Abstract
Renal cell carcinoma is the tenth most common malignancy in the USA, with upwards of 61,000 new cases and resulting in more than 14,000 deaths annually. Although partial nephrectomy remains the standard treatment, image-guided nephron-sparing ablative techniques including cryoablation, radiofrequency ablation, and microwave ablation have emerged as treatment options in certain patient populations. Ablative therapies have high technical successes, low tumor recurrence rates, and preserve renal parenchymal volume. The purpose of this article is to provide an update on ablation therapies for small renal masses.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Renal cancer accounts for 2–3 % of all malignant disease, affecting 61,560 individuals annually and resulting in 14,080 deaths in the USA alone [1, 2]. With widespread and increasing use of cross sectional imaging, namely computed tomography (CT) and magnetic resonance imaging (MRI), there have been more incidentally detected small renal malignancies less than 4 cm in size (stage T1a) [3, 4]. Concomitantly, coupled with increased detection, our understanding of renal cell carcinoma tumor biology, molecular pathogenesis, and those syndromes associated with renal malignancies has also increased [5]. There is, for instance, a more complete understanding of certain syndromes, including the Von Hippel-Lindau and Birt-Hogg-Dubé, and their association with hereditary renal cell carcinomas [2]. While the gold standard of treatment remains definitive surgical excision with nephron-sparing partial nephrectomy for tumors less than 4 cm (stage T1a), medical advances have brought about additional viable treatment options, including percutaneous ablative therapies, for the management of small renal malignancies [6, 7].
The American Urological Association guidelines consider percutaneous ablative therapies, including cryoablation, radiofrequency ablation, and microwave ablation, viable treatment options for small renal malignancies less than 4 cm (stage T1a) [8]. Similarly, multiple international consensus panels, including the European Association of Urology, support the use of ablative techniques for renal cell carcinomas less than 4 cm as well as for those with syndromes increasing the likelihood of multiple renal cell carcinomas, including the Von Hippel-Lindau and Birt-Hogg-Dubé [9, 10]. Moreover, for patients with small localized renal cell carcinomas who are not deemed surgical candidates, including those with morbid obesity, advanced age, multiple comorbidities, or a solitary kidney, ablation is an effective nephron-sparing treatment option [8, 11–13].
As percutaneous ablation is minimally invasive and requires only moderate procedural sedation in many instances, potential advantages include preservation of the renal parenchyma and renal function and decreased procedural-related morbidity. Post-operatively patients experience faster recovery, with many patients having the treatment on an outpatient basis.
Pre-procedural Evaluation
Proper patient preparation and complete renal mass evaluation are requisites to successful percutaneous renal ablative therapies, requiring a multidisciplinary approach including input from diagnostic radiologists, interventional radiologists, urologists, and oncologists. Patients should undergo a thorough medical and surgical evaluation, physical examination, risk factor evaluation (smoking and workplace exposures including asbestos, aniline dyes, and cadmium), family history screening including evaluation for known genetic disorders (Von Hippel-Lindau, Birt-Hogg-Dubé, and hereditary papillary carcinoma syndromes), and functional status evaluation. In addition, laboratory studies, including platelet count, international normalized ratio (INR), partial thromboplastin time, and estimation of renal function (creatinine and estimated glomerular filtration rate) should be evaluated. At our institution, we require platelets greater than 50,000/uL and an INR less than 1.5.
As stated, percutaneous ablation is particularly suited for individuals with small renal tumors less than 4 cm (stage T1a) [13]. Larger tumors may be treated with ablation; however, the literature suggests an increased recurrence rate [5, 6]. Because metastatic renal cell carcinomas would require systemic therapy, extra-renal disease and osseous metastases must be identified on staging chest, abdominal, and pelvic CT or MRI, with nuclear medicine skeletal scintigraphy and intracranial imaging performed in some instances [13].
Pre-procedure cross sectional imaging with CT or MR is crucial for characterizing the renal mass itself and optimizing treatment planning. With regard to renal mass characterization, renal masses referred for ablation are usually small, often requiring careful evaluation for benign etiologies including oncocytomas and angiomyolipomas. In one series, for instance, ten of 27 patients who underwent referral for MR-guided cryotherapy were found to have benign pathologies based imaging characteristics or biopsy results, thus highlighting the need for careful evaluation of all available imaging studies or tissues prior to ablative procedures [14••]. With respect to treatment planning, CT and MR imaging may demonstrate if a lesion is safely accessible. Moreover, the need for adjunctive maneuvers such as creation artificial saline windows or the placement of retrograde catheters may be revealed on the pre-procedure imaging. Pre-procedural imaging helps tailor the discussion of the risks and benefits of the procedure for individual patients. Schmit et al. developed the ABLATE (A, axial tumor diameter; B, bowel proximity; L, location within the kidney; A, adjacency to ureter; T, touching renal sinus fat; E, endophytic or exophytic position) planning algorithm to systematically evaluate renal masses for ablation [15]. An additional helpful planning scoring algorithm, the RENAL nephrometry score, is a quantitative scoring system based on five components (R, maximal radius; E, exophytic or endophytic properties; N, nearness to the collecting system or sinuses; A, anterior or posterior location; L, location relative to the polar lines), each scored on a 1 to 3 point scale [16]. This scoring system has been shown to predict treatment efficacy and complications after percutaneous renal ablation, with a mean score of 6.8 versus 8.1 for those without and with major complications, respectively [17•]. Taking all factors into account, the ideal renal mass for percutaneous ablation is a small exophytic posterior mass less than 4 cm (stage T1a) [18].
Pre-procedural Biopsy
Historically pre-ablation biopsy has been described as having a limited role due to poor tumor targeting, sampling error, and false-negative results. As increasing numbers of small renal masses are referred for ablation, however, there has been an increasing role of biopsy for definitive tissue diagnosis. In contrast to surgical excision, the renal mass itself is destroyed during the ablation process thereby mandating pre-procedure biopsy and precluding post-procedure pathological evaluation [19]. Furthermore, as previously discussed, according to one study, upwards of 37 % of renal masses referred for ablation were determined to be benign based on imaging findings or pathologic analyses [14, 19]. As false-negative rates of renal biopsies have decreased to 1 % with associated low complication rates, pre-ablation biopsies have become more routine as part of the ablation procedure [20]. While the renal mass biopsy may be performed as a separate procedure prior to ablation, it is often performed immediately prior to ablation, with results available after completion of the ablation. The results of the biopsy may help guide the frequency and length of follow-up imaging.
Ablation Techniques
The most prevalent ablative techniques for small renal masses include cryoablation, radiofrequency ablation, and microwave ablation, with each having potential advantages and disadvantages (Table 1) [21].
Cold-Based Ablation Techniques
Cryoablation, a cold-based technique, involves the use of one or usually multiple needle-like cyroprobes. These cryoprobes are connected to a liquid gas, usually argon, for rapid cooling to temperatures of nearly −190 °C [18]. Tissue destruction is achieved at temperatures below −20 °C and through multiple freeze-thaw cycles [18]. During freezing cycles, ice crystals form in the intracellular and extracellular spaces causing cellular disruption and death [22]. At the same time, freeze-thaw cycles cause cellular dehydration, membrane rupture, and vascular thrombosis [22]. A major advantage of cryoablation, over heat-based ablation techniques, is direct visualization of the destructive ablation zone or “ice ball” during intra-procedural CT or MR monitoring. Moreover, the size and shape of the ablation zone may be refined using various types and numbers of cryoprobes. This allows the operator to shape an “ice ball” with adequate tumor coverage. Similarly, if the treatment zone begins encroaching on a vital non-tumor structure, such as the ureter or colon, accommodations may be made to stop progression of the “ice ball.” Cryoablation offers less intra-procedural pain and some studies suggest decreased risk of collecting system damage when compared to heat-based therapies. Cryoablation, however, is more time consuming when compared to radiofrequency and microwave-based therapies as it requires multiple freeze-thaw cycles. At our institution, CT is the dominant imaging modality for percutaneous cryoablation. Ultrasound is sometimes performed for initial cryoprobe insertion or intra-procedural biopsy. CT imaging is then performed to confirm placement and for direct monitoring of the ablation zone or “ice ball” (Fig. 1). Two freeze-thaw cycles, each lasting 8–10 minutes, are then performed. As a guideline, the number of cyroprobes inserted for cryoablation is equal to the maximal diameter of the renal mass centimeters plus one.
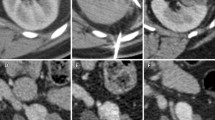
Cryoablation. An 84-year-old male with multiple comorbidities including right lung adenocarcinoma status post upper lobectomy with incidentally discovered right renal cell carcinoma for cryoablation. Single axial computed tomography image with intravenous contrast material (a) demonstrating a 4.5 × 4.7 cm heterogeneously, mostly peripherally enhancing, soft tissue mass with an irregular central hypodense necrotic center within the right interpolar region consistent with renal cell carcinoma (arrows). Intra-procedural axial computed tomography image (b) showing two cryoprobes within the mass (arrows). Intra-procedural axial computed tomography image (c) demonstrating the hypodense ablation zone or “ice ball” enveloping the right renal mass (arrows). Follow-up axial computed tomography image with contrast material (d) obtained 1 year after the procedure showing no enhancement to suggest residual or recurrent disease (arrows)
Heat-Based Ablation Techniques
Heat-based ablations, including radiofrequency and microwave ablation, are also used to treat small renal masses. Radiofrequency ablation uses high-frequency alternating currents, resulting in electric current and heat generation [23]. This results in tissue necrosis as well as tissue coagulation, decreasing post-procedural bleeding. Disadvantages of radiofrequency ablation, however, include grounding pad requirements and sensitivity to tissue characteristics, tissue impendence, and charring. Microwave ablation, which is similar to radiofrequency ablation, utilizes electromagnetic energy with frequencies greater than 900 MHz [24]. Microwave electromagnetic energy interacts with nearby water molecules resulting in rapid molecular oscillation, heat generation, coagulative necrosis, and tissue death [24]. Advantages of microwave ablation over other heat-based technologies include higher intratumoral temperatures, larger ablation zones, and less likely to be influenced by tissue characteristics, impendence, and charring [25]. With both radiofrequency and microwave ablations, tissue death begins to occur at 50 to 60 °C [25]. With respect to radiofrequency and microwave ablations, initial percutaneous access is similar to the previously described cryoablation (Fig. 1). Instead of a cryoprobe, however, a radiofrequency or microwave ablation probe is inserted just beyond the lesion (Fig. 2). A single heating cycle, based on the size of the lesion, is performed, thus the time to perform the procedure is quicker than for cryoablation.
Radiofrequency ablation. A 56-year-old man with lymphoma and incidentally discovered right renal cell carcinoma for radiofrequency ablation. Single axial computed tomography image with intravenous contrast material (a) showing a 1.6 × 1.4 cm homogenously enhancing mass within the right renal lower pole consistent with renal cell carcinoma (arrow). Intra-procedural axial computed tomography image (b) showing a single radiofrequency probe within the mass (arrow). Follow-up axial computed tomography image with contrast material (c) obtained 2 years after the procedure demonstrating no enhancement to suggest residual or recurrent disease (arrow)
Adjunctive Displacement and Embolization Techniques
Special modifications, displacement maneuvers, and embolization techniques are required in certain ablation cases. Peripheral or exophytic renal masses located near critical structures such as the ureter or bowel, for instance, require special attention due to potential risk of critical injury. Hydrodissection, or displacement, may be performed using a thin-gauge needle, with instillation of a dilute contrast mixture or a gas, such as air or carbon dioxide, for direct visualization and displacement of the critical structures away from the ablation zone (Fig. 3). Alternatively, if the renal mass is large, typically larger than 3 cm, generating substantial concern for intra-procedural or post-procedural hemorrhage, pre-ablation selective embolization with particles or ethanol/lipiodol mixtures may be performed prior to ablation to limit hemorrhage (Fig. 4).
Adjunctive displacement techniques. A 60-year-old male with the Von Hippel-Lindau syndrome and bilateral renal cell carcinomas status post right nephrectomy and prior left renal radiofrequency ablation now with 2.9 × 2.5 cm left renal cell carcinoma, in close proximity to the colon, for radiofrequency ablation (a–c). Intra-procedural axial computed tomography image (a) showing a single radiofrequency probe (arrow) within the left renal mass. Note the close proximity of the colon (asterisk). Given the close proximity of the colon, hydrodissection was employed for protection. Intra-procedural axial computed tomography image (b) demonstrating the insertion of a 21-gauge needle (arrow) between the left renal mass and colon (asterisk). Intra-procedural axial computed tomography image (c) demonstrating the introduction of dilute contrast material (arrow) to provide a buffer and protect the colon (asterisk) during the ablation procedure. An 87-year-old female with 2.5 × 2.0 cm left renal cell carcinoma, in close proximity to the ureter, for cryoablation (d–f). Intra-procedural axial computed tomography image (d) showing the left renal mass (arrow) in close proximity to the ureter (dashed arrow). Intra-procedural axial computed tomography image (e) demonstrating the introduction of a 21-gauge needle (arrow) adjacent to the left ureter (dashed arrow). Intra-procedural axial computed tomography image (f) showing instillation of dilute contrast material (arrows) bathing the ureter and providing protection during cryoablation
Adjunctive embolization techniques. A 73-year-old male with pulmonary fibrosis and large left upper pole renal mass measuring 4.8 cm for embolization prior to cryoablation. Single axial T1 post contrast magnetic resonance image (a) showing the large hypervascular left upper mass (arrows). Selective left renal arteriogram (b) demonstrating a subtle tumor blush indicative of the mass (arrows) prior to ethiodized oil embolization. Axial cone beam computed tomography (c), completed after selective injection of ethiodized oil, showing embolization material throughout the left renal mass (arrows), consistent with satisfactory embolization. Intra-procedural axial computed tomography image from cryoablation completed the following day (d) demonstrating a cryoprobe within the mass as well as hyperdense ethiodized oil (arrows) from prior embolization procedure. The mass was ablated successfully without hemorrhagic complication
Potential Complications
There are several known complications associated with ablation of small renal masses [26]. Despite the use of imaging-guidance, there is risk of injury to surrounding structures such as the ureter or large bowel resulting in a urinoma or perforation, respectively [26]. While ablation is a relative nephron-sparing procedure and is often successfully performed in patients with pre-existing renal insufficiency, there is the risk for worsening renal function after ablation. Moreover, given the vascularity of the kidneys and renal masses in general, there is a risk of post-procedural hemorrhage and hematoma [27]. Complications have been reported in 3–10 % of cryoablation cases and 4.7 % of radiofrequency ablation cases [18, 26, 27]. There have also been neuromuscular complications, including transient paresthesias and flank muscle laxity, from nerve injury during ablation [28]. Other uncommonly reported complications include tract tumor seeding, skin burns, and pneumothoraces [28].
Post-Ablation Surveillance
There are no evidence-based or official guidelines for post-ablation follow-up. At our institution, patients undergo contrast-enhanced CT or MR imaging 1 month after ablation, then every 6 months for 2 years, and then annually for 5 years. Follow-up continues, but is variable in time, after 5 years depending on the pathology of the tumor and clinical scenario [29]. As renal cell carcinomas tends to grow slowly (3–5 mm/year), long-term surveillance is essential to detect and treat residual or recurrent disease [30]. On follow-up imaging, technical success is defined as complete necrosis of the treated renal mass, without residual enhancement [31]. The initial post-ablation study is essential as it serves as a baseline for comparison with subsequent cross sectional imaging [31]. Moreover, immediate complications, including urinomas, perforations, or hematomas, may be detected [31]. Also, any residual tumor identified on the immediate post-ablation imaging may be treated before significant recurrence [31].
Outcomes
To date, there have been no randomized controlled trials comparing percutaneous ablation with partial nephrectomy. However, there are many notable non-randomized comparative studies showing comparable treatment efficacy for primarily small renal cell carcinomas treated with ablation and partial nephrectomy (Table 2). The majority of studies have compared radiofrequency ablation with partial nephrectomy. Stern et al. showed that for sporadic T1a renal tumors, radiofrequency ablation resulted in similar disease-free probability compared to partial nephrectomy in a 3-year actuarial analysis [32]. In second study with 5-year follow-up, Olweny et al. demonstrated similar overall survival (97 versus 100 %) and disease-free survival (89 versus 89 %) for radiofrequency ablation when compared to partial nephrectomy, respectively, for solitary T1a renal cell carcinomas [33]. A recent study by Chang et al. also demonstrated similar 5-year overall survival (85 versus 96 %) and disease free survival (81 versus 89 %) for radiofrequency versus partial nephrectomy, respectively, for stage cT1b renal cell carcinomas [34].
With regard to cryoablation, in one of the largest retrospective series to date, Thompson et al. compared both percutaneous cryoablation and radiofrequency ablation with partial nephrectomy to for cT1 renal masses [35••]. This series demonstrated a similar local recurrence-free survival and metastases-free survival for cryoablation versus partial nephrectomy. Moreover, throughout the surgical literature, cryoablation has been shown to be effective for renal malignancies with mean sizes of 2.3 cm, resulting in disease-specific survival rates of 92 % at 5 years [36, 37]. In a retrospective series comparing percutaneous and surgical cryoablation for small renal masses, no differences in overall or recurrence-free survival was found at an average 3–4 years follow-up [38•]. The 5-year overall and recurrence-free survival for percutaneous cryoablation was 77 and 95 %, respectively [39].
As percutaneous microwave ablation is a relatively new technique, there are fewer comparative studies in the published literature [40, 41•]. Guan et al. compared microwave ablation with partial nephrectomy for small renal masses and demonstrated a similar overall local recurrence-free survival at 3 years with 91 % for microwave ablation and 96 % for partial nephrectomy [42].
Conclusion
Image-guided ablative therapies, including cryoablation, radiofrequency ablation, and microwave ablation, are safe and effective nephron-sparing therapies for small renal masses less than 4 cm. Although larger randomized trials are necessary, current data suggests excellent oncologic tumor control and low complication profiles making percutaneous ablation an important tool for treating renal cell carcinoma in selected patient populations.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. Lancet. 2009;373(9669):1119–32. doi:S0140-6736(09)60229-4 [pii]10.1016/S0140-6736(09)60229-4
Cohen HT, McGovern FJ. Renal-cell carcinoma. N Engl J Med. 2005;353(23)):2477. doi:10.1056/NEJMra043172.
Jemal A, Bray F, Center MM, Ferlay J, Ward E, Forman D. Global cancer statistics. CA Cancer J Clin. 2011;61(2):69. doi:10.3322/caac.20107.
Sahni VA, Silverman SG. Imaging management of incidentally detected small renal masses. Semin Intervent Radiol. 2014;31(1):9. doi:10.1055/s-0033-136383800813.
Finley DS, Pantuck AJ, Belldegrun AS. Tumor biology and prognostic factors in renal cell carcinoma. Oncologist. 2011;16(2):4. doi:10.1634/theoncologist.2011-S2-04.
Jonasch E, Gao J, Rathmell WK. Renal cell carcinoma. BMJ. 2014;349:g4797.
Bakal CW, Cynamon J, Lakritz PS, Sprayregen S. Value of preoperative renal artery embolization in reducing blood transfusion requirements during nephrectomy for renal cell carcinoma. J Vasc Interv Radiol. 1993;4(6):727.
Campbell SC, Novick AC, Belldegrun A, Blute ML, Chow GK, Derweesh IH. Guideline for management of the clinical T1 renal mass. J Urol. 2009;182((4):1271. doi:10.1016/j.juro.2009.07.004.
Ljungberg B, Bensalah K, Canfield S, Dabestani S, Hofmann F, Hora M, et al. EAU guidelines on renal cell carcinoma: 2014 update. Eur Urol. 2015;67(5):913. doi:10.1016/j.eururo.2015.01.005.
Van Poppel H, Becker F, Cadeddu JA, Gill IS, Janetschek G, Jewett MA, et al. Treatment of localised renal cell carcinoma. Eur Urol. 2011;60(4):662. doi:10.1016/j.eururo.2011.06.040.
Trudeau V, Larcher A, Boehm K, Dell'Oglio P, Sun M, Tian Z. Comparison of Postoperative Complications and Mortality Between Laparoscopic and Percutaneous Local Tumor Ablation for T1a Renal Cell Carcinoma: a Population-Based Study. Urology. 2015. doi:10.1016/j.urology.2015.08.043.
Ogan K, Jacomides L, Dolmatch BL, Rivera FJ, Dellaria MF, Josephs SC, et al. Percutaneous radiofrequency ablation of renal tumors: technique, limitations, and morbidity. Urology. 2002;60(6):954.
Escudier B, Porta C, Schmidinger M, Algaba F, Patard JJ, Khoo V. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2014;25(3:ii):49. doi:10.1093/annonc/mdu259.
Tuncali K, van Sonnenberg E, Shankar S, Mortele KJ, Cibas ES, Silverman SG. Evaluation of patients referred for percutaneous ablation of renal tumors: importance of a preprocedural diagnosis. AJR Am J Roentgenol. 2004;18(3):575. doi:10.2214/ajr.183.3.1830575. A study that highlights the importance of biopsy prior to ablation procedures.
Schmit GD, Kurup AN, Weisbrod AJ, Thompson RH, Boorjian SA, Wass CT, et al. ABLATE: a renal ablation planning algorithm. AJR Am J Roentgenol. 2014;202(4):894. doi:10.2214/AJR.13.11110.
Kutikov A, Uzzo RG. The R.E.N.A.L. nephrometry score: a comprehensive standardized system for quantitating renal tumor size, location and depth. J Urol. 2009;182(3):844. doi:10.1016/j.juro.2009.05.035.
Schmit GD, Thompson RH, Kurup AN, Weisbrod AJ, Boorjian SA, Carter RE. Usefulness of R.E.N.A.L. nephrometry scoring system for predicting outcomes and complications of percutaneous ablation of 751 renal tumors. J Urol. 2013;89(1):30. doi:10.1016/j.juro.2012.08.180. A study that introduces the RENAL nephrometry algorithm to predict the outcomes and complications associated with ablation procedures.
Allen BC, Remer EM. Percutaneous cryoablation of renal tumors: patient selection, technique, and postprocedural imaging. Radiographics. 2010;30(4):887. doi:10.1148/rg.304095134.
Sahni VA, Silverman SG. Biopsy of renal masses: when and why. Cancer Imaging. 2009;9:44. doi:10.1102/1470-7330.2009.0005.
Ramanathan R, Leveillee RJ. Ablative therapies for renal tumors. Ther Adv Urol. 2010;2(2):51. doi:10.1177/175628721036670810.1177_1756287210366708.
Venkatesan AM, Wood BJ, Gervais DA. Percutaneous ablation in the kidney. . 2011;26(2):375. doi:10.1148/radiol.11091207.
Dominguez-Escrig JL, Sahadevan K, Johnson P. Cryoablation for small renal masses. Adv Urol. 2008:479495. doi:10.1155/2008/479495
Zagoria RJ. Imaging-guided radiofrequency ablation of renal masses. Radiographics. 2004;24(1):S59. doi:10.1148/rg.24si045512.
Simon CJ, Dupuy DE, Mayo-Smith WW. Microwave ablation: principles and applications. Radiographics. 2005;25(1):S69. doi:10.1148/rg.25si055501.
Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Mu MJ. US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology. 2012;263:900. doi:10.1148/radiol.12111209.
Weizer AZ, Raj GV, O'Connell M, Robertson CN, Nelson RC, Polascik TJ. Complications after percutaneous radiofrequency ablation of renal tumors. Urology. 2005;66(6):1176. doi:10.1016/j.urology.2005.06.125.
Kurup AN. Percutaneous ablation for small renal masses-complications. Semin Intervent Radiol. 2014;31(1):42. doi:10.1055/s-0033-136384200817.
Bhayani SB, Allaf ME, Su LM, Solomon SB. Neuromuscular complications after percutaneous radiofrequency ablation of renal tumors. Urology. 2005;65(3):592. doi:10.1016/j.urology.2004.09.053.
Higgins LJ, Hong K. Renal ablation techniques: state of the art. AJR Am J Roentgenol. 2015;205(4):735. doi:10.2214/AJR.15.14752.
Siu W, Hafez KS, 3rd Johnston WK, Jr. Wolf JS. Growth rates of renal cell carcinoma and oncocytoma under surveillance are similar. Urol Oncol. 2007;25(2):115. doi:10.1016/j.urolonc.2006.07.018.
Iannuccilli JD, Grand DJ, Dupuy DE, Mayo-Smith WW. Percutaneous ablation for small renal masses-imaging follow-up. Semin Intervent Radiol. 2014;31(1):50. doi:10.1055/s-0033-136384300818.
Stern JM, Svatek R, Park S, Hermann M, Lotan Y, Sagalowsky AI. Intermediate comparison of partial nephrectomy and radiofrequency ablation for clinical T1a renal tumours. BJU Int. 2007;100(2):287. doi:10.1111/j.1464-410X.2007.06937.x.
Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA, et al. Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol. 2012;61(6):1156. doi:10.1016/j.eururo.2012.01.001.
Chang X, Zhang F, Liu T, Ji C, Zhao X, Yang R. Radio frequency ablation versus partial nephrectomy for clinical T1b renal cell carcinoma: long-term clinical and oncologic outcomes. J Urol. 2015;193(2):430. doi:10.1016/j.juro.2014.07.112.
Thompson RH, Atwell T, Schmit G, Lohse CM, Kurup AN, Weisbrod A. Comparison of partial nephrectomy and percutaneous ablation for cT1 renal masses. Eur Urol. 2015;67(2):252–9. doi:10.1016/j.eururo.2014.07.021. A study comparing partial nephrectomy to both radiofrequency and cryoablation ablation for cT1 renal masses.
Klatte T, Grubmuller B, Waldert M, Weibl P, Remzi M. Laparoscopic cryoablation versus partial nephrectomy for the treatment of small renal masses: systematic review and cumulative analysis of observational studies. Eur Urol. 2011;60(3):435. doi:10.1016/j.eururo.2011.05.002.
Aron M, Kamoi K, Remer E, Berger A, Desai M, Gill I, et al. Laparoscopic renal cryoablation: 8-year, single surgeon outcomes. J Urol. 2010;183(3):889. doi:10.1016/j.juro.2009.11.041.
Zargar H, Samarasekera D, Khalifeh A, Remer EM, O'Malley C, Akca O. Laparoscopic vs percutaneous cryoablation for the small renal mass: 15-year experience at a single center. Urology. 2015;85(4):850. doi:10.1016/j.urology.2015.01.004. A study comparing laparoscopic and cryoablation for small renal masses showing no difference between overall or recurrence-free survival.
Goyal J, Verma P, Sidana A, Georgiades CS, Rodriguez R. Single-center comparative oncologic outcomes of surgical and percutaneous cryoablation for treatment of renal tumors. J Endourol. 2012;26(11):1413. doi:10.1089/end.2012.0244.
Moreland AJ, Ziemlewicz TJ, Best SL, Hinshaw JL, Lubner MG, Alexander ML, et al. High-powered microwave ablation of t1a renal cell carcinoma: safety and initial clinical evaluation. J Endourol. 2014;28(9):1046. doi:10.1089/end.2014.0190.
Yu J, Liang P, Yu XL, Cheng ZG, Han ZY, Zhang X. US-guided percutaneous microwave ablation versus open radical nephrectomy for small renal cell carcinoma: intermediate-term results. Radiology. 2014;270(3):880. doi:10.1148/radiol.13130275. A study comparing radical nephrectomy and microwave ablation showing comparable oncologic results for nephrectomy and microwave ablation and no major complications for microwave ablation.
Guan W, Bai J, Liu J, Wang S, Zhuang Q, Ye Z, et al. Microwave ablation versus partial nephrectomy for small renal tumors: intermediate-term results. J Surg Oncol. 2012;106(3):316. doi:10.1002/jso.23071.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Benjamin J. Shin, Jeffrey Forris Beecham Chick, and S. William Stavropoulos each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Minimally Invasive Surgery
Rights and permissions
About this article
Cite this article
Shin, B.J., Chick, J.F.B. & Stavropoulos, S.W. Contemporary Status of Percutaneous Ablation for the Small Renal Mass. Curr Urol Rep 17, 23 (2016). https://doi.org/10.1007/s11934-016-0581-7
Published:
DOI: https://doi.org/10.1007/s11934-016-0581-7