrate possibly salvageable brain tissue. This is the basis of the DWI-PWI mismatch concept used for MRI-guided intravenous thrombolysis in acute ischemic stroke. Nowadays, there are different MRI techniques in use to display brain perfusion, the most common being dynamic susceptibility contrast (DSC) perfusion MRI and arterial spin labeled (ASL) perfusion MRI. In DSC perfusion MRI, a series of fast T2*-weighted images is acquired while a gadolinium-based contrast agent is injected and passes through the brain, where it induces a reduction of T2* intensity depending on the local concentration. The acquired image series is used to generate different perfusion maps such as time to peak (TTP), mean transit time (MTT), cerebral blood flow (CBF), and cerebral blood volume (CBV). Although DSC perfusion MRI is fast, easily applicable, and provides whole brain coverage, few studies have employed DSC in systematic approaches. Even considering that spatial resolution is not optimal to detect very small ischemic lesions and possible susceptibility effects in the posterior fossa, there is still a lack of DSC studies of the posterior circulation.
-
Weidauer et al. [16] used DSC perfusion MRI for a more detailed characterization of paramedian thalamic infarction and were able to demonstrate a hypoperfusion in the PCA territory due to occlusion or severe stenosis of the distal basilar artery and/or the P1-segments of the PCA.
-
In a more recent prospective study, we demonstrated the feasibility and value of DSC perfusion MRI in acute pontine stroke [17••]. In about half of patients, visual analysis of PWI showed persisting hypoperfusion, and in one patient hyperperfusion indicating recanalization was found. Figure 1d gives an example of hypoperfusion in acute pontine stroke. Persistent hypoperfusion was frequently associated with pathology of the basilar artery and a more severe clinical deficit. DSC perfusion MRI is of particular use in acute pontine stroke as the small penetrating branches of the basilar artery are regularly missed by MR angiography [17••]. In another case series that focused upon supra- and infratentorial lacunar infarction, hypoperfusion matching the infarction area was associated with early clinical deterioration but not with infarction growth [18].
-
Kim et al. [19••] examined patients with acute medullary infarction with DSC perfusion MRI and detected hypoperfusion in the medulla oblongata and/or the inferior cerebellum in about half of patients. Despite comparable infarction patterns on DWI, patients with proven hypoperfusion had poor clinical outcomes after 7 days and 1 month after onset of symptoms. Paralleling the results of our own investigation of hypoperfusion in pontine stroke, stenosis or occlusion of the ipsilateral vertebral artery was frequently observed in patients with perfusion deficit on PWI. In the multivariate analysis, altered perfusion was independently associated with poor early and late outcomes besides infarction pattern on DWI. Consequently, the authors concluded that DSC perfusion MRI may be useful to predict the clinical outcome after acute ischemic stroke in the medulla oblongata [19••].
-
At the time of the writing of this article, there were only three articles on intravenous or intra-arterial thrombolysis in posterior circulation stroke with evidence of a DWI-PWI mismatch. Ostrem et al. [20] described intra-arterial rtPA treatment in five patients with acute basilar artery occlusion with marked DWI-PWI mismatch. However, in these cases treatment was not guided by the DWI-PWI mismatch concept. In two patients, a significant improvement was observed [20]. Köhrmann et al. [21] reported on two cases with isolated cerebellar infarction treated with intravenous rtPA guided by the DWI-PWI mismatch concept in an extended time window. Initial MRI demonstrated only minor DWI lesions but superior cerebellar artery (SCA) occlusion with hypoperfusion of the respective territory. After thrombolysis, both patients recovered within a few hours and follow-up MRI demonstrated only minor infarction [21].
-
More recently, we presented a case series of patients with PCA stroke treated with intravenous thrombolysis in an extended time window using MRI criteria [22••]. Baseline MRI showed circumscribed ischemic lesions in the thalamus or hippocampus, whereas MR angiography showed PCA occlusion with corresponding hypoperfusion in the PCA territory on DSC perfusion MRI. Follow-up MRI showed partial or complete recanalization in four patients with minor infarction growth, whereas in one patient PCA occlusion persisted, resulting in a large PCA infarction. In three patients the clinical symptoms improved within 2 h, and at 3-month follow-up four patients had a favorable clinical outcome. These results provide motivation to evaluate systematically an approach to treat PCA stroke patients with thrombolysis based on the mismatch concept [22••].
-
ASL perfusion MRI is a completely noninvasive technique that uses arterial blood as an endogenous tracer for perfusion imaging by magnetic labeling of blood water proximal to the tissue of interest. Contributions to a specific vascular territory can be visualized very elegantly with territorial ASL perfusion MRI. Hendrikse et al. [23••] used this approach to investigate the effects of variations in vascular anatomy of the circle of Willis on perfusion territories and could demonstrate a large variability of perfusion territory contributions. All patients with a fetal origin of the PCA showed an arterial blood supply of the thalamus from the ipsilateral internal carotid artery, whereas this was the case in only about half of patients without fetal origin of the PCA [23••]. The same method was used for investigation of blood flow distribution in patients with internal carotid artery (ICA) stenosis. The thalamus was supplied predominantly by the basilar artery, but in some cases contributions from the ipsilateral and even from the contralateral ICA could be shown. This finding indicates a substantially larger variety of perfusion territories in the thalamus compared to the classical vascular territories we know from anatomic studies in the 1970s. Consequently, the authors concluded that ASL perfusion MRI could be particularly useful for investigation of the vascular supply of the thalamus [24].
Vascular imaging: MR angiography and high-resolution MRI
-
MR angiography is another technique of special interest for stroke neurologists because it directly reveals the underlying vascular pathology, such as intracranial artery stenosis or occlusion, and provides several advantages over CT or conventional angiography because it is noninvasive, works without ionizing radiation, and is not obscured by bony structures of the posterior fossa. A major disadvantage is its limited spatial resolution; very small arteries (eg, the penetrating pontine branches of the basilar artery or the tiny arteries supplying the thalamus) could not be displayed until recently with MR angiography. However, with increase of magnetic field strength, MR angiography now allows imaging of very small vessels. Figure 1a gives an example of a small penetrating artery originating from the P1-segment of the left PCA.
-
Conijn et al. [25•] used time-of-flight (TOF) MR angiography at 7.0 Tesla to investigate the perforating branches of the posterior communicating artery (PCoA) and the P1-segment of the PCA. In approximately two thirds of study participants a perforating artery from the PCoA was found and its presence was associated with a significantly larger diameter of the PCoA. Similarly, a solitary small perforating artery originating from the P1-segment of the PCA was found in about two thirds of examined subjects, and two penetrating vessels were found in about one fifth. In combination with other techniques such ASL perfusion MRI, assessment of the perforating arteries with MR angiography is likely to increase our understanding of infarcts in the deep brain structures supplied by these arteries [25•].
-
Other studies focused rather on vascular variants and pathologic changes related to acute ischemic stroke in the posterior circulation. As mentioned above, there are a large number of vessel variations in the posterior circulation, and in particular hypoplasia of one of the vertebral arteries can be found very often. Hong et al. [26] investigated whether an unequal vertebral artery flow contributes to the development of basilar artery curvature and indicates the laterality of pontine or cerebellar infarction occurring around the vertebrobasilar junction. Most patients had a basilar artery curvature in the opposite direction to the dominant vertebral artery. Pontine infarctions were located contralateral to the side of basilar artery curvature, whereas posterior inferior cerebral artery (PICA) infarctions were on the same side as the nondominant vertebral artery. The authors concluded that an unequal vertebral artery flow might influence basilar artery curvature and incidence of infarctions in the vicinity of the vertebrobasilar junction [26].
-
Basilar artery hypoplasia and its possible relation to posterior circulation stroke is another vascular finding not well studied or understood. Olindo et al. [27] demonstrated a higher frequency of basilar artery hypoplasia in patients with posterior circulation stroke with small vessel disease or undetermined etiology. Infarctions were found predominantly in the pons and cerebellum [27]. Similarly, Aoki et al. [28] found that clinical deterioration in acute pontine infarction is more likely in patients with smaller diameter of the basilar artery. A more classic risk factor for acute ischemic stroke is atherosclerosis, and several studies centered on this aspect of vascular pathology and its relation to stroke, infarction growth, and outcome. Kim et al. [29] examined the extent of atherosclerosis in the basilar artery in patients with pontine stroke and were able to show that the presence of atherosclerotic changes in the basilar artery is closely related to infarction growth. In another study, patients with single small subcortical infarctions in the anterior and posterior circulation were analyzed with regard to indicators for small vessel disease (chronic white matter lesions, microbleeds) and atherosclerosis (cerebral or cardiac atherosclerosis). Most interestingly, patients with infarction in the basilar artery and vertebral artery territory had fewer lesions extending to the surface, and more often they had atherosclerotic changes in the supplying large cerebral artery as well as cerebral atherosclerosis in general [30].
-
High-resolution MRI is a powerful tool to examine intracranial vessels in detail for alterations of the vessel wall and atherosclerotic plaques. Klein et al. [31] used this approach to investigate the degree of basilar artery atherosclerosis in patients with paramedian and lacunar pontine stroke. Surprisingly, even in patients with unremarkable MR angiography and initially assumed small vessel disease as etiology, high-resolution MRI revealed basilar artery plaques, possibly indicating another underlying pathomechanism [31]. Arterial remodeling characterizes a compensatory enlargement (positive remodeling) or paradoxical shrinkage (negative remodeling) in atherosclerotic altered vessels and has been described recently in high-grade basilar artery stenosis. Positive remodeling was found more often compared to negative remodeling and was associated with larger plaque size and greater plaque burden [32].
T2*-weighted imaging and susceptibility weighted imaging
-
At the present moment, there are only two articles exploring the possibilities and value of T2*-weighted and susceptibility weighted imaging (SWI) in posterior circulation stroke. On T2*-weighted images, hemosiderin deposits (eg, in intracerebral hemorrhage or microbleeds) cause a marked hypointensity. This effect is caused by increased sensitivity to magnetic susceptibility induced by static field inhomogeneities induced by paramagnetic blood breakdown products.
-
Assouline et al. [33] described vascular susceptibility artifacts in acute intracranial artery occlusion on T2*-weighted images and in three patients who also had acute PCA occlusion. In nearly all the examined patients, the authors demonstrated an artifactual ovoid hypointense signal extending beyond the vascular boundaries at the location where the vessel was truncated on MR angiography. This signal seems to be caused by accumulation of deoxyhemoglobin within the fresh thrombotic material, which always decreases in extension or disappears completely after partial or complete recanalization of the occluded artery. The vascular susceptibility artifact appears more sensitive and more specific in middle cerebral artery occlusion compared to a hyperdensity of the middle cerebral artery on CT, but more studies are needed to confirm this result and ascertain that this holds true for posterior circulation vascular occlusion [33].
-
SWI, originally called BOLD venographic imaging, is a technique dependent on blood oxygen level and therefore very sensitive to venous blood. Bilateral thalamic infarction may be caused either by occlusion of an unpaired paramedian thalamic artery, as discussed above, or by internal cerebral vein thrombosis, and in daily practice these two etiologies may be indistinguishable with conventional MRI. In a recently published case report of a patient with bilateral thalamic infarction, it was demonstrated that SWI may be useful to detect internal cerebral vein thrombosis, facilitating the differentiation between the two possible etiologies [34].
Conclusions
-
MRI provides an abundance of different modalities that may be used for diagnosis (DWI, vascular imaging, SWI), prognosis (DTI, PWI), and prognosis-oriented assessment for acute treatment (DWI-PWI mismatch concept) in posterior circulation stroke and underlying vascular pathologies. In addition, new MRI techniques (territorial ASL perfusion MRI) and refinement of already routinely used MRI sequences (MR angiography with higher magnetic field strength, high-resolution MRI) are available for a more precise characterization of perfusion territories and intracranial vessels and vascular pathologies. In specific fields of stroke research, it seems even more advantageous to focus upon the posterior circulation because of its anatomic and vascular characteristics (eg, investigation of Wallerian degeneration in pontine infarction or assessment of small vascular territories in the thalamus with territorial ASL perfusion MRI). The number of articles covering different aspects of MRI in posterior circulation stroke is still increasing and it is hoped that this will motivate an active patient management approach and that more studies will improve the choice of the best treatment and preventive measures.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Caplan L. Posterior circulation ischemia: then, now, and tomorrow. The Thomas Willis Lecture-2000. Stroke. 2000;31:2011–23.
De Marchis GM, Kohler A, Renz N et al. Posterior versus anterior circulation strokes: comparison of clinical, radiological and outcome characteristics. J Neurol Neurosurg Psychiatry 2010.
De Keyser J, Gdovinova Z, Uyttenboogaart M, et al. Intravenous alteplase for stroke: beyond the guidelines and in particular clinical situations. Stroke. 2007;38:2612–8.
Hacke W, Kaste M, Fieschi C, et al. Intravenous thrombolysis with recombinant tissue plasminogen activator for acute hemispheric stroke. The European Cooperative Acute Stroke Study (ECASS). JAMA. 1995;274:1017–25.
Hacke W, Kaste M, Fieschi C, et al. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). Second European-Australasian Acute Stroke Study Investigators. Lancet. 1998;352:1245–51.
Röther J, Schellinger PD, Gass A, et al. Effect of intravenous thrombolysis on MRI parameters and functional outcome in acute stroke <6 h. Stroke. 2002;33:2438–45.
Albers GW, Thijs VN, Wechsler L, et al. Magnetic resonance imaging profiles predict clinical response to early reperfusion: the diffusion and perfusion imaging evaluation for understanding stroke evolution (DEFUSE) study. Ann Neurol. 2006;60:508–17.
Gass A, Ay H, Szabo K, Koroshetz WJ. Diffusion-weighted MRI for the “small stuff”: the details of acute cerebral ischaemia. Lancet Neurol. 2004;3:39–45.
Lee E, Kang DW, Kwon SU, Kim JS. Posterior cerebral artery infarction: diffusion-weighted MRI analysis of 205 patients. Cerebrovasc Dis. 2009;28:298–305.
Lazzaro NA, Wright B, Castillo M, et al. Artery of percheron infarction: imaging patterns and clinical spectrum. AJNR Am J Neuroradiol. 2010;31:1283–9.
Szabo K, Förster A, Jager T, et al. Hippocampal lesion patterns in acute posterior cerebral artery stroke: clinical and MRI findings. Stroke. 2009;40:2042–5. This article describes clinical, neuropsychological, and MRI findings in patients with hippocampal infarction as a subtype of PCA territory stroke.
Kim JS, Han YS. Medial medullary infarction: clinical, imaging, and outcome study in 86 consecutive patients. Stroke. 2009;40:3221–5.
Liang Z, Zeng J, Zhang C, et al. Longitudinal investigations on the anterograde and retrograde degeneration in the pyramidal tract following pontine infarction with diffusion tensor imaging. Cerebrovasc Dis. 2008;25:209–16.
Grassel D, Ringer TM, Fitzek C, et al. Wallerian degeneration of pyramidal tract after paramedian pons infarct. Cerebrovasc Dis. 2010;30:380–8.
Jang SH, Bai D, Son SM, et al. Motor outcome prediction using diffusion tensor tractography in pontine infarct. Ann Neurol. 2008;64:460–5.
Weidauer S, Nichtweiss M, Zanella FE, Lanfermann H. Assessment of paramedian thalamic infarcts: MR imaging, clinical features and prognosis. Eur Radiol. 2004;14:1615–26.
Förster A, Ottomeyer C, Wolf ME, et al. Dynamic susceptibility contrast perfusion MRI identifies persistent vessel pathology in acute pontine stroke. Cerebrovasc Dis. 2010;29:389–94.
Poppe AY, Coutts SB, Kosior J, et al. Normal magnetic resonance perfusion-weighted imaging in lacunar infarcts predicts a low risk of early deterioration. Cerebrovasc Dis. 2009;28:151–6.
Kim SJ, Ryoo S, Bang OY, et al. Perfusion-weighted MRI as a predictor of clinical outcomes following medullary infarctions. Cerebrovasc Dis. 2010;29:382–8.
Ostrem JL, Saver JL, Alger JR, et al. Acute basilar artery occlusion: diffusion-perfusion MRI characterization of tissue salvage in patients receiving intra-arterial stroke therapies. Stroke. 2004;35:e30–4.
Köhrmann M, Sauer R, Huttner HB, et al. MRI mismatch-based intravenous thrombolysis for isolated cerebellar infarction. Stroke. 2009;40:1897–9.
Förster A, Gass A, Kern R et al. MR Imaging-Guided Intravenous Thrombolysis in Posterior Cerebral Artery Stroke. AJNR Am J Neuroradiol 2011, In Press. This article describes a series of patients with acute PCA territory stroke treated with thrombolysis guided by MRI criteria based on the DWI-PWI mismatch concept.
Hendrikse J, Petersen ET, Chng SM, et al. Distribution of cerebral blood flow in the nucleus caudatus, nucleus lentiformis, and thalamus: a study of territorial arterial spin-labeling MR imaging. Radiology. 2010;254:867–75.
Hartkamp NS, Bokkers RP, van der Worp HB et al. Distribution of cerebral blood flow in the caudate nucleus, lentiform nucleus and thalamus in patients with carotid artery stenosis. Eur Radiol 2010.
Conijn MM, Hendrikse J, Zwanenburg JJ et al. Perforating arteries originating from the posterior communicating artery: a 7.0-Tesla MRI study. Eur Radiol 2009. This article reports on the prospects of MR angiography with higher magnetic field strength to clearly image the smallest penetrating arteries supplying the thalamus.
Hong JM, Chung CS, Bang OY, et al. Vertebral artery dominance contributes to basilar artery curvature and peri-vertebrobasilar junctional infarcts. J Neurol Neurosurg Psychiatry. 2009;80:1087–92.
Olindo S, Khaddam S, Bocquet J, et al. Association between basilar artery hypoplasia and undetermined or lacunar posterior circulation ischemic stroke. Stroke. 2010;41:2371–4.
Aoki J, Iguchi Y, Kimura K, et al. Diameter of the basilar artery may be associated with neurological deterioration in acute pontine infarction. Eur Neurol. 2010;63:221–6.
Kim JS, Cho KH, Kang DW, et al. Basilar artery atherosclerotic disease is related to subacute lesion volume increase in pontine base infarction. Acta Neurol Scand. 2009;120:88–93.
Nah HW, Kang DW, Kwon SU, Kim JS. Diversity of single small subcortical infarctions according to infarct location and parent artery disease. Analysis of indicators for small vessel disease and atherosclerosis. Stroke 2010.
Klein IF, Lavallee PC, Mazighi M, et al. Basilar artery atherosclerotic plaques in paramedian and lacunar pontine infarctions: a high-resolution MRI study. Stroke. 2010;41:1405–9.
Ma N, Jiang WJ, Lou X, et al. Arterial remodeling of advanced basilar atherosclerosis: a 3-tesla MRI study. Neurology. 2010;75:253–8.
Assouline E, Benziane K, Reizine D, et al. Intra-arterial thrombus visualized on T2* gradient echo imaging in acute ischemic stroke. Cerebrovasc Dis. 2005;20:6–11.
Thomas B, Somasundaram S, Thamburaj K, et al. Clinical applications of susceptibility weighted MR imaging of the brain - a pictorial review. Neuroradiology. 2008;50:105–16.
Disclosure
The authors report no potential conflicts of interest relevant to this article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Förster, A., Griebe, M., Gass, A. et al. Recent Advances in Magnetic Resonance Imaging in Posterior Circulation Stroke: Implications for Diagnosis and Prognosis. Curr Treat Options Cardio Med 13, 268–277 (2011). https://doi.org/10.1007/s11936-011-0119-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11936-011-0119-8