Abstract
Bladder cancer is the most common neoplasm of the urinary tract and imaging has become vital in both the diagnosis and management of these patients. There are many different imaging modalities (e.g. ultrasound, computed tomography, magnetic resonance imaging, positron emission tomography, bone scans), each with their specific applications. It is important to understand the merits of each modality to use them in the proper clinical setting. As the use of neoadjuvant chemotherapy becomes more prevalent, accurate clinical staging of patients with advanced disease is paramount. This chapter discusses the role of multiple imaging modalities in the setting of both non-muscle invasive and muscle invasive bladder cancer, as well as in the setting of neoadjuvant chemotherapy and surveillance.
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Urothelial carcinoma
- Bladder cancer
- Radiology
- Imaging
- Staging
- Computed tomography
- Positron emission tomography
- Magnetic resonance imaging
1 Introduction
Bladder cancer is the most common neoplasm of the urinary tract and the sixth most common malignancy in the United States [1]. While most are detected at the clinically localized stage, approximately 25 % of patients will initially present with regional or metastatic disease. Radiographic imaging is a vital part of the evaluation of both local and advanced bladder cancer as it assists the urologist in the determination of appropriate management. It furthermore plays an important role after definitive treatment for long-term cancer surveillance and in some cases management of surgical complications.
It is important to recognize that at the time of most patients having an established diagnosis of bladder cancer, they will have already undergone some form of imaging as part of a hematuria evaluation. In this chapter, we discuss the different imaging modalities used in diagnosis and staging in the context of both localized and advanced bladder cancer. We will also review the role of imaging in the setting of neoadjuvant chemotherapy and post-treatment cancer surveillance. Newer imaging techniques utilizing cystoscopy (narrow band imaging, confocal laser microendoscopy, and optimal coherence tomography) are not presently used in standard practice and will not be discussed.
2 Role of Imaging for Non-muscle Invasive Bladder Cancer (CIS, Ta, T1)
The majority of patients (approximately 75 %) presenting with urothelial cancers of the bladder are diagnosed with non-muscle invasive disease, that is, confined to the mucosal or lamina propria layers. Nonetheless, imaging is still an important part of this evaluation as it helps ensure accurate clinical staging while also evaluating the upper urothelial tracts for synchronous or metachronous lesions. There are several modalities that may be used in this evaluation including ultrasonography, contrast-enhanced radiography (i.e. intravenous pyelogram, retrograde pyelogram), computed tomography, and magnetic resonance imaging.
2.1 Radiography, Intravenous Pyelography, and Ultrasound
Plain radiography has no role in the evaluation of non-muscle invasive bladder cancer (NMIBC) given its lack of soft tissue contrast or definition. While there have been some case reports describing appearance of calcifications in the bladder showing up on plain x-ray, these are merely of historical perspective and will not be described. On the other hand, the addition of intravenous contrast to plain radiography (intravenous pyelography, IVP) has been used for many years in the field of urology. An IVP involves injection of intravenous contrast followed by serial radiographic and tomographic images obtained of the kidneys, ureter, and bladder as the contrast media moves through the urinary tract. For many years IVP was the study of choice along with cystoscopy in the evaluation of hematuria, however in the era of computed tomography and magnetic resonance imaging, this test has fallen out of favor. In the most recent version of the AUA Clinical Guidelines for asymptomatic microhematuria, IVP and ultrasound were considered less optimal imaging tests given their low sensitivity compared with magnetic resonance imaging (MRI) or computed tomography (CT) and high likelihood of missing a diagnosis [2]. When present on IVP, urothelial cancers may appear as a filling defect within the bladder or upper urinary tracts (Fig. 5.1) [3]. Papillary lesions, as often seen with non-invasive tumors, will have frond-like projections into the bladder lumen, giving the appearance of a poorly marginated filling defect within the bladder. A thickened bladder wall on the IVP may be seen in some cases of carcinoma in situ. One potential pitfall with IVP is that a large median lobe may be mistaken for a filling defect or bladder tumor.
Intravenous pyelogram demonstrating left hydroureteronephrosis and large filling defect within the bladder. Reprinted with permission [3]
Ultrasonography has several theoretical advantages as an imaging modality of the bladder and urinary tract. It is readily available, requires no patient preparation, is inexpensive, and not associated with radiation exposure. In addition, it allows for simultaneous evaluation of the upper tracts and may demonstrate hydronephrosis, renal calculi, or renal masses. The accuracy of ultrasound in visualizing bladder tumors depends on the degree of distention and tumor characteristics (size, morphology, and location) and is operator-dependent. Newer contrast-enhanced techniques show some promise for improving diagnostic accuracy in the imaging of bladder tumors for evaluation of hematuria. That being said, cystoscopy remains the gold standard diagnostic procedure and should be performed regardless of ultrasound findings.
Ultrasonography is a poor tool for staging of bladder cancer and it is rarely used after histologic confirmation. More commonly, ultrasound of the bladder is performed as part of the evaluation of the kidneys and an incidental lesion is noted. Bladder tumors on ultrasound typically appear as hypoechoic, plaque-like or polpypoid lesions projecting into the bladder lumen [4]. Doppler studies may demonstrate blood flow, especially in larger papillary lesions. Shadowing may also be present if there is calcification. Bladder wall thickening may also be apparent although this is a nonspecific finding.
The diagnostic accuracy of ultrasound for detection of bladder tumors is highly variable and dependent on size and location. Datta et al. showed that US had an overall sensitivity of 63 % and specificity of 99 % for detection of bladder cancer in a series of over 1,000 patients presenting with hematuria [5]. Smaller lesions are in particular more difficult to evaluate. For lesions less than 5 mm in diameter, Malone et al. found that US detected only 38 % of tumors compared to 82 % of those greater than 5 mm as confirmed by cystoscopy [6]. Several series have shown the importance of tumor location and sensitivity of US. The bladder neck, dome, and anterior bladder are all sites where US visualization is limited and may miss lesions [7, 8].
Contrast-enhanced ultrasonography (CEUS) is a newer form of ultrasonography that attempts to improve the diagnostic accuracy of ultrasound. This modality relies on intravenous microbubble contrast agents and a specialized ultrasound probe. The microbubbles are entirely intravascular and their properties result in high echogenicity when visualized on sonogram. This allows for evaluation of vasculature and neovascularity (i.e. tumors). It has been used for imaging of the spleen, liver, and kidneys [9]. Previous studies using CEUS of the bladder revealed that the mucosal and submucosal layers had early enhancement and the detrusor was relatively hypoechoic in comparison. Using CEUS of the bladder, the presence of a hypoechoic layer between the bladder tumor and bladder wall was predictive of non-invasive disease in a small study [10]. Most recently, Nicolau reported their experience using contrast-enhanced ultrasound in a cohort of 43 patients undergoing transurethral resection of bladder tumors. CEUS and routine US were performed the day prior to a transurethral resection of bladder tumor (TURBT). CEUS was more accurate than routine US in detection of bladder cancer (88.3 % vs. 72.09 %) and was particularly helpful in non-conclusive US cases. Sensitivity of CEUS was highest with lesions >5 mm (94.7 % vs. 20 % if <5 mm) [11]. More recently, three-dimensional ultrasound has been combined with CEUS in efforts to not only improve detection but also predict invasiveness. This new technique, as shown in Fig. 5.2, results in contrast-enhanced sonographic images in multiple planes allowing for a three-dimensional reconstruction of a bladder tumor. Of 60 bladder lesions evaluated with combined 3D US and CEUS prior to TURBT, all 16 muscle invasive tumors were correctly diagnosed. Inter-reader agreement was highest when these images were combined as compared to individual use (kappa = 0.914) [12].
Contrast-enhanced 3D ultrasound of patients with non-invasive bladder tumor. (a) Conventional 2D ultrasound; (b) 3D image from three rectangular planes; (c) Contrast enhanced 2D ultrasound with homogenous enhancement; (d) 3D contrast enhanced image. Reprinted with permission [12]
Despite the advantages of ultrasound for bladder cancer imaging, it still lacks in diagnostic accuracy, especially for smaller lesions. Routine grey scale ultrasound is still considered inferior to other imaging modalities such as CT and MRI for evaluation and staging of bladder cancer. For the evaluation of hematuria, ultrasound is considered a suboptimal test based on the AUA guidelines [2]. With evolving technologies utilizing microbubble contrast and three-dimensional imaging, ultrasound imaging may play a more important role in imaging bladder tumors, but not without further refinement of the modality.
2.2 Computed Tomography and Urography for Non-invasive Disease
Multidetector row computed tomography (CT) is currently the most widely used imaging modality for bladder cancer. In practice many patients will have had some form of CT imaging prior to undergoing office cystoscopy or transurethral resection of a bladder tumor as this is usually performed as part of a hematuria evaluation. It is important to delineate the use of CT in the context of a hematuria evaluation versus staging after a confirmatory diagnosis of bladder cancer. In this section, we discuss the role of CT imaging as it relates to NMIBC. Later in this chapter, we will discuss CT imaging in the setting of invasive bladder cancer.
CT imaging for bladder cancer should be performed with intravenous contrast unless contraindicated due to renal insufficiency or allergy. Delayed images are essential and allow for assessment of the collecting system, ureters, and bladder. Advances in post-processing computerized technology now allow for reconstructed CT images in coronal and sagittal planes, which provide further improvements in evaluation of the urinary tract.
2.2.1 Low-Grade NMIBC
Most non-invasive bladder tumors are low grade and of papillary architecture. Malignant potential of these tumors is extremely low, with rates of progression less than 5 %. These tumors may vary in size, number, and characteristics. Papillary lesions may be seen as a focal filling defect projecting into the bladder or an area of asymmetric bladder wall thickening. Larger lesions may appear as an enhancing soft tissue density projecting into a relatively hypodense background of the urine filled bladder, or on delayed images as a filling defect (Fig. 5.3).
In the setting of non-invasive urothelial bladder cancer, ongoing surveillance after treatment is necessary to ensure there has been no disease recurrence. Cystoscopy is the mainstay of post-treatment cancer surveillance in low-grade non-invasive disease. Upper tract imaging should be performed every 1–2 years or more frequently in cases of high-grade recurrences, therefore CT urography continues to play a prominent role even at this stage.
2.2.2 High-Risk Non-invasive Disease
High-risk NMIBC includes carcinoma in situ, T1 (lamina propria invasion), and high-grade Ta. Morphologically the latter two may be indistinguishable from low-grade NMIBC on CT imaging while the former is virtually never seen on imaging [13]. High-risk disease is associated with higher rates of recurrence and progression and adjuvant intravesical treatment after resection is recommended in order to reduce the chance of recurrence and progression. The role of imaging as part of surveillance after treatment is no different than that with low-grade NMIBC.
2.2.3 CT: Conclusion
Computed tomography urography (CTU) has become the imaging test of choice for NMIBC and is often performed prior to cystoscopy during hematuria evaluation. Contemporary sensitivity and specificity for bladder cancer detection using CTU range from 79 to 95 % and 83–99 %, respectively [14–16]. As will be discussed later, despite this diagnostic capability, CTU falls short when it comes to staging accuracy. Furthermore, CTU is unable to demonstrate carcinoma in situ and may miss small lesions, particularly when less than 1 cm [13]. Cystoscopic examination with transurethral resection therefore remains essential and to date cannot be replaced by CT.
2.3 Magnetic Resonance Imaging in NMIBC
Magnetic resonance imaging provides without question the best soft tissue contrast quality of all imaging modalities. This technique requires no radiation exposure but is more time-consuming and costly. The ability of MRI to produce images with such spatial resolution and detail rely on the effects of proton alignment within tissues when exposed to a magnetic field. In the most basic form, there are two phases in which images are constructed, T1 and T2. The contrast agent gadolinium is also used for better enhancement of tissues. As with computed tomography, multiplanar images in the axial, coronal, and sagittal planes can be constructed.
There are little data available in using MRI to evaluate the urinary bladder in NMIBC. However, MRI has been used to assess the urinary tract during a hematuria evaluation in patients who cannot tolerate iodinated contrast due to allergy. Magnetic resonance urography (MRU) is much similar to CTU, using gadolinium contrast to evaluate the urinary system in a delayed phase. On T1-weighted images, fluid (such as urine) is of low signal intensity and therefore the bladder appears hypointense. On T2-weighted images, fluid is of high signal intensity. On either of these phases, a bladder tumor may be demonstrated by an area of signal intensity contrasting that of urine and the bladder wall. For example, on T1-weighted imaging, a bladder tumor will likely appear as an area of intermediate intensity and on T2 phase the tumor will appear hypointense to the surrounding urine. The detrusor layer is of low signal intensity and should be intact for non-invasive tumors, as depicted in Fig. 5.4 [17]. With diffusion-weighted (DW) MRI (discussed later) the “inchworm sign,” characterized as a low signal intensity stalk invaginating into a high signal intensity tumor, has shown to be highly accurate in predicting non-muscle invasive bladder cancer [18].
(Left) T2 MRI showing non-invasive tumor with low signal intensity (black arrow). (Right) T1 MRI shows same tumor with high signal intensity compared to urine. White arrows denote intact detrusor layer. Reprinted with permission [17]
3 Role of Imaging in the Setting of Neoadjuvant Chemotherapy
The use of neoadjuvant systemic chemotherapy (NC) has been shown to result in improved overall survival [19]. However, controversy is still ongoing in regards to which patients should be initially managed with NC. For patients with organ confined disease (≤clinical T2N0) radical cystectomy alone will likely cure most patients without the need for cytotoxic chemotherapy. On the other hand, clinical staging is notoriously inaccurate, with a discrepancy between clinical and pathologic staging in up to 50 % of cases [20]. Therefore, many of these patients will actually have more advanced disease after cystectomy and may require chemotherapy in an adjuvant setting. It has been our practice to treat patients with clinically advanced disease with neoadjuvant chemotherapy prior to radical cystectomy, although practice patterns and opinions vary on this matter. Nonetheless, radiographic imaging plays an important role in patient selection for neoadjuvant chemotherapy as well as monitoring response to treatment.
There are several findings on cross-sectional imaging with either CT or MRI that may suggest locally advanced disease. The most notable of these is hydronephrosis, which has been shown to be an independent predictor of both extravesical and node positive disease [18, 21]. Given this finding after staging one should give strong consideration to neoadjuvant chemotherapy prior to surgical resection. Hydronephrosis is apparent in both CT and MRI and is evident by dilation of the collecting system to varying degrees. Most commonly there is ureteral dilation to the level of the ureterovesical junction. It is important to evaluate the delayed images as hydroureteronephrosis may be seen as a result of upper tract TCC. If any of these findings are seen during staging of bladder cancer, one should strongly consider the use of neoadjuvant chemotherapy. Other findings suggestive of locally advanced disease include evidence of perivesical fat involvement and extension into other organs. Both of these radiographic findings are highly suspicious for T3 or T4 disease, although the lack of these findings does not necessarily rule out extravesical disease.
The use of positron emission tomography to better select patients who may benefit from NC has been evaluated in recent years. This functional study relies on the use of radio-labeled metabolic substrates to identify areas of abnormal uptake, which may suggest tumor metastasis (Fig. 5.5). To date, fludeoxyglucose (F 18) and [11C] choline have been the primary radiotracers used for bladder cancer staging with a diagnostic accuracy of 65–94 % in predicting lymph node metastasis. While it has been proposed that [11C] choline may be better for bladder cancer owing to lack of urinary excretion, no randomized controlled trials comparing the two have been conducted.
More recently, diffusion-weighted MRI has been added to conventional MRI to improve tumor staging in patients with bladder cancer. This technique relies on the relative movement of water through tissues, resulting in an apparent diffusion coefficient (ADC). It has previously been demonstrated that the ADC of bladder tumors is lower than that of the surrounding tissue [22]. Overall accuracy of predicting tumor stage was improved from 67 to 88 % with the addition of DW images to MRI T2 phase in one series [23]. Another single center experience found that use of DW-MRI with T1/T2-MRI reduced upstaging after cystectomy to 5 % [24]. While DW-MRI appears to improve diagnostic accuracy, to date most studies have been limited to small single institution experiences. Nonetheless, this newer technology holds promise in efforts to improve clinical staging to better select those patients who may benefit from NC.
Monitoring the response to neoadjuvant chemotherapy with imaging is important as well. Indeed, not all patients respond to NC and under those circumstances consideration should be given to proceeding with cystectomy. Furthermore, patients with nodal involvement who have an incomplete or no response to NC in particular have poor outcomes [25]. CT, MR, and positron emission tomography (PET) techniques have all been described as useful imaging studies during and after treatment with NC to evaluate for treatment response. At present there is no universally agreed upon strategy on how to best use imaging to monitor patients on NC. In our practices, we obtain imaging with CT or MRI after two cycles of treatment to evaluate for response. Patients in whom there appears to be objective response to chemotherapy receive 1–2 additional cycles, while those that show any signs of progression have chemotherapy interrupted and proceed to cystectomy as clinically indicated.
In efforts to better predict response of neoadjuvant chemotherapy in patients prior to cystectomy, newer modalities have been studied. Diffusion-weighted imaging (DWI) MRI had higher specificity and accuracy in predicting complete vs. partial response to chemoradiation prior to surgery when compared to T2-weighted and dynamic contrast-enhanced MRI phases in a small study of 20 patients with organ confined disease [26]. To date, this is the only experience using DWI in this setting. Martens and colleagues compared FDG-PET to conventional contrast-enhanced CT in a group of patients with lymph node positive bladder cancer undergoing NC. In this small cohort FDG-PET was no better than CT imaging in distinguishing responders from non-responders or complete vs. partial response [27].
4 Role of Imaging for Muscle Invasive Bladder Cancer (Clinical T2, T3, or T4)
While only about 25 % of urothelial carcinomas are found to be muscle invasive at the time of diagnosis, imaging plays a crucial role in the management of these patients. It is paramount to determine the presence of extravesical, nodal, and/or metastatic disease [28, 29] as these findings will affect treatment recommendations. This is particularly relevant as discussed previously; treatment paradigms are changing with increasing administration of neoadjuvant chemotherapy for locally advanced urothelial carcinoma [30–32].
Generally, plain radiography and ultrasound are not used routinely in the evaluation of muscle invasive urothelial carcinoma due to diminished sensitivity, the lack of high-resolution images and the absence of whole body imaging. While IVP has historically been used for detecting upper tract disease, it has a very limited role in the evaluation of locally advanced urothelial carcinoma. Like in NMIBC, CT and MRI have a predominant role in muscle invasive disease as these cross-sectional imaging modalities provide information on the overall clinical stage of the patient [33]. Additional imaging modalities include positron emission tomography (PET) and whole body bone scans. It is imperative that these studies be conducted prior to any radical extirpative surgery, as surgery may not be the initial treatment of choice for patients with advanced disease (non-organ confined extravesical tumor, regional node positive disease, or the presence of distant nodal or visceral metastases). Additionally, as most patients with muscle invasive disease will proceed to radical cystectomy, whole body imaging allows for surgical planning and facilitates the detection of anatomic variations such as duplicated ureters or ectopic kidneys.
4.1 Computed Tomography for Muscle Invasive Bladder Cancer
Although the primary method of in-situ clinical staging for urothelial carcinoma is with a transurethral resection, multidetector row computed tomography (CT) has become the most common modality for evaluating muscle invasive bladder cancers [34, 35]. This is due to its speed, widespread availability, and advantage of imaging multiple organs simultaneously. Considerations with the use of CT include radiation exposure and the need for an intravenous contrast agent which may be difficult in patients with contrast allergies or renal insufficiency.
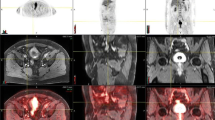
Despite its widespread use, CT has a limited ability to accurately stage urothelial carcinoma. While complete intravesical lesions (stage T1) are usually apparent, there can be significant ambiguity in differentiating T2 from T3 disease [36] (Figs. 5.6 and 5.7). Several studies have demonstrated limited accuracy for CT in determining the depth of bladder invasion [37–39]. Paik et al. [40] reported an overall accuracy of 54.9 %, and under- and over-staging in 39 % and 6.1 % of patients respectively. Eight of their patients were found to have extravesical disease on CT, confirmed pathologically in only four. More recently, Baltaci et al. [41] reported their series of 100 cases, in which only 22 of 57 (38.6 %) patients with extravesical invasion on CT were confirmed pathologically. Ambiguity of extravesical disease can be present after cystoscopic intervention and bladder tumor resection, which can cause perivesical fibrosis and mimic extravesical extension. To avoid this, imaging ideally should be obtained prior to any transurethral resection.
The presence of node positive disease is also an important prognostic factor in muscle invasive bladder cancer [42]. When examining nodal involvement with CT, it is purely an anatomic study without any functional assessment, thus lymph nodes are evaluated based on their anatomic architecture (Fig. 5.8). Size ≥10 mm in the short axis dimension is the most suggestive finding to determine nodal involvement. Lymph nodes also may become more rounded if involved with metastatic disease [36]. CT however, remains a poor predictor of lymph node involvement with accuracy between 5 and 50 % [42]. This poor predictive value is secondary to the inability to detect micrometastatic disease using CT.
4.2 Magnetic Resonance Imaging for Muscle Invasive Bladder Cancer
The role of MRI in muscle invasive bladder cancer is identical to CT: evaluation of extravesical, nodal, and metastatic disease. MRI is thought to be superior to CT in terms of local staging [34, 35, 43], however its slower speed, decreased availability, and discomfort for symptomatic patients and for those with claustrophobia make it a less accessible or optimal imaging modality for many. Despite the ability to more accurately evaluate soft tissue, there is no reported advantage in the staging of urothelial carcinoma. A recent study by Tekes et al. [44] reported on 67 patients with urothelial carcinoma staged using a dynamic MRI with gadolinium contrast and comparing results of clinical radiographic staging to pathologic stage. The overall accuracy of MRI was 62 %, with over-staging occurring in 32 % of patients. While this was found to be lower than previously published studies (72–95 % accuracy) [44], it is consistent with Vargas et al. [45] who in a prospective study found that MRI correctly staged 56 % of patients in their series while over-staging occurred in 38 % of cases.
With MRI, pelvic lymph nodes are more visible due to the surrounding adipose tissue, but this does not translate into higher accuracy in determining lymph node involvement [35]. Vargas et al. [45] report a sensitivity and specificity of only 50 % and 71 % respectively. As with CT, the determination of lymph node involvement is most reliant on a size criterion of ≥10 mm in shortest dimension. Normal and tumor bearing lymph nodes demonstrate similar enhancement on gadolinium-enhanced MRI.
4.3 Bone Scans for Muscle Invasive Bladder Cancer
Along with the lung and liver, bone is one of the more common sites of distant metastasis for urothelial carcinoma. Similar to PET scans, bone scans utilize an intravenous radio-isotope, which leads to “hot spots” or uptake seen on whole body scan. Not only metastatic disease, but also benign etiologies such as inflammation or previous trauma can create uptake noticeable on bone scan. Despite their high sensitivity for bone abnormalities, the low specificity of bone scans requires a skillful interpretation. Historically, preoperative bone scans were routinely ordered prior to radical cystectomy, which if positive would result in the patient’s surgeon canceling the procedure. In contrast, Braendengen et al. [46] reviewed 91 patients following radical cystectomy who had undergone preoperative bone scans. The bone scans were scored on a scale indicating how likely it was the patient had bone metastases. They found no significant relationship between findings on preoperative bone scan and the development of subsequent bone metastases. Additionally, they evaluated 54 patients with muscle invasive urothelial carcinoma who underwent preoperative bone scan. Only three patients had positive studies that resulted in a change to their management strategy from primary surgery to primary systemic chemotherapy. Follow up revealed that one of these patients had a false-positive study, leaving only two patients who truly had metastatic bone progression. They concluded that preoperative bone scans were unnecessary as they did not contribute to the overall clinical decision making process. In today’s practice, a bone scan should be ordered in the presence of symptoms such as new onset bone pain. An elevated serum alkaline phosphatase is another widely accepted indication for a bone scan, although this was not supported in the aforementioned study.
4.4 Positron Emission Tomography for Muscle Invasive Bladder Cancer
Positron emission tomography (PET) is a functional study with limited anatomic detail that detects the uptake of radio-isotopes by metabolically active cells in the body, such as tumor cells. In the initial diagnosis of urothelial carcinoma, the utility of PET scans is limited due to urinary excretion of the radio-isotope [43]. PET scans theoretically provide the ability to detect disease at an earlier stage, before any anatomic variation may be present. In practice however, PET scans have not routinely demonstrated superiority to detecting nodal involvement compared to CT, and may not be sensitive enough to detect node positivity unless the disease burden equates to a ≥10 mm lymph node [47]. There is a paucity of published data of the use of PET in urothelial carcinoma. One of the largest series published reported a sensitivity of 67 %, specificity of 86 %, and accuracy of 80 % [48] for staging of urothelial carcinoma.
Today, radiographic advancements have allowed PET scans to be fused with CT scans in order to create simultaneous anatomic and functional imagery (PET/CT). This combined image has allowed for greater accuracy than with either PET or CT alone. In their series, Kibel et al. [49] compared CT and bone scintigraphy vs. PET/CT in patients with urothelial carcinoma using [18F] fluorodeoxyglucose (FDG) and found occult metastatic disease in 7 of 42 patients found to be negative on conventional CT scan. They reported a positive predictive value of 78 %, negative predictive value of 91 %, and sensitivity and specificity of 70 % and 94 % respectively for PET/CT, which is consistent with previously reported sensitivity and specificity of 60 % and 88 % respectively [47]. In addition, some authors have suggested that PET scans may provide prognostic information. Drieskens et al. [47] reported in their series a median overall survival of 32 months in patients with urothelial carcinoma and a negative PET/CT scan as opposed to 13.5 months with a positive PET/CT, which is reflective of the studies with a high positive predictive value.
One of the pitfalls of PET scans is the detection of false-positive lesions (Fig. 5.9). A false-positive evaluation can occur with intestinal uptake that mimics a metastatic lesion [47] or with areas of inflammation due to benign etiologies. Such findings must be clarified through clinical correlation and comparison or combination with CT scans or other diagnostic procedures.
In the post-treatment setting, PET scans can be utilized to detect disease response or progression after neoadjuvant chemotherapy (Fig. 5.10a, b). Post-cystectomy patients at risk for either locoregional or distant recurrence can be evaluated with PET if any suspicious or ambiguous lesions are found [50].
(a) PET/CT reveals a large hypermetabolic pre-sacral lymph node in a male patient with muscle invasive urothelial carcinoma prior to neoadjuvant chemotherapy. Also apparent is a focus of false-positive uptake in the small intestine (arrow). (b) Resolution of the pre-sacral node after neoadjuvant chemotherapy as seen on PET/CT
5 Surveillance Imaging After Radical Surgery
Guidelines detailing a specific outline of follow up after radical cystectomy do not exist. Choice of imaging modality and timing is at the discretion of the surgeon, but should take into account the final pathologic tumor stage as more locally advanced disease warrants a stricter surveillance schedule. Generally, imaging of the chest, abdomen and pelvis should be performed every 3–12 months for at least 2 years with either CT or MRI [51]. A chest radiograph is considered a sufficient evaluation of the lungs; however, further work up with a CT Thorax needs to be obtained if there is a lesion of concern. Clinical suspicion should always prompt an evaluation with whole body imaging and additional studies as indicated including bone scintigraphy or PET/CT.
References
Siegel R, Naishadham D, Jemal A. Cancer statistics, 2012. CA Cancer J Clin. 2012;2(1):10–29 [Comparative Study].
Davis R, Jones JS, Barocas DA, Castle EP, Lang EK, Leveillee RJ, et al. Diagnosis, evaluation and follow-up of asymptomatic microhematuria (AMH) in adults: AUA guideline. J Urol. 2012;188(6 Suppl):2473–81.
Amling CL. Diagnosis and management of superficial bladder cancer. Curr Probl Cancer. 2001;25(4):219–78.
Kundra V, Silverman PM. Imaging in oncology from the University of Texas M. D. Anderson Cancer Center. Imaging in the diagnosis, staging, and follow-up of cancer of the urinary bladder. Am J Roentgenol. 2003;180(4):1045–54.
Datta SN, Allen GM, Evans R, Vaughton KC, Lucas MG. Urinary tract ultrasonography in the evaluation of haematuria – a report of over 1,000 cases. Ann R Coll Surg Engl. 2002;84(3):203–5.
Malone PR, Weston-Underwood J, Aron PM, Wilkinson KW, Joseph AE, Riddle PR. The use of transabdominal ultrasound in the detection of early bladder tumours. Br J Urol. 1986;58(5):520–2.
Ozden E, Turgut AT, Turkolmez K, Resorlu B, Safak M. Effect of bladder carcinoma location on detection rates by ultrasonography and computed tomography. Urology. 2007;69(5):889–92.
Abu-Yousef MM, Narayana AS, Franken Jr EA, Brown RC. Urinary bladder tumors studied by cystosonography. Part I: detection. Radiology. 1984;153(1):223–6.
Quaia E. Microbubble ultrasound contrast agents: an update. Eur Radiol. 2007;17(8):1995–2008.
Caruso G, Salvaggio G, Campisi A, Melloni D, Midiri M, Bertolotto M, et al. Bladder tumor staging: comparison of contrast-enhanced and gray-scale ultrasound. Am J Roentgenol. 2010;194(1):151–6.
Nicolau C, Bunesch L, Peri L, Salvador R, Corral JM, Mallofre C, et al. Accuracy of contrast-enhanced ultrasound in the detection of bladder cancer. Br J Radiol. 2011;84(1008):1091–9.
Li QY, Tang J, He EH, Li YM, Zhou Y, Zhang X, et al. Clinical utility of three-dimensional contrast-enhanced ultrasound in the differentiation between noninvasive and invasive neoplasms of urinary bladder. Eur J Radiol. 2012;81(11):2936–42.
Wang LJ, Wong YC, Ng KF, Chuang CK, Lee SY, Wan YL. Tumor characteristics of urothelial carcinoma on multidetector computerized tomography urography. J Urol. 2010;183(6):2154–60.
Turney BW, Willatt JM, Nixon D, Crew JP, Cowan NC. Computed tomography urography for diagnosing bladder cancer. BJU Int. 2006;98(2):345–8.
Blick CG, Nazir SA, Mallett S, Turney BW, Onwu NN, Roberts IS, et al. Evaluation of diagnostic strategies for bladder cancer using computed tomography (CT) urography, flexible cystoscopy and voided urine cytology: results for 778 patients from a hospital haematuria clinic. BJU Int. 2012;110(1):84–94.
Sadow CA, Silverman SG, O’Leary MP, Signorovitch JE. Bladder cancer detection with CT urography in an Academic Medical Center. Radiology. 2008;249(1):195–202.
Rajesh A, Sokhi HK, Fung R, Mulcahy KA, Bankart MJ. Bladder cancer: evaluation of staging accuracy using dynamic MRI. Clin Radiol. 2011;66(12):1140–5.
Green DA, Rink M, Hansen J, Cha EK, Robinson B, Tian Z, et al. Accurate preoperative prediction of non-organ-confined bladder urothelial carcinoma at cystectomy. BJU Int. 2013;111(3):404–11.
Winquist E, Kirchner TS, Segal R, Chin J, Lukka H. Genitourinary Cancer Disease Site Group CCOPiE-bCPGI. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol. 2004;171(2 Pt 1):561–9.
Svatek RS, Shariat SF, Novara G, Skinner EC, Fradet Y, Bastian PJ, et al. Discrepancy between clinical and pathological stage: external validation of the impact on prognosis in an international radical cystectomy cohort. BJU Int. 2011;107(6):898–904.
Stimson CJ, Cookson MS, Barocas DA, Clark PE, Humphrey JE, Patel SG, et al. Preoperative hydronephrosis predicts extravesical and node positive disease in patients undergoing cystectomy for bladder cancer. J Urol. 2010;183(5):1732–7.
Matsuki M, Inada Y, Tatsugami F, Tanikake M, Narabayashi I, Katsuoka Y. Diffusion-weighted MR imaging for urinary bladder carcinoma: initial results. Eur Radiol. 2007;17(1):201–4.
Takeuchi M, Sasaki S, Ito M, Okada S, Takahashi S, Kawai T, et al. Urinary bladder cancer: diffusion-weighted MR imaging–accuracy for diagnosing T stage and estimating histologic grade. Radiology. 2009;251(1):112–21 [Evaluation Studies Research Support, Non-U.S. Gov’t].
Watanabe H, Kanematsu M, Kondo H, Goshima S, Tsuge Y, Onozuka M, et al. Preoperative T staging of urinary bladder cancer: does diffusion-weighted MRI have supplementary value? Am J Roentgenol. 2009;192(5):1361–6.
Jensen JB, Ulhoi BP, Jensen KM. Prognostic value of lymph-node dissection in patients undergoing radical cystectomy following previous oncological treatment for bladder cancer. Scand J Urol Nephrol. 2011;45(6):436–43.
Yoshida S, Koga F, Kawakami S, Ishii C, Tanaka H, Numao N, et al. Initial experience of diffusion-weighted magnetic resonance imaging to assess therapeutic response to induction chemoradiotherapy against muscle-invasive bladder cancer. Urology. 2010;75(2):387–91.
Mertens LS, Fioole-Bruining A, van Rhijn BW, Kerst JM, Bergman AM, Vogel WV, et al. FDG-positron emission tomography/computerized tomography for monitoring the response of pelvic lymph node metastasis to neoadjuvant chemotherapy for bladder cancer. J Urol. 2013;189(5):1687–91.
Dighe MK, Bhargava P, Wright J. Urinary bladder masses: techniques, imaging spectrum, and staging. J Comput Assist Tomogr. 2011;35(4):411–24 [Review].
Ficarra V, Dalpiaz O, Alrabi N, Novara G, Galfano A, Artibani W. Correlation between clinical and pathological staging in a series of radical cystectomies for bladder carcinoma. BJU Int. 2005;95(6):786–90.
Grossman HB, Natale RB, Tangen CM, Speights VO, Vogelzang NJ, Trump DL, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349(9):859–66.
Sherif A, Holmberg L, Rintala E, Mestad O, Nilsson J, Nilsson S, et al. Neoadjuvant cisplatinum based combination chemotherapy in patients with invasive bladder cancer: a combined analysis of two Nordic studies. Eur Urol. 2004;45(3):297–303 [Research Support, Non-U.S. Gov’t].
Malmstrom PU, Rintala E, Wahlqvist R, Hellstrom P, Hellsten S, Hannisdal E. Five-year followup of a prospective trial of radical cystectomy and neoadjuvant chemotherapy: nordic cystectomy trial I. The Nordic Cooperative Bladder Cancer Study Group. J Urol. 1996;155(6):1903–6.
Husband JE. Computer tomography and magnetic resonance imaging in the evaluation of bladder cancer. J Belge Radiol. 1995;78(6):350–5 [Research Support, Non-U.S. Gov’t Review].
Beyersdorff D, Zhang J, Schoder H, Bochner B, Hricak H. Bladder cancer: can imaging change patient management? Curr Opin Urol. 2008;18(1):98–104 [Review].
Cowan NC, Crew JP. Imaging bladder cancer. Curr Opin Urol. 2010;20(5):409–13 [Review].
Setty BN, Holalkere NS, Sahani DV, Uppot RN, Harisinghani M, Blake MA. State-of-the-art cross-sectional imaging in bladder cancer. Curr Probl Diagn Radiol. 2007;36(2):83–96 [Review].
Voges GE, Tauschke E, Stockle M, Alken P, Hohenfellner R. Computerized tomography: an unreliable method for accurate staging of bladder tumors in patients who are candidates for radical cystectomy. J Urol. 1989;142(4):972–4 [Comparative Study].
Bryan PJ, Butler HE, LiPuma JP, Resnick MI, Kursh ED. CT and MR imaging in staging bladder neoplasms. J Comput Assist Tomogr. 1987;11(1):96–101 [Comparative Study].
Yaman O, Baltaci S, Arikan N, Yilmaz E, Gogus O. Staging with computed tomography, transrectal ultrasonography and transurethral resection of bladder tumour: comparison with final pathological stage in invasive bladder carcinoma. Br J Urol. 1996;78(2):197–200 [Comparative Study].
Paik ML, Scolieri MJ, Brown SL, Spirnak JP, Resnick MI. Limitations of computerized tomography in staging invasive bladder cancer before radical cystectomy. J Urol. 2000;163(6):1693–6 [Research Support, Non-U.S. Gov’t].
Baltaci S, Resorlu B, Yagci C, Turkolmez K, Gogus C, Beduk Y. Computerized tomography for detecting perivesical infiltration and lymph node metastasis in invasive bladder carcinoma. Urol Int. 2008;81(4):399–402.
Tilki D, Brausi M, Colombo R, Evans CP, Fradet Y, Fritsche HM, et al. Lymphadenectomy for bladder cancer at the time of radical cystectomy. Eur Urol. 2013;64(2):266–76.
Verma S, Rajesh A, Prasad SR, Gaitonde K, Lall CG, Mouraviev V, et al. Urinary bladder cancer: role of MR imaging. Radiographics. 2012;32(2):371–87 [Research Support, Non-U.S. Gov’t Review].
Tekes A, Kamel I, Imam K, Szarf G, Schoenberg M, Nasir K, et al. Dynamic MRI of bladder cancer: evaluation of staging accuracy. Am J Roentgenol. 2005;184(1):121–7.
Vargas HA, Akin O, Schoder H, Olgac S, Dalbagni G, Hricak H, et al. Prospective evaluation of MRI, (1)(1)C-acetate PET/CT and contrast-enhanced CT for staging of bladder cancer. Eur J Radiol. 2012;81(12):4131–7 [Comparative Study].
Braendengen M, Winderen M, Fossa SD. Clinical significance of routine pre-cystectomy bone scans in patients with muscle-invasive bladder cancer. Br J Urol. 1996;77(1):36–40.
Drieskens O, Oyen R, Van Poppel H, Vankan Y, Flamen P, Mortelmans L. FDG-PET for preoperative staging of bladder cancer. Eur J Nucl Med Mol Imaging. 2005;32(12):1412–7 [Clinical Trial].
Bachor R, Kotzerke J, Reske SN, Hautmann R. Lymph node staging of bladder neck carcinoma with positron emission tomography. Urologe A. 1999;38(1):46–50.
Kibel AS, Dehdashti F, Katz MD, Klim AP, Grubb RL, Humphrey PA, et al. Prospective study of [18F]fluorodeoxyglucose positron emission tomography/computed tomography for staging of muscle-invasive bladder carcinoma. J Clin Oncol. 2009;27(26):4314–20.
Schoder H, Larson SM. Positron emission tomography for prostate, bladder, and renal cancer. Semin Nucl Med. 2004;34(4):274–92 [Review].
Network NCC. Bladder cancer. Fort Washington, PA, Version I. 2013 [cited 2013 September 1]; Available from: www.nccn.org
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2015 Springer Science+Business Media New York
About this chapter
Cite this chapter
Kim, T., Griffin, J.G., Holzbeierlein, J.M., Sexton, W.J. (2015). Imaging in Localized and Advanced Bladder Cancer. In: Konety, B., Chang, S. (eds) Management of Bladder Cancer. Springer, New York, NY. https://doi.org/10.1007/978-1-4939-1881-2_5
Download citation
DOI: https://doi.org/10.1007/978-1-4939-1881-2_5
Published:
Publisher Name: Springer, New York, NY
Print ISBN: 978-1-4939-1880-5
Online ISBN: 978-1-4939-1881-2
eBook Packages: MedicineMedicine (R0)