Abstract
Accurate preoperative staging of bladder cancer is essential in determining the extent of disease and optimal treatment. The current gold standard of transurethral resection of bladder tumor (TURBT) followed by computed tomography (CT) imaging provides excellent staging specificity, but often understages the disease, leading to pathologic upstaging and adverse outcomes in patients undergoing radical cystectomy. Newer imaging modalities, such as multiparametric magnetic resonance (MR) imaging and positron emission tomography (PET) combined with CT or MR provides promising imaging alternatives which may improve accuracy of staging both local and distant disease.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Bladder cancer is the sixth most commonly diagnosed malignancy in the USA, with an incidence of about 75,000 new cases and 16,000 deaths annually [1]. Diagnosis is generally made via transurethral resection of a bladder mass found on evaluation for hematuria. Approximately 20–25 % of patients initially present with muscle-invasive bladder cancer (MIBC), the gold-standard treatment for which is radical cystectomy (RC) with pelvic lymphadenectomy. Prior to surgery, these patients must be evaluated with cross-sectional imaging to assess for local tumor invasion and to detect metastatic spread. Should the patient prove to have extravesical or distant disease, the optimal treatment may vary significantly. Herein, we will review the currently recommended imaging protocols and explore recent data for alternative or investigational imaging modalities that may improve diagnostic and prognostic accuracy for bladder cancer patients.
Currently Recommended Bladder Cancer Staging
For non-muscle-invasive bladder cancer (NMIBC), the American Urologic Association (AUA) and National Comprehensive Cancer Network (NCCN) guidelines recommend upper tract imaging with ultrasound, computed tomography (CT), magnetic resonance (MR), or intravenous pyelogram, and pelvic imaging if the tumor is sessile or high grade [2]. For staging of newly diagnosed MIBC, AUA and NCCN guidelines recommend contrasted CT urography or MR urography of the abdomen and pelvis, and either plain film x-ray or non-contrast CT of the chest, and bone scan for patients with symptoms and/or an elevated alkaline phosphatase [2, 3]. Contrast-enhanced CT is the current standard of care for the assessment of nodal or distant disease, but CT is poor in its evaluation of depth of bladder invasion. MR excels in assessing bladder invasion due to better tissue differentiation when compared to CT, and limited data regarding assessment of nodal disease indicate that MR is comparable to CT. Multiple studies of CT and MR have demonstrated modest sensitivity in detection of pelvic lymph node involvement, varying with size cutoffs [4, 5].
Despite advances in radiological imaging over the last several decades, current modalities are plagued by clinical understaging of MIBC in a significant number of patients, as high as 42 % in a sizable recent series [6, 7] (Table 1). Large, well-designed trials of newer imaging modalities are essential to advance the field and improve the care of bladder cancer patients.
Imaging Modalities
Computed Tomography
Though the technology is aging, computed tomography (CT) remains the gold standard in radiologic staging of MIBC. Advantages include rapid image acquisition, relatively low cost, wide availability, and reasonable accuracy in assessing for nodal or distant disease. Disadvantages include exposure to ionizing radiation, poor assessment of the primary tumor, and intolerability of IV contrast in patients with chronic kidney disease (CKD). Because CT is an anatomic rather than a functional study, detection of extravesical disease is limited to lesions above certain size thresholds, which leads to understaging patients with small nodal metastases. Current recommendations suggest that pelvic nodes ≥8 mm and abdominal nodes ≥1 cm measured on the short axis be considered pathologic [9]. While sensitive to detection of enlarged loco-regional and distant lymph nodes, CT provides no data regarding metabolic activity or function of the lymph node, and thus is unable to discriminate between nodes harboring metastatic disease versus those that are inflammatory or of other benign etiology [5, 10]. CT provides poor discrimination between non-muscle-invasive versus muscle-invasive bladder tumors, as tissue differentiation is inadequate. Though CT has a lower sensitivity than MR for detecting perivesical invasion, it has a higher specificity, as a T2 tumor may cause extravesical inflammation that is detected by MR and is mistaken for perivesical invasion, leading to overstaging with MR [11]. Studies have demonstrated that CT is able to successfully differentiate T3b and T4 disease from non-muscle-invasive, but overall is inaccurate for local tumor staging [12].
Magnetic Resonance Imaging
MR imaging (MRI) has emerged as an exciting and possibly more versatile option for MIBC staging when compared to CT. MR has no ionizing radiation and soft tissue contrast is excellent, providing more anatomic data than CT, especially in assessing the primary tumor. Newer MR protocols also add functional data which may assist in more accurate staging in these patients.
T1-weighted MRI is useful for identifying extravesical fat infiltration, pelvic lymphadenopathy, and bone metastases [13••]. However, normal detrusor and bladder tumor both have similar, intermediate signal intensity, making the depth of bladder wall invasion difficult to discern. T2-weighted imaging excels in demonstrating tumor depth (NMIBC versus MIBC), and extravesical extension. On T2 imaging, the normal detrusor muscle appears as a hypointense line; thus, interruption in the line is indicative of muscle-invasive disease [13••, 14]. While these standard MR modalities show some advantages over CT in assessing the primary tumor, functional MR imaging protocols are where potential substantive advancements lie in improving overall staging accuracy.
Dynamic contrast-enhanced MR (DCE-MR) utilizes paramagnetic contrast agents and can be used to detect differences in blood flow within a tumor, including areas of ischemia and necrosis [15]. Several studies investigating DCE-MR have demonstrated an accuracy of 85 % in distinguishing non-muscle-invasive bladder cancer (NMIBC) from MIBC, 82 % accuracy in determining organ-confined from non-organ-confined disease, and 80 % accuracy in detecting nodal disease [16••, 17]. Using DCE-MR as a marker of angiogenesis may predict disease recurrence; a 24-patient pilot study suggested that patients with stronger, faster enhancement were more likely to recur [18]. DCE-MR has proven useful in predicting complete response to chemotherapy in breast and rectal cancer [19, 20], and in a 30-patient pilot study of patients undergoing neoadjuvant chemotherapy prior to radical cystectomy, certain pharmacokinetic parameters can characterize the microcirculatory changes within the tumor, providing information regarding the early chemotherapeutic response [21•]. Gadolinium must be used with caution in patients with severe renal insufficiency, as there is increased risk of nephrogenic systemic fibrosis (NSF). The American College of Radiologists suggests discussing NSF risks with those with a glomerular filtration rate (GFR) <40 mL/min/1.73 m2, though it is exceedingly rare in patients with GFR >15 mL/min/1.73 m2 [22].
Diffusion-weighted MR (DW-MR) measures water diffusion across the cell membrane, which varies between normal tissues and tumor, as tumor tissues tend to have greater cellularity, reducing water diffusion [23, 24]. While primarily used in assessing local tumor spread, recent studies have demonstrated DW-MRs utility in predicting histologic grade and aggressiveness. In a prospective study of 51 patients with suspected bladder cancer, the area under the receiver operating characteristic (ROC) curve for predicting MIBC and high-grade disease using DW-MR apparent diffusion coefficient (ADC) thresholds was 0.884 and 0.906, respectively [25]. Another study of 132 patients undergoing transurethral resection of bladder tumor (TURBT) demonstrated strong correlation between lower ADC and high-grade, high-stage, sessile tumors [26•]. DW-MR is also more accurate than T2-MR in staging organ-confined (≤T2) disease, with staging accuracy of 69.7 versus 15.1 %, respectively [11].
In addition to providing functional information about the primary tumor, DW-MR may have utility in monitoring response to treatment. With good response, ADC will often decrease, indicating decreased cellularity. In hepatocellular carcinoma, cervical cancer, and several metastatic disease states, response to chemotherapy and radiation is accurately monitored with DW-MR [27–30]. In patients with MIBC undergoing neoadjuvant chemotherapy, monitoring for change in ADC may provide additive information that could guide treatment decisions toward early cystectomy or continued chemotherapy based on radiologic tumor response [19].
Lymphotropic nanoparticle-enhanced MR (Ultra-small super-paramagnetic particles of iron oxide (USPIO)-MR) is a functional study that allows for differentiation of benign and malignant enlarged lymph nodes. USPIO are administered intravenously and are phagocytosed by macrophages within the lymph nodes. Given the higher density of functioning macrophages in benign lymph nodes, benign nodes have higher signal intensity than malignant nodes on T2 imaging. Early studies have demonstrated excellent sensitivity (96 %), specificity (95 %), and accuracy (95 %) [31]. Recently, USPIO-MR combined with DW-MR has demonstrated improved detection of lymph node metastases compared to USPIO-MR imaging alone, and it shortened interpretation times, which is important for potential widespread implementation [32•]. Data remain preliminary, but USPIO-MR imaging is a promising modality that may improve accuracy of lymph node staging compared to conventional techniques.
Positron Emission Tomography
Positron emission tomography (PET) imaging, especially when combined with CT (PET/CT) for anatomic localization, has a myriad of applications within oncology due to its ability to locate metabolically active tissues that may represent foci of cancer that cannot be visualized on standard cross-sectional imaging due to small size. In bladder cancer, its role is not well defined. Previous meta-analysis of the diagnostic accuracy of PET/CT demonstrate a global accuracy of 0.92, but the studies were small and heterogeneous in nature [33–38]. In a larger, more recent study of 233 patients undergoing cystectomy for bladder cancer, PET/CT was evaluated against CT in staging MIBC and high-risk NMIBC. PET/CTs accuracy in detection of pelvic lymph nodes was 0.87 compared to 0.83 for CT, and in detection of distant disease, the accuracy was 0.86 versus 0.83, respectively. In this study, only 3 % of patients were found to have metastatic disease on PET/CT that was missed on CT, possibly changing their treatment course [39••]. The role of PET/CT after chemotherapy for bladder cancer appears limited, as sensitivity in detecting LN metastases decreases to ~50 % [40]. One of the biggest hurdles in PET/CT for bladder cancer is that the most commonly used radiotracer, 18F-fluorodeoxyglucose (FDG), is excreted in the urine, masking FDG uptake by the primary bladder tumor and hindering visualization of perivesical lymph nodes. To counteract the inherent limitations of FDG imaging of the bladder, various protocols use increased hydration, catheterization, delayed images, and forced diuresis with some improvement in accuracy [35, 41].
Novel PET radiotracers are also under investigation to enhance staging accuracy in bladder cancer [12].C-acetate PET/CT has demonstrated promise in a small number of patients, but has demonstrated modest overall accuracy (~0.65–0.75) [42]. 11C-choline and 11C-methionine are alternative options as they are not excreted in the urine. In a study of 27 patients, 11C-choline PET has demonstrated efficacy in detecting residual disease after TURBT that is comparable to CT, and was superior in detecting pathologic pelvic lymph nodes [43]. However, a 44 patient study of 11C-choline PET/CT in patients with MIBC scheduled for cystectomy demonstrated no improvement in staging accuracy compared to CT alone [44]. A disadvantage of 11C-choline is a very short half-life, limiting its use to facilities with an on-site cyclotron. Data is limited for 11C-methionine in bladder cancer staging, and small studies suggest non-superiority to conventional imaging [45]. In patients with suspected bony lesions from metastatic bladder cancer, 18F-flouride PET has demonstrated superior accuracy in detection of lytic lesions compared to (99 m)Tc-MDP bone scan. Most data regarding 18F-flouride PET is from non-bladder solid malignancies [46, 47], but a recent 48-patient study in patients with bladder cancer with suspected bony metastases found that 18F-flouride PET was more sensitive, specific, and accurate than (99 m)Tc-MDP bone scan [48].
Receptor-specific radiolabeled biomarkers for PET/CT provide the attractive possibility of targeting tumor markers specific to a bladder tumor, theoretically increasing the specificity of PET/CT. In some bladder cancers, EGFR and HER2 are overexpressed, thus radiolabeled trastuzumab could allow for in vivo monitoring of tumor HER2 expression [49].
Magnetic Resonance/Positron Emission Tomography
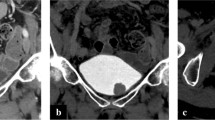
In an effort to capitalize on the excellent soft tissue contrast seen in MR imaging and the imaging of metabolically active tissues suspicious for malignancy with PET, the hybrid magnetic resonance/positron emission tomography (MR/PET) was developed and first introduced clinically in 2006 (Fig. 1). Protocols for different tumor types are still in development, but initial experiences suggest that image quality is at least comparable to PET/CT [50, 51]. Currently, at our center, we are investigating the sensitivity and specificity of preoperative MR/PET using surgical pathology as the gold standard in patients undergoing radical cystectomy (NCT01655745). We hypothesize that MR/PET, with the combination of improved spatial resolution of soft tissue and functional imaging provided by PET, will improve staging relative to CT.
Fused MR/PET in a 72-year-old male with bladder cancer. The cross hairs are placed on a right external iliac metastatic lymph node. Row 1 shows PET scan, there is no increased uptake to suggest metastatic disease. Row 2 shows contrast-enhanced MRI with enhancement of the bladder and vessels. Again, the lymph node shows minimal enhancement. Row 3 shows fused MR and PET images with bright enhancement of the lymph node indicating tumor involvement. (Image courtesy of Julia Fielding, MD. Department of Radiology, The University of North Carolina at Chapel Hill)
Ultrasound
While not presently used in bladder cancer staging algorithms, ultrasound is widely used to assist in diagnosis though evaluation of gross hematuria. Conventional 2D transabdominal ultrasound technology (two-dimensional ultrasound (2D US)) is limited by subjectivity and expertise of the examiner, and in its ability to assess local depth of invasion. Three-dimensional ultrasound (3D US) allows for more systematic visualization of the tumor in multiple planes, increasing accuracy [52•]. In a pilot study of 14 patients with bladder tumors visualized on cystoscopy, 3D ultrasound was significantly more sensitive in detection of the tumor (78.6 versus 67.9 %), and was 100 % accurate in detecting serosal invasion of the tumor (≥T3b), compared to 88.9 % accuracy with 2D US [53].
Contrast-enhanced ultrasound (CE-US) uses intravenous microbubble contrast to help delineate vasculature on ultrasound and has been shown in small studies to compare favorably to conventional ultrasound in bladder cancer staging. A 34-patient study of CE-US versus 2D US demonstrated the ROC area under the curve of 0.996 for CE-US, compared to 0.613 for 2D US in detection of muscle-invasive disease [54]. Combining 3D and CE-US appears to have additive benefits as well. In a trial comparing 60 patients with the diagnosis of bladder cancer, CE-US, 3D US, and CE+3D US were used and compared to final pathology after TURBT. Combined CE+3D US was 100 % sensitive and 93 % specific in diagnosing MIBC versus NMIBC, and there was better intra-reader agreement when compared to CE-US and 3D US alone [52•].
Cystoscopic ultrasound is an investigational technique utilizing a flexible ultrasound bronchoscope as a cystoscope, allowing for local ultrasound of the primary bladder tumor. In a small pilot study, this technique was 95.7 % accurate in detection of MIBC, and sensitivity of MIBC detection was significantly higher with cystoscopic ultrasound compared to initial TURBT [55].
Despite technologic advances that have overcome some of the limitations of 2D ultrasound, the role of ultrasound in the staging of bladder cancer has not been well-defined, which has limited widespread clinical utility.
Conclusion
The management of bladder cancer varies depending upon clinical staging of the disease, and the mainstay imaging modality for preoperative staging is CT, though MR imaging has shown promise. CT remains the gold standard as a fast, relatively inexpensive study that provides a good deal of anatomic information and can easily identify enlarged lymph nodes, but is limited in its ability to accurately evaluate local tumor burden and invasion, and provides no functional information about suspicious lymph nodes. Multiparametric MR complements some weaknesses of CT, providing excellent soft tissue discrimination and accurate assessment of the primary tumor, and some protocols such as DCE-MR and DW-MR provide functional data, possibly increasing staging accuracy. However, the exact role of MR in staging bladder cancer is unclear, given a current paucity of large, well-powered studies. PET imaging, when combined with CT and MR, allows for assessment of metabolic activity of tissue, and may help with restaging and assessing response to chemotherapy. The urinary excretion of FDG makes bladder cancer staging with PET difficult due to interference in visualizing the bladder and surrounding tissues, but novel protocols and radiotracers may minimize that hurdle in the future, possibly expanding the indication and utility of PET in this population.
Abbreviations
- MIBC:
-
Muscle-invasive bladder cancer
- NMIBC:
-
Non-muscle-invasive bladder cancer
- MR:
-
Magnetic resonance
- DCE-MR:
-
Dynamic contrast-enhanced MR
- DWI-MR:
-
Diffusion-weighted image MR
- CT:
-
Computed tomography
- PET:
-
Positron emission tomography
- NSF:
-
Nephrogenic systemic fibrosis
- TURBT:
-
Transurethral resection of bladder tumor
- 2D US:
-
Two-dimensional ultrasound
- 3D US:
-
Three-dimensional ultrasound
- CE-US:
-
Contrast-enhanced ultrasound
- USPIO:
-
Ultra-small super-paramagnetic particles of iron oxide
- ADC:
-
Apparent diffusion coefficient
- FDG:
-
18F-fluorodeoxyglucose
- GFR:
-
Glomerular filtration rate
- ROC:
-
Receiver operating characteristic
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
National Cancer Institute. Surveillance, epidemiology, and end results program (SEER) fact sheet. www.seer.cancer.gov. Updated 2014. Accessed 05/23, 2014.
Clark P. NCCN clinical practice guidelines in oncology: bladder cancer - version 2. 2014.
Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65(4):778–92.
Shariat SF, Palapattu GS, Karakiewicz PI, et al. Discrepancy between clinical and pathologic stage: impact on prognosis after radical cystectomy. Eur Urol. 2007;51(1):137–51.
Tritschler S, Mosler C, Straub J, et al. Staging of muscle-invasive bladder cancer: can computerized tomography help us to decide on local treatment? World J Urol. 2012;30(6):827–31.
Gray PJ, Lin CC, Jemal A, et al. Clinical–pathologic stage discrepancy in bladder cancer patients treated with radical cystectomy: results from the national cancer data base. Int J Radiat Oncol Biol Phys. 2014;88(5):1048–56.
Bostrom PJ, van Rhijn BW, Fleshner N, et al. Staging and staging errors in bladder cancer. Eur Urol Suppl. 2010;9(1):2–9.
Liedberg F, Bendahl PO, Davidsson T, et al. Preoperative staging of locally advanced bladder cancer before radical cystectomy using 3 tesla magnetic resonance imaging with a standardized protocol. Scand J Urol. 2013;47(2):108–12.
Barentsz J, Engelbrecht M, Witjes J, De la Rosette J, Van der Graaf M. MR imaging of the male pelvis. Eur Radiol. 1999;9(9):1722–36.
Paik ML, Scolieri MJ, Brown SL, Spirnak JP, Resnick MI. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000;163(6):1693–6.
El-Assmy A, Abou-El-Ghar ME, Mosbah A, et al. Bladder tumour staging: comparison of diffusion-and T2-weighted MR imaging. Eur Radiol. 2009;19(7):1575–81.
Kundra V, Silverman PM. Imaging in the diagnosis, staging, and follow-up of cancer of the urinary bladder. Am J Roentgenol. 2003;180(4):1045–54.
de Haas RJ, Steyvers MJ, Fütterer JJ. Multiparametric MRI of the bladder: ready for clinical routine? Am J Roentgenol. 2014;202(6):1187–95. An excellent review of recent studies of the multiparametric MR imaging in bladder cancer, with clear explanations of various MR techniques and their strengths and weaknesses.
Tekes A, Kamel IR, Imam K, Chan TY, Schoenberg MP, Bluemke DA. MR imaging features of transitional cell carcinoma of the urinary bladder. Am J Roentgenol. 2003;180(3):771–7.
Kim B, Semelka RC, Ascher SM, Chalpin DB, Carroll PR, Hricak H. Bladder tumor staging: comparison of contrast-enhanced CT, T1- and T2-weighted MR imaging, dynamic gadolinium-enhanced imaging, and late gadolinium-enhanced imaging. Radiology. 1994;193(1):239–45.
Daneshmand S, Ahmadi H, Huynh LN, Dobos N. Preoperative staging of invasive bladder cancer with dynamic gadolinium-enhanced magnetic resonance imaging: results from a prospective study. Urology. 2012;80(6):1313–8. The largest prospective study to date of bladder cancer T and N staging using dynamic contrast-enhanced MR.
Tekes A, Kamel I, Imam K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. Am J Roentgenol. 2005;184(1):121–7.
Tuncbilek N, Kaplan M, Altaner S, et al. Value of dynamic contrast-enhanced MRI and correlation with tumor angiogenesis in bladder cancer. Am J Roentgenol. 2009;192(4):949–55.
Hafeez S, Huddart R. Advances in bladder cancer imaging. BMC Med. 2013;11:104-7015-11-104. doi:10.1186/1741-7015-11-104.
Ah-See ML, Makris A, Taylor NJ, et al. Early changes in functional dynamic magnetic resonance imaging predict for pathologic response to neoadjuvant chemotherapy in primary breast cancer. Clin Cancer Res. 2008;14(20):6580–9. doi:10.1158/1078-0432.CCR-07-4310.
Nguyen HT, Jia G, Shah ZK et al. Prediction of chemotherapeutic response in bladder cancer using k‐means clustering of dynamic contrast‐enhanced (DCE)‐MRI pharmacokinetic parameters. J Magn Reson Imaging. 2014. Pilot study evaluating DCE-MR characteristics as a surrogate for tumor blood flow in assessing early response to chemotherapy. Further studies may provide early guidance in management of patients undergoing neoadjuvant chemotherapy.
ACR committee on Drugs and Contrast Media. ACR manual on contrast media. 2013: http://www.acr.org/Quality-Safety/Resources/Contrast-Manual.
Yoshida S, Koga F, Kobayashi S, et al. Diffusion-weighted magnetic resonance imaging in management of bladder cancer, particularly with multimodal bladder-sparing strategy. World J Radiol. 2014;6(6):344.
Papalia R, Simone G, Grasso R, et al. Diffusion‐weighted magnetic resonance imaging in patients selected for radical cystectomy: detection rate of pelvic lymph node metastases. BJU Int. 2012;109(7):1031–6.
Sevcenco S, Ponhold L, Heinz-Peer G et al. Prospective evaluation of diffusion-weighted MRI of the bladder as a biomarker for prediction of bladder cancer aggressiveness. 2014.
Kobayashi S, Koga F, Kajino K, et al. Apparent diffusion coefficient value reflects invasive and proliferative potential of bladder cancer. J Magn Reson Imaging. 2014;39(1):172–8. Confirms DW-MR characteristics correlate with bladder cancers’ biologic aggressiveness.
Chen C, Li C, Kuo Y, et al. Early response of hepatocellular carcinoma to transcatheter arterial chemoembolization: choline levels and MR diffusion constants—initial experience 1. Radiology. 2006;239(2):448–56.
Cui Y, Zhang X, Sun Y, Tang L, Shen L. Apparent diffusion coefficient: potential imaging biomarker for prediction and early detection of response to chemotherapy in hepatic metastases 1. Radiology. 2008;248(3):894–900.
Harry VN, Semple SI, Gilbert FJ, Parkin DE. Diffusion-weighted magnetic resonance imaging in the early detection of response to chemoradiation in cervical cancer. Gynecol Oncol. 2008;111(2):213–20.
Kyriazi S, Collins DJ, Messiou C, et al. Metastatic ovarian and primary peritoneal cancer: assessing chemotherapy response with diffusion-weighted MR imaging—value of histogram analysis of apparent diffusion coefficients. Radiology. 2011;261(1):182–92.
Deserno WM, Harisinghani MG, Taupitz M, et al. Urinary bladder cancer: preoperative nodal staging with ferumoxtran-10–enhanced MR imaging 1. Radiology. 2004;233(2):449–56.
Birkhäuser FD, Studer UE, Froehlich JM, et al. Combined ultrasmall superparamagnetic particles of iron oxide–enhanced and diffusion-weighted magnetic resonance imaging facilitates detection of metastases in normal-sized pelvic lymph nodes of patients with bladder and prostate cancer. Eur Urol. 2013;64(6):953–60. Pilot study combining DW-MR and lymphotrophic nanoparticle-enhanced MR in clinical staging of bladder, demonstrating excellent accuracy in staging bladder cancer.
Lu Y, Chen J, Liang J, et al. Clinical value of FDG PET or PET/CT in urinary bladder cancer: a systemic review and meta-analysis. Eur J Radiol. 2012;81(9):2411–6.
Apolo AB, Riches J, Schoder H, et al. Clinical value of fluorine-18 2-fluoro-2-deoxy-D-glucose positron emission tomography/computed tomography in bladder cancer. J Clin Oncol. 2010;28(25):3973–8. doi:10.1200/JCO.2010.28.7052.
Harkirat S, Anand S, Jacob M. Forced diuresis and dual-phase F-fluorodeoxyglucose-PET/CT scan for restaging of urinary bladder cancers. Indian J Radiol Imaging. 2010;20(1):13–9. doi:10.4103/0971-3026.59746.
Kibel AS, Dehdashti F, Katz MD, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27(26):4314–20. doi:10.1200/JCO.2008.20.6722.
Jadvar H, Quan V, Henderson RW, Conti PS. [F-18]-fluorodeoxyglucose PET and PET-CT in diagnostic imaging evaluation of locally recurrent and metastatic bladder transitional cell carcinoma. Int J Clin Oncol. 2008;13(1):42–7.
Drieskens O, Oyen R, Van Poppel H, Vankan Y, Flamen P, Mortelmans L. FDG-PET for preoperative staging of bladder cancer. Eur J Nucl Med Mol Imaging. 2005;32(12):1412–7.
Goodfellow H, Viney Z, Hughes P, et al. Role of fluorodeoxyglucose positron emission tomography (FDG PET)‐computed tomography (CT) in the staging of bladder cancer. BJU Int. 2014;114(3):389–95. The largest head-to-head comparison to date of CT versus PET/CT for staging bladder cancer. Demonstrates modest staging benefit with PET/CT.
Liu IJ, Lai YH, Espiritu JI, et al. Evaluation of fluorodeoxyglucose positron emission tomography imaging in metastatic transitional cell carcinoma with and without prior chemotherapy. Urol Int. 2006;77(1):69–75.
Anjos DA, Etchebehere EC, Ramos CD, Santos AO, Albertotti C, Camargo EE. 18F-FDG PET/CT delayed images after diuretic for restaging invasive bladder cancer. J Nucl Med. 2007;48(5):764–70.
Schöder H, Ong SC, Reuter VE, et al. Initial results with 11C-acetate positron emission tomography/computed tomography (PET/CT) in the staging of urinary bladder cancer. Mol Imaging Biol. 2012;14(2):245–51.
Picchio M, Treiber U, Beer AJ, et al. Value of 11C-choline PET and contrast-enhanced CT for staging of bladder cancer: correlation with histopathologic findings. J Nucl Med. 2006;47(6):938–44.
Maurer T, Souvatzoglou M, Kübler H, et al. Diagnostic efficacy of [11C] choline positron emission tomography/computed tomography compared with conventional computed tomography in lymph node staging of patients with bladder cancer prior to radical cystectomy. Eur Urol. 2012;61(5):1031–8.
Ahlström H, Malmström P, Letocha H, Andersson J, Långström B, Nilsson S. Positron emission tomography in the diagnosis and staging of urinary bladder cancer. Acta Radiol. 1996;37(2):180–5.
Even-Sapir E, Metser U, Flusser G, et al. Assessment of malignant skeletal disease: initial experience with 18F-fluoride PET/CT and comparison between 18F-fluoride PET and 18F-fluoride PET/CT. J Nucl Med. 2004;45(2):272–8.
Hillner BE, Siegel BA, Hanna L, et al. Impact of 18F-fluoride PET on intended management of patients with cancers other than prostate cancer: results from the national oncologic PET registry. J Nucl Med. 2014;55(7):1054–61.
Chakraborty D, Bhattacharya A, Mete UK, Mittal BR. Comparison of 18F fluoride PET/CT and 99mTc-MDP bone scan in the detection of skeletal metastases in urinary bladder carcinoma. Clin Nucl Med. 2013;38(8):616–21. doi:10.1097/RLU.0b013e31828da5cc.
Orlova A, Tran TA, Ekblad T, Karlström AE, Tolmachev V. 186Re-maSGS-ZHER2: 342, a potential affibody conjugate for systemic therapy of HER2-expressing tumours. Eur J Nucl Med Mol Imaging. 2010;37(2):260–9.
Kjær A, Loft A, Law I, et al. PET/MRI in cancer patients: first experiences and vision from Copenhagen. MAGMA. 2013;26(1):37–47.
Partovi S, Robbin MR, Steinbach OC, et al. Initial experience of MR/PET in a clinical cancer center. J Magn Reson Imaging. 2014;39(4):768–80.
Li Q, Tang J, He E, et al. Clinical utility of three-dimensional contrast-enhanced ultrasound in the differentiation between noninvasive and invasive neoplasms of urinary bladder. Eur J Radiol. 2012;81(11):2936–42. Small study comparing 3D CE-US to 3D US and CE-US alone, demonstrating good accuracy in clinical staging of the primary bladder cancer.
Park HJ, Hong SS, Kim JH, et al. Tumor detection and serosal invasion of bladder cancer: role of three-dimensional volumetric reconstructed US. Abdom Imaging. 2010;35(3):265–70.
Caruso G, Salvaggio G, Campisi A, et al. Bladder tumor staging: comparison of contrast-enhanced and gray-scale ultrasound. Am J Roentgenol. 2010;194(1):151–6.
Xu C, Zhang Z, Wang H, et al. A new tool for distinguishing muscle invasive and non-muscle invasive bladder cancer: the initial application of flexible ultrasound bronchoscope in bladder tumor staging. PLoS One. 2014;9(4):e92385.
Compliance with Ethics Guidelines
Conflict of Interest
Dr. Maxim J. McKibben and Dr. Michael E. Woods each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of Topical Collection on New Imaging Techniques
Rights and permissions
About this article
Cite this article
McKibben, M.J., Woods, M.E. Preoperative Imaging for Staging Bladder Cancer. Curr Urol Rep 16, 22 (2015). https://doi.org/10.1007/s11934-015-0496-8
Published:
DOI: https://doi.org/10.1007/s11934-015-0496-8