Abstract
Purpose of Review
Since recognition in 1975, Lyme disease has become the most common vector-borne illness in North America and Europe. The clinical features are well-characterized and treatment is usually curative, but misperceptions about morbidity persist. The purpose of this review is to examine advances in the diagnosis and treatment of Lyme disease, as well as ongoing management challenges.
Recent Findings
It is useful to recognize that Lyme disease occurs in stages, with early- and late-stage disease. Clinical expression is in part determined by Borrelial variability. For example, some strains of Borrelia burgdorferi, the causative organism in North America, are particularly arthritogenic. Most patients with early Lyme disease can be cured with a single course of oral antibiotic therapy, in contrast to some patients with Lyme arthritis, a late-stage manifestation, who are more antibiotic refractory and require other treatment strategies.
Summary
Successful treatment of Lyme disease begins with successful diagnosis and with an understanding of the emergence, clinical features, and impact of Lyme disease over the past half century.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Lyme disease (Lyme borreliosis) (LD) was recognized in the USA in 1976 in Lyme, Connecticut, in children with inflammatory oligo-articular arthritis [1]. In these patients, Lyme arthritis (LA) was usually preceded by a characteristic rash, erythema migrans (EM) [2]. Some patients had systemic symptoms, including peripheral neuropathy associated with meningitis [3]. Also reported were cardiac disease and mild hepatitis [4]. LD was found to be a transmitted by Ixodes ticks and the primary causative organism, Borrelia burgdorferi, was isolated from the tick vector in 1983 [5] and from patients with early LD the following year [6, 7].
It became clear that LD is an emerging infection found throughout the world-wide distribution of Ixodes ticks [8•]. In the USA, LD is caused by B. burgdorferi sensu stricto [6, 7]. In Europe and elsewhere, two other Borrelial genospecies, Borrelia afzelii and Borrelia garinii, cause somewhat different clinical syndromes [9]. B. burgdorferi is associated with more frequent arthritis in the USA than in Europe [10•, 11], but in Europe, B. afzelii causes a characteristic late-stage rash, acrodermatitis chronica atrophicans [12], not seen in the USA, and B. garinii causes more neurological disease than other genospecies [10•].
LD is now the most common vector-borne illness in Europe and North America [13]. In the USA, most cases occur in the mid-Atlantic and southern New England (Maine to Virginia), upper Midwest (Wisconsin and Minnesota), and western states (northern California, Oregon, Nevada [8•, 13]. Over the 40 years since LD was first reported, changes in habitat favorable to the vector have resulted in geographic expansion of Ixodes ticks (Fig. 1) [14] and an increase in cases of LD (Fig. 2) [15].
Ixodes Ticks, United States, 1998 and 2015(14). The red-colored areas are where Ixodes scapularis are established. The blue-colored areas are where Ixodes scapularis are reported. The green-colored areas are where Ixodes pacificus are established. The yellow-colored areas are where Ixodes pacificus are reported
Lyme disease cases reported to the Centers for Disease Control, United States [15]
LD has also been over-diagnosed, over-treated, and often sensationalized [16]. At the same time, surveillance has been hampered by under-reporting to health authorities [17, 18] and actual cases (350,000/annually in the USA) may exceed reported cases (35,000) by 10-fold [19, 20]. All of these factors: (a) the rapid emergence of a new vector-borne infection, (b) inaccurate perceptions by the media and some health providers, and (c) incomplete surveillance have made LD a misunderstood and controversial illness.
But over the half century since LD was recognized, progress in diagnosis and treatment has been substantial. The diagnosis of LD is facilitated by dividing the illness into early- and late-stage disease [21]. Both early LD, which can be localized or disseminated, and late LD (primarily LA in the USA) have well-described clinical manifestations and confirmatory diagnostic testing. For the rheumatologist, LA can be diagnosed by the pattern of joint involvement in the same way that gout or rheumatoid arthritis is recognized.
But uncertainties persist. Overlap can occur between stages. In addition, more prompt diagnosis and treatment, which are almost always curative, are changing the presentation in many patients. Fewer patients move through different stages of the illness, since early treatment is curative and prevents progression. As a result, most LA patients now report no antecedent history of EM [22].
Successful treatment of LD begins with successful diagnosis. For early-stage disease, it is important to have a high index of suspicion and recognize that the sentinel rash, EM, does not always present in a typical pattern [23]. On the other hand, late-stage, “chronic” LD is often over-diagnosed in patients with depression and other disorders [16]. A well-grounded understanding of the clinical manifestations of LD is critical for both diagnosis and a favorable treatment. This review considers the epidemiology, clinical manifestations, diagnosis, both clinical and laboratory, and treatment of LD. Areas of incomplete understanding and controversy are also considered.
The Emergence of Lyme Disease
LD is an emerging infection, but it is probably also a much older disease. There is evidence of B. garinii infection in nineteenth century museum specimen Ixodes ticks, and a European patient with probable LD was reported in 1884 [24]. More remarkably, complete genome sequencing of a 5300-year-old Tyrolean “iceman” demonstrated 60% homology between this individual and the B. burgdorferi genome, consistent with an ancient infection [25] and spirochetes resembling modern Borrelia have been observed in 15-million-year-old amber-fossilized ticks, suggesting the antiquity of the tick-spirochete parasitic relationship [26]. The re-emergence of LD in the modern era is probably the result of habitat modification, especially in Europe and the northeastern USA, that facilitated the enzootic spread of B. burgdorferi infection [27]. Re-forestation of farm land and the rise of suburbs have created open spaces that exponentially increase host species populations [27].
LD occurs throughout the distribution of Ixodes ticks in North America, Europe, and Asia [28]. In North America, 90% of cases occur in New England, mid-Atlantic states, and the upper mid-West [8•], but LD is spreading from high-incidence states to neighboring, low-incidence states and to Canada [29,30,31]. Within endemic regions, smaller geographic areas of very high-incidence B. burgdorferi infection are also found [8•]. The age distribution of LD is bimodal, with peaks at ages 5–15 and 45–55, consistent with risk of tick exposure [8•]. Similarly, activities such as forestry work, hiking, and camping increase risk of infection [14].
Ixodes ticks that transmit LD have a three-stage life cycle; larvae, nymph, and adult, feeding once to progress to the next stage [21]. Rodents, including white-footed mice and chipmunks, are the preferred hosts for both larvae and nymphs and maintain the life cycle of infection [10•]. White-tailed deer are not directly involved in the tick life cycle, but as the primary host for adult I. scapularis mating maintain tick populations [32]. Humans and domestic animals are dead-end hosts [21]. In North America and Europe, the tick life cycle defines the onset of LD in the late spring and early summer, because this is when nymphs actively feed (Fig. 3) [33].
CDC: seasonal incidence of onset of early LD [33]
Diagnosis
The Spectrum of Early Lyme Disease
Tick Bites
All cases of LD are acquired from the bite of an infected tick [10•], but most patients are unaware of the bite which is usually not painful [23, 34]. Even in LD-endemic regions, most Ixodes ticks are not infected and most bites from infected ticks do not transmit LD [35]. Among 247 individuals in a highly endemic area of Westchester County, New York, with untreated, engorged I. scapularis bites, only 3.2% developed LD when followed prospectively [35]. In addition, in this cohort, the risk of acquiring LD was reduced to 0.4% by the prophylactic administration of doxycycline 200 mg orally, if given within 72 h after exposure [35].
Early Lyme Disease
Once LD develops, the clinical manifestations are well-defined. It is useful to recognize stages of illness: (1) Early localized infection, (2) early disseminated infection, and (3) late infection [21]. LD begins in most patients (80%) with EM that only occurs at disease onset [36].
Early Localized Infection
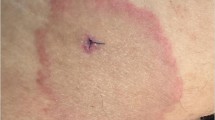
EM develops within a few days (sometimes up to 30 days) after the bite. In early localized infection, the disease is confined to the skin and other manifestations, except mild fever and constitutional symptoms are absent [37]. EM is an expanding erythematous rash, often with a well-demarcated outer border. The primary lesion develops as a centrifugally expanding erythema. The rash is large (typically greater than 5 cm) and may expand over several days (Fig. 4) [23]. The lesion is usually raised, slightly itchy, but not extremely painful. Even in the absence of treatment, EM resolves in less than 30 days [23, 38].
Patterns of erythema migrans [23]. a. Homogeneous. b. Central clearing
EM should not be confused with local irritation from an insect bite, but not all EM rashes have a classical appearance [23]. In addition, a minority of patients do not report EM. In these patients, the rash may be faint, evanescent, or hidden on the scalp. If EM occurs on a toe or on the ear, it will cause only non-specific erythematous swelling. In the past, many EM rashes had a “bulls-eye” appearance, but central clearing takes time to develop and with prompt treatment, this pattern is less common [23]. Sometimes EM causes necrosis and vesicle formation or mimics cellulitis, all of which can be confusing. So in addition to evaluating the appearance of the rash, it is important to diagnose early LD based on a variety of factors: geographic location, seasonality, tick exposure, and extra-cutaneous features [23, 38].
Early Disseminated Infection
In early disseminated infection, there is hematogenous dissemination of the organism, so patients can develop prominent, sometimes severe, constitutional symptoms, including fever, headache, stiff neck, and migratory arthralgias [39]. Secondary skin lesions can develop anywhere on the body. They tend to be smaller than the primary lesion and more transient, with resolution independent of EM [39]. In early disseminated infection, the liver and spleen are affected in 15% of patients, with mild to moderate transaminitis (aspartate transaminase less than 400) that resolves over several weeks [39]. Also seen is dissemination to the heart (myocarditis) and the peripheral nervous system and meninges (cranial and peripheral neuropathies, meningitis and, less commonly, encephalitis) [10•]. Arthralgias occur early, but frank arthritis is considered a late-stage disease manifestation [39]. LD does not cause sino-pulmonary infection or involve the kidney or gastrointestinal tract. Whether early disseminated LD impacts the fetus is unclear, but prompt treatment of pregnant women is recommended [40].
Lyme Carditis
Lyme carditis is a well-characterized manifestation of early disseminated LD [41]. Most patients develop mild myocarditis and AV nodal dysfunction. Fluctuating heart block from 1st degree, to Wenckebach, to complete heart block can occur with Lyme carditis [41]. Although complete heart block develops in 50% of carditis patients, it is usually well tolerated, although fatalities have been reported [42•]. Heart block is always reversible and permanent pacemaker insertion is not recommended [41].
Lyme carditis causing chronic cardiomyopathy in a European patient has been reported [43], but no cases of chronic cardiomyopathy in North America have been validated and a recent French study failed to detect evidence of Borrelial exposure in a large cohort of cardiomyopathy patients in an endemic region [44]. Conceivably, species variability could explain differences in disease expression.
Neurological Disease
In early disseminated LD, there may be neurological involvement [45]. This includes disease of the peripheral nervous system [45]. Cranial neuropathies can occur, particularly facial nerve palsies. This manifestation usually resolves completely, but persistent fascial nerve damage is a distressing morbidity of Lyme disease [46]. Facial nerve palsy may rarely be bilateral [47, 48]. Sixth nerve involvement may present as diplopia [45]. Patients may also have peripheral neuropathy, typically in a mononeuritis pattern, presenting with features such as foot drop or radiculoneuropathy presenting as back pain [3, 45, 49, 50].
Lyme meningitis or, rarely in North America, encephalitis can also occur. Meningitis patients have headache, stiff neck, and fever. Cerebrospinal fluid (CSF) demonstrates a mild to moderate lymphocytic pleocytosis (less than 400 lymphocytes), elevated CSF protein, and an increased CSF antibody index (ratio of CSF to serum B. burgdorferi antibody titers > 1) [10•]. Patients with meningoencephalitis can have acute cognitive impairment, emotional lability, or other alterations in higher cortical function [10•].
A distinction is made between early- and late-stage neurological disease. In North America, late-stage neurological disease is uncommon, but can affect the central nervous system [10•, 50, 51]. There may be cognitive dysfunction, memory loss, and fatigue. Usually, there is evidence of antecedent clinical manifestations, including EM, constitutional symptoms, meningitis, and peripheral neuropathy [49, 52]. Guillian-Barre syndrome [53] and cerebrovascular accidents [54] have been rarely associated with LD. Upper motor neuron disease and demyelinating syndromes have been reported, but causation has not been established [55, 56].
Clinical Features of Lyme Arthritis
LD was recognized in the USA as LA, because of a characteristic pattern of oligo-arthritis [1]. In the past, most patients with LA reported antecedent EM, but this is now less likely, since recognition of early-stage disease leads to curative treatment [22]. Sixty percent of untreated patients with early LD will progress to LA [57] and among LA patients reported to the US Centers for Disease Control and Prevention (CDC) between 1992 and 2006, 32% had LA [58].
LA can cause frank arthritis within weeks of disease onset. More typically, there is an asymptomatic period of several months after early disease, followed by arthritis. This may begin as migratory arthralgias, lasting for months, followed by frank arthritis, or frank arthritis may begin acutely. Most patients have monoarthritis, usually affecting the knee, but an oligo-articular pattern is also seen. LA almost always affects fewer than 5 joints, typically large joints, especially the knee [57].
In a distinct pattern, most LA patients have intermittent attacks of arthritis lasting for days to weeks with resolution between episodes. In about 10%, arthritis is chronic (lasting > 1 year). Large, relatively non-painful, joint effusions, which reaccumulate when aspirated, are typical. In the knee, popliteal cyst rupture is common [57]. In most patients, LA does not cause permanent joint damage, although this can occur in patients with chronic unremitting arthritis [10•].
LA is an infection, but B. burgdorferi has rarely been cultured from synovial fluid [10•]. In a research setting, it is possible, however, to detect B. burgdorferi DNA by polymerase chain reaction (PCR) in synovial fluid in most untreated LA patients. After antibiotic treatment, synovial fluid PCR testing is usually negative [59]. Antibiotic therapy is effective in the majority of cases of Lyme arthritis, but not all patients respond immediately. In some patients, arthritis resolution after treatment may take months [59].
Since not all patients with LA respond to antibiotic treatment, it is useful to divide patients into two groups: antibiotic responsive (successful response to antibiotic treatment <3 months) and antibiotic refractory (treatment response requires > 3 months) [60, 61•]. It is unclear why LA responds differently in the two groups. Emerging evidence suggests that certain North American B. burgdorferi genotypes have exceptional arthritogenic virulence [62]. There may also be host differences in immunogenetic susceptibility to infection [63] and in some patients, persistent LA may result from a post-infectious immune process rather than persistent infection [64]. MicroRNA expression in synovial fluid in LA patients prior to treatment shows a response consistent with bacterial killing, while microRNA expression from antibiotic-treated patients with persistent synovial effusions demonstrates a chronic inflammatory pattern, similar to the signature seen in rheumatoid arthritis (RA) [65••]. It is not possible to prospectively anticipate which patients who will not respond to antibiotic therapy, but the management of antibiotic refractory LA may require non-antibiotic strategies to achieve arthritis resolution [61•].
Several conditions may be considered in the differential diagnosis of LA. Because it often presents as an acute monoarticular knee effusion in an active individual, LA may be confused with an internal derangement, but LA usually does not cause structural damage or exacerbate osteoarthritis. Compared with other forms of bacterial arthritis, LA is usually less painful and less likely to be associated with fever or other constitutional symptoms. Because it is asymmetrical and oligo-articular, LA can mimic juvenile idiopathic arthritis and spondyloarthropathy. Since it usually does not involve small joints in the hands and feet and is neither poly-articular nor symmetrical, LA should not be confused with RA. Occasional patients with RA in LD-endemic areas develop LD. In these patients, the clinical features of LA are the same as in individuals without RA. Some patients with LA subsequently develop RA or other auto-immune inflammatory arthritis, but there is no evidence of causation [66].
Diagnostic Testing
Direct Methods
It is possible to culture B. burgdorferi from EM skin lesions, but such testing is not routinely available or necessary [10•]. In a research setting, B. burgdorferi has been cultured from blood samples in patients with early disseminated LD. As would be expected, in these patients, clinical severity predicts the likelihood of hematogenous disease [67]. In contrast, reports of positive culture results as the sole means to diagnose LD in patients tested after extensive antibiotic treatment have not been substantiated [68]. There are only rare reports of culture of B. burgdorferi from synovial fluid, but B. burgdorferi DNA detected by PCR has been demonstrated in synovial fluid and less reliably in CSF [10•]. Because of lack of standardization, LD PCR testing is not recommended for routine clinical practice [59]. There is ongoing interest in new direct diagnostic techniques, including detection of Borrelial antigens, nucleic acid amplification, and genomic sequencing [69].
Indirect Methods
In spite of inherent limitations, assessment of antibody response against B. burgdorferi is currently the only validated diagnostic testing available for LD [69,70,71,72]. Criteria have been established by the CDC/Association of State and Territorial Public Health Laboratory Directors to determine positive tests for B. burgdorferi by enzyme-linked immunosorbent assay (ELISA) against whole cell sonicate preparations and by Western blot (WB) for both early (IgM)- and late (IgG)-stage disease [73]. These criteria were devised to maximize the sensitivity and specificity of all stages of LD. Testing relies on a two-step algorithm in which WB is used to confirm a positive ELISA, not as a separate test [74]. Simpler, more sensitive, next generation, serologic assays based on recombinant peptides are in development [69]. Two FDA-approved tests that target the immune response against cell surface variable-major-protein-like sequenced expressed (VlsE) and its sixth invariable region, the C6 peptide, have been found to be effective in the diagnosis of early LD [75, 76]. In the future, these assays, alone or in combination, may supplant currently used serological testing [69, 75].
For patients with early disease, it may take up to 4 weeks for a detectable ELISA antibody response to occur. In these patients, early LD should be diagnosed and treated based on clinical suspicion, including geographic location, seasonality, tick exposure, and features of illness whether or not the patient is seropositive. With prompt treatment, some patients, particularly those with early local infection, will remain seronegative [77].
After 4 weeks, virtually, all patients with early-disseminated and late-stage disease seroconvert [10•]. Most patients with LA demonstrate markedly positive ELISA and WB reactions and remain seropositive for years, even after curative antibiotic treatment [10•]. Persistently, positive LD tests, in the absence of other evidence for infection, are not an indication for retreatment. In fact, these patients probably have some degree of protective immunity.
LD testing should be an adjunct to a well-formulated clinical suspicion and not used indiscriminately [78]. At the present time, the predictive value of LD testing is greatly degraded by testing in patients in whom the prevalence of LD is low [79,80,81]. In 2008, the 7 largest US diagnostic laboratories conducted almost 3 million tests for tick-borne diseases [82]. Over-testing and misinterpretation of test results, including positive IgM immunoblots, frequently results in over-treatment [83]. False negative results are possible at disease onset, but the bigger problem is testing individuals with a low likelihood of LD, generating false positive results [81].
Treatment of Lyme Disease
Early Disease Treatment
For early LD (local or disseminated), the goal of antibiotic therapy is to shorten the duration of EM and associated symptoms and to prevent the development of late-stage disease. Most patients with uncomplicated early disease can be cured with oral antibiotic therapy. For EM, the drugs of choice for adults (except pregnant women) are doxycycline, 100 mg orally twice daily for 10 to 21 days or amoxicillin, 250 to 500 mg orally three times daily for 10 to 21 days. The drugs of choice for children with permanent dentition are doxycycline 1 to 2 mg/kg twice daily or amoxicillin 25 to 50 mg/kg three times daily. Amoxicillin is recommended for children lacking permanent dentition [10•, 84].
The range of treatment duration recommended reflects the variability of disease severity in patients with early LD. We treat most patients with mild illness for 10 to 14 days. Because the severity of disease at onset predicts the risk for late-stage manifestations in inadequately treated patients, we recommend two to 3 weeks of therapy for individuals with more-than-mild illness. Some studies suggest that a shorter duration is adequate [85•].
Both doxycycline and amoxicillin are effective. Doxycycline may have better CNS penetration, but is not superior in preventing early neurologic LD [86]. Also, doxycycline is effective against Anaplasma phagocytophilum, the causative organism of human granulocytic anaplasmosis, a possible co-infection [85•]. For patients who cannot be treated with doxycycline or amoxicillin, cefuroxime axetil is an alternative. Azithromycin, clarithromycin, or erythromycin is less effective. First-generation cephalosporins, quinolone antibiotics, and sulfa drugs are ineffective [21].
Neurological Disease
There are limited comparative efficacy studies in Lyme neuroborreliosis [87]. For patients with mild neurological disease, such as isolated facial palsy, oral doxycycline may be an adequate therapy. For most patients with neurological disease, ceftriaxone 2 g intravenously daily for 14 to 28 days is recommended [10•]. Cefotaxime, penicillin G, and doxycycline are alternatives [88]. It should be noted that corticosteroid usage has been associated with adverse long-term outcomes in patients with LD facial palsies [89, 90].
Lyme Carditis
Patients with first-degree heart block and PR interval < 0.3 s are treated orally like other individuals with early LD. For patients with higher grade AV nodal block and PR interval > 0.3 s, parenteral antibiotic therapy (similar to neurological patients) and cardiac monitoring is recommended. Because Lyme carditis resolves without conduction system abnormality, even in patients with complete heart block, permanent pacemaker insertion is not recommended [10•].
Lyme Arthritis
The majority of LA patients (75%) can be treated successfully with oral antibiotic therapy, including doxycycline 100 mg orally twice daily for 30 to 60 days or amoxicillin 500 mg orally three times daily for 30 to 60 days. In patients who do not respond to oral antibiotic therapy, intravenous ceftriaxone, as described for neurological disease, may be effective. Despite oral and intravenous therapy, approximately 10% of patients will be antibiotic refractory. These patients may benefit from anti-inflammatory agents, arthroscopic synovectomy, and disease-modifying drugs, such as methotrexate [58].
Post-treatment Lyme Disease, Chronic Lyme Disease, and Fibromyalgia
Early LD is by far the most common manifestation of LD. Early LD patients are almost always cured, but occasionally, they incur permanent neurological morbidity, such as residual facial palsy or foot drop. Because it is usually a benign disorder, early LD is under-reported to public health authorities [18].
Most cases of late LD in North America involve the joints only [58]. Late CNS LD is rare, over-diagnosed and over-treated [91]. Some patients are diagnosed with “chronic Lyme disease” who do not have LD and some providers make this diagnosis in the absence of well-defined clinical criteria or validated laboratory studies [91,92,93,94]. Not uncommonly, these patients have other medical conditions, including depression and fibromyalgia [16, 95]. They are often given long-term antibiotic therapy or unsubstantiated alternative therapies [91]. There is no evidence that such treatment is beneficial [96, 97•]. In fact, long-term antibiotic treatment and unconventional treatment for “chronic Lyme disease” have been repeatedly associated with adverse events [91]. For this group of patients, supportive care, reassurance, and in some cases, treatment of the underlying condition, including depression, are more likely to be helpful.
Another issue is the problem of “post-Lyme disease treatment syndrome” [98]. In most, but not all studies, patients with early LD disease, including neuroborreliosis, have symptom resolution after treatment with standard regimens and their quality of life is similar to the general population [93, 99, 100]. In one study of 100 culture-confirmed EM patients, fibromyalgia was seen in long-term follow-up in only 1% after antibiotic treatment and fatigue due to LD in 3% [100, 101]. A minority of patients, however, continue to experience common subjective symptoms, such as fatigue, widespread pain, and neurocognitive symptoms after treatment [94]. These patients are likely to continue to seek medical attention [16, 102]. Some investigators differentiate between LD patients with “post-Lyme disease treatment syndrome” who remain symptomatic after 6 months and those whose symptoms resolve more promptly [101]. Is depression a factor? Is Lyme disease any different from other infections (such as pneumonia) where there may be a gradient among patients for the time necessary for symptom resolution?
Conclusion
Over the past half century, LD has emerged in North America, Europe, and throughout the distribution of Ixodes ticks, causing a complex, vector-borne illness. In most patients, LD can be successfully managed, but is important to understand the illness in context; its emergence, geographic spread, clinical features, and treatment. In endemic areas, early LD is a common illness, often requiring a high index of suspicion, particularly in patients with atypical EM. LA presents in a pattern which should be familiar to the rheumatologist and serological confirmation is virtually always present. In some patients, LD is over-diagnosed and over-treated. For these patients, understanding of the spectrum of disease allows more accurate diagnosis and appropriate treatment. As a result of world-wide habitat modification, favorable to the tick vector, LD is here to stay. Progress in diagnosis and treatment should lead to satisfactory outcomes in most patients.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Steere AC, Malawista SE, Snydman DR, Shope RE, Andiman WA, Ross MR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20(1):7–17.
Asbrink E, Hovmark A, Olsson I. Clinical manifestations of acrodermatitis chronica atrophicans in 50 Swedish patients. Zentralbl Bakteriol Mikrobiol Hyg A. 1986;263(1–2):253–61.
Ogrinc K, Lusa L, Lotric-Furlan S, Bogovic P, Stupica D, Cerar T, et al. Course and outcome of early European Lyme neuroborreliosis (Bannwarth syndrome): clinical and laboratory findings. Clin Infect Dis. 2016;63(3):346–53.
Steere AC, Bartenhagen NH, Craft JE, Hutchinson GJ, Newman JH, Rahn DW, et al. The early clinical manifestations of Lyme disease. Ann Intern Med. 1983;99(1):76–82.
Burgdorfer W, Barbour AG, Hayes SF, Benach JL, Grunwaldt E, Davis JP. Lyme disease-a tick-borne spirochetosis? Science. 1982;216(4552):1317–9.
Benach JL, Bosler EM, Hanrahan JP, Coleman JL, Habicht GS, Bast TF, et al. Spirochetes isolated from the blood of two patients with Lyme disease. N Engl J Med. 1983;308(13):740–2.
Steere AC, Grodzicki RL, Kornblatt AN, Craft JE, Barbour AG, Burgdorfer W, et al. The spirochetal etiology of Lyme disease. N Engl J Med. 1983;308(13):733–40.
• Mead PS. Epidemiology of Lyme disease. Infect Dis Clin North Am. 2015;29(2):187–210. This review provides a comprehensive discussion of Lyme disease epidemiology.
Margos G, Vollmer SA, Ogden NH, Fish D. Population genetics, taxonomy, phylogeny and evolution of Borrelia burgdorferi sensu lato. Infect Genet Evol. 2011;11(7):1545–63.
• Steere AC, Strle F, Wormser GP, Hu LT, Branda JA, Hovius JW, et al. Lyme borreliosis. Nat Rev Dis Primers. 2016;2:16090. This review is a thorough update by experts.
Grillon A, Scherlinger M, Boyer PH, De Martino S, Perdriger A, Blasquez A, et al. Characteristics and clinical outcomes after treatment of a national cohort of PCR-positive Lyme arthritis. Semin Arthritis Rheum. 2019;48(6):1105–12.
Maraspin V, Mrvic T, Ruzic-Sabljic E, Jurcic V, Strle F. Acrodermatitis chronica atrophicans in children: report on two cases and review of the literature. Ticks Tick Borne Dis. 2019;10(1):180–5.
Bacon RM, Kugeler KJ, Mead PS. Surveillance for Lyme disease--United States, 1992–2006. Morbidity and mortality weekly report surveillance summaries (Washington, DC : 2002). 2008;57(10):1–9.
Eisen RJ, Eisen L, Beard CB. County-scale distribution of Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the continental United States. J Med Entomol. 2016;53(2):349–86.
Centers for Disease Control. Data and surveillance Lyme disease 2019 [5/18/19]. Available from: https://www.cdc.gov/lyme/stats/index.html. Accessed 1 July 2019.
Reid MC, Schoen RT, Evans J, Rosenberg JC, Horwitz RI. The consequences of overdiagnosis and overtreatment of Lyme disease: an observational study. Ann Intern Med. 1998;128(5):354–62.
Schiffman EK, McLaughlin C, Ray JAE, Kemperman MM, Hinckley AF, Friedlander HG, et al. Underreporting of Lyme and other tick-borne diseases in residents of a high-incidence county, Minnesota, 2009. Zoonoses Public Health. 2018;65(2):230–7.
White J, Noonan-Toly C, Lukacik G, Thomas N, Hinckley A, Hook S, et al. Lyme disease surveillance in New York state: an assessment of case underreporting. Zoonoses Public Health. 2018;65(2):238–46.
Doyle TJ, Glynn MK, Groseclose SL. Completeness of notifiable infectious disease reporting in the United States: an analytical literature review. Am J Epidemiol. 2002;155(9):866–74.
Nelson CA, Saha S, Kugeler KJ, Delorey MJ, Shankar MB, Hinckley AF, et al. Incidence of clinician-diagnosed Lyme disease, United States, 2005-2010. Emerg Infect Dis. 2015;21(9):1625–31.
Steere AC. Lyme disease. N Engl J Med. 1989;321(9):586–96.
Schoen RT. A case revealing the natural history of untreated Lyme disease. Nat Rev Rheumatol. 2011;7(3):179–84.
Smith RP, Schoen RT, Rahn DW, Sikand VK, Nowakowski J, Parenti DL, et al. Clinical characteristics and treatment outcome of early Lyme disease in patients with microbiologically confirmed erythema migrans. Ann Intern Med. 2002;136(6):421–8.
Matuschka FR, Ohlenbusch A, Eiffert H, Richter D, Spielman A. Antiquity of the Lyme-disease spirochaete in Europe. Lancet. 1995;346(8986):1367.
Keller A, Graefen A, Ball M, Matzas M, Boisguerin V, Maixner F, et al. New insights into the Tyrolean Iceman’s origin and phenotype as inferred by whole-genome sequencing. Nat Commun. 2012;3:698.
Poinar G. Spirochete-like cells in a Dominican amber Ambylomma tick (Arachnida: Ixodidae). Hist Biol. 2015;27(5):565–70.
Spielman A. The emergence of Lyme disease and human babesiosis in a changing environment. Ann N Y Acad Sci. 1994;740:146–56.
Stanek G, Wormser GP, Gray J, Strle F. Lyme borreliosis. Lancet. 2012;379(9814):461–73.
Clow KM, Leighton PA, Ogden NH, Lindsay LR, Michel P, Pearl DL, et al. Northward range expansion of Ixodes scapularis evident over a short timescale in Ontario. Canada PLoS One. 2017;12(12):e0189393.
Gasmi S, Ogden NH, Lindsay LR, Burns S, Fleming S, Badcock J, et al. Surveillance for Lyme disease in Canada: 2009-2015. Canada communicable disease report =. Releve des maladies transmissibles au Canada. 2017;43(10):194–9.
Schwartz AM, Hinckley AF, Mead PS, Hook SA, Kugeler KJ. Surveillance for Lyme disease - United States, 2008–2015. Morbidity and mortality weekly report surveillance summaries (Washington, DC : 2002). 2017;66(22):1–12.
Rand PW, Lubelczyk C, Lavigne GR, Elias S, Holman MS, Lacombe EH, et al. Deer density and the abundance of Ixodes scapularis (Acari: Ixodidae). J Med Entomol. 2003;40(2):179–84.
Centers for Disease Control. Lyme disease—confirmed cases by month of disease onset, United States, 2001-2017 2018 [Available from: https://www.cdc.gov/lyme/stats/graphs.html.
Nigrovic LE, Neville DN, Balamuth F, Bennett JE, Levas MN, Garro AC. A minority of children diagnosed with Lyme disease recall a preceding tick bite. Ticks Tick Borne Dis. 2019;10(3):694–6.
Nadelman RB, Nowakowski J, Fish D, Falco RC, Freeman K, McKenna D, et al. Prophylaxis with single-dose doxycycline for the prevention of Lyme disease after an Ixodes scapularis tick bite. N Engl J Med. 2001;345(2):79–84.
Steere AC, Sikand VK. The presenting manifestations of Lyme disease and the outcomes of treatment. N Engl J Med. 2003;348(24):2472–4.
Wormser GP, Sudhindra P, Lopez E, Patel L, Rezai S, Brumbaugh AD, et al. Fatigue in patients with erythema migrans. Diagn Microbiol Infect Dis. 2016;86(3):322–6.
Nadelman RB. Erythema migrans. Infect Dis Clin N Am. 2015;29(2):211–39.
Steere AC. Lyme disease. N Engl J Med. 2001;345(2):115–25.
Waddell LA, Greig J, Lindsay LR, Hinckley AF, Ogden NH. A systematic review on the impact of gestational Lyme disease in humans on the fetus and newborn. PLoS One. 2018;13(11):e0207067.
Robinson ML, Kobayashi T, Higgins Y, Calkins H, Melia MT. Lyme carditis. Infect Dis Clin N Am. 2015;29(2):255–68.
• Three sudden cardiac deaths associated with Lyme carditis - United States, November 2012–July 2013. MMWR morbidity and mortality weekly report. 2013;62(49):993–6. A report of 3 deaths attibutable to Lyme disease.
Stanek G, Klein J, Bittner R, Glogar D. Isolation of Borrelia burgdorferi from the myocardium of a patient with longstanding cardiomyopathy. N Engl J Med. 1990;322(4):249–52.
N’Guyen Y, Lesaffre F, Metz D, de Martino S, Jaulhac B, Andreoletti L. No serological evidence for Borrelia burgdorferi sensu lato infection in patients with dilated cardiomyopathy in Northern France. Infectious diseases (London, England). 2016;48(10):763–4.
Pachner AR. CNS Lyme disease. Neurology. 1992;42(9):1849–50.
Clark JR, Carlson RD, Sasaki CT, Pachner AR, Steere AC. Facial paralysis in Lyme disease. Laryngoscope. 1985;95(11):1341–5.
Wong K, Sequeira S, Bechtel K. Pediatric bilateral facial paralysis: an unusual presentation of Lyme disease. Pediatric emergency care. 2018.
Gaudin RA, Jowett N, Banks CA, Knox CJ, Hadlock TA. Bilateral facial paralysis: a 13-year experience. Plast Reconstr Surg. 2016;138(4):879–87.
Tseng YJ, DeMaria A, Jr., Goldmann DA, Mandl KD. Claims-based diagnostic patterns of patients evaluated for Lyme disease and given extended antibiotic therapy. Vector borne and zoonotic diseases (Larchmont, NY). 2017;17(2):116–22.
Marques AR. Lyme neuroborreliosis. Continuum (Minneap Minn). 2015;21(6 Neuroinfectious Disease):1729–44.
Halperin JJ. Lyme neuroborreliosis. Curr Opin Infect Dis. 2019;32(3):259–64.
Halperin JJ. Neuroborreliosis. Neurol Clin. 2018;36(4):821–30.
Patel K, Shah S, Subedi D. Clinical association: Lyme disease and Guillain-Barre syndrome. The American Journal of Emergency Medicine. 2017;35(10):1583.e1–2.
Monteventi O, Steinlin M, Regenyi M, Roulet-Perez E, Weber P, Fluss J. Pediatric stroke related to Lyme neuroborreliosis: data from the Swiss NeuroPaediatric Stroke Registry and literature review. Eur J Paediatr Neurol. 2018;22(1):113–21.
Visser AE, Verduyn Lunel FM, Veldink JH, van den Berg LH. No association between Borrelia burgdorferi antibodies and amyotrophic lateral sclerosis in a case-control study. Eur J Neurol. 2017;24(1):227–30.
Logigian EL, Kaplan RF, Steere AC. Chronic neurologic manifestations of Lyme disease. N Engl J Med. 1990;323(21):1438–44.
Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107(5):725–31.
Schoen R. Musculoskeletal manifestations of Lyme disease. Waltham, MA2009-Present.
Li X, McHugh GA, Damle N, Sikand VK, Glickstein L, Steere AC. Burden and viability of Borrelia burgdorferi in skin and joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 2011;63(8):2238–47.
Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54(10):3079–86.
• Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin North Am. 2015;29(2):269–80. A review of the patterns of Lyme arthritis.
Jones KL, McHugh GA, Glickstein LJ, Steere AC. Analysis of Borrelia burgdorferi genotypes in patients with Lyme arthritis: high frequency of ribosomal RNA intergenic spacer type 1 strains in antibiotic-refractory arthritis. Arthritis Rheum. 2009;60(7):2174–82.
Steere AC, Klitz W, Drouin EE, Falk BA, Kwok WW, Nepom GT, et al. Antibiotic-refractory Lyme arthritis is associated with HLA-DR molecules that bind a Borrelia burgdorferi peptide. J Exp Med. 2006;203(4):961–71.
Borchers AT, Keen CL, Huntley AC, Gershwin ME. Lyme disease: a rigorous review of diagnostic criteria and treatment. J Autoimmun. 2015;57:82–115.
•• Lochhead RB, Strle K, Kim ND, Kohler MJ, Arvikar SL, Aversa JM, et al. MicroRNA expression shows inflammatory dysregulation and tumor-like proliferative responses in joints of patients with postinfectious Lyme arthritis. Arthritis Rheumatol (Hoboken, NJ). 2017;69(5):1100–10. MicroRNA expression in Lyme arthritis synovium changes with disease persistence.
Arvikar SL, Crowley JT, Sulka KB, Steere AC. Autoimmune arthritides, rheumatoid arthritis, psoriatic arthritis, or peripheral spondyloarthritis following Lyme disease. Arthritis Rheumatol (Hoboken, NJ). 2017;69(1):194–202.
Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D, et al. Brief communication: hematogenous dissemination in early Lyme disease. Ann Intern Med. 2005;142(9):751–5.
Wormser GP, Shapiro ED, Strle F. Studies that report unexpected positive blood cultures for Lyme borrelia - are they valid? Diagn Microbiol Infect Dis. 2017;89(3):178–81.
Branda JA, Body BA, Boyle J, Branson BM, Dattwyler RJ, Fikrig E, et al. Advances in serodiagnostic testing for Lyme disease are at hand. Clin Infect Dis. 2018;66(7):1133–9.
Theel ES. The past, present, and (possible) future of serologic testing for Lyme disease. J Clin Microbiol. 2016;54(5):1191–6.
Alby K, Capraro GA. Alternatives to serologic testing for diagnosis of Lyme disease. Clin Lab Med. 2015;35(4):815–25.
Alasel M, Keusgen M. Promising alternatives for one-tier testing of Lyme borreliosis. Clinica chimica acta; international journal of clinical chemistry. 2018;479:148–54.
Recommendations for test performance and interpretation from the Second National Conference on Serologic Diagnosis of Lyme Disease. MMWR Morbidity and mortality weekly report. 1995;44(31):590–1.
Marques AR. Laboratory diagnosis of Lyme disease: advances and challenges. Infect Dis Clin N Am. 2015;29(2):295–307.
Moore A, Nelson C, Molins C, Mead P, Schriefer M. Current guidelines, common clinical pitfalls, and future directions for laboratory diagnosis of Lyme disease, United States. Emerg Infect Dis. 2016;22(7).
Nigrovic LE, Lipsett SC, Molins CR, Wormser GP, Bennett JE, Garro AC, et al. Higher C6 enzyme immunoassay index values correlate with a diagnosis of noncutaneous Lyme disease. Diagn Microbiol Infect Dis. 2019;94(2):160–4.
Rebman AW, Crowder LA, Kirkpatrick A, Aucott JN. Characteristics of seroconversion and implications for diagnosis of post-treatment Lyme disease syndrome: acute and convalescent serology among a prospective cohort of early Lyme disease patients. Clin Rheumatol. 2015;34(3):585–9.
Markowicz M, Kivaranovic D, Stanek G. Testing patients with non-specific symptoms for antibodies against Borrelia burgdorferi sensu lato does not provide useful clinical information about their aetiology. Clin Microbiol Infect. 2015;21(12):1098–103.
Lee-Lewandrowski E, Chen Z, Branda J, Baron J, Kaufman HW. Laboratory blood-based testing for Lyme disease at a national reference laboratory. Am J Clin Pathol. 2019;152(1):91–6.
Leeflang MM, Ang CW, Berkhout J, Bijlmer HA, Van Bortel W, Brandenburg AH, et al. The diagnostic accuracy of serological tests for Lyme borreliosis in Europe: a systematic review and meta-analysis. BMC Infect Dis. 2016;16:140.
Lantos PM, Branda JA, Boggan JC, Chudgar SM, Wilson EA, Ruffin F, et al. Poor positive predictive value of Lyme disease serologic testing in an area of low disease incidence. Clin Infect Dis. 2015;61(9):1374–80.
Connally NP, Hinckley AF, Feldman KA, Kemperman M, Neitzel D, Wee SB, et al. Testing practices and volume of non-Lyme tickborne diseases in the United States. Ticks Tick Borne Dis. 2016;7(1):193–8.
Webber BJ, Burganowski RP, Colton L, Escobar JD, Pathak SR, Gambino-Shirley KJ. Lyme disease overdiagnosis in a large healthcare system: a population-based, retrospective study. Clin Microbiol Infect. 2019.
Treatment of Lyme disease. Jama. 2016;315(22):2461–2.
• Sanchez E, Vannier E, Wormser GP, Hu LT. Diagnosis, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: a review. JAMA. 2016;315(16):1767–77. A review focusing on Lyme disease treatment.
Strle F, Stupica D, Bogovic P, Visintainer P, Wormser GP. Is the risk of early neurologic Lyme borreliosis reduced by preferentially treating patients with erythema migrans with doxycycline? Diagn Microbiol Infect Dis. 2018;91(2):156–60.
Cadavid D, Auwaerter PG, Rumbaugh J, Gelderblom H. Antibiotics for the neurological complications of Lyme disease. The Cochrane database of systematic reviews. 2016;12:Cd006978.
Dersch R, Freitag MH, Schmidt S, Sommer H, Rauer S, Meerpohl JJ. Efficacy and safety of pharmacological treatments for acute Lyme neuroborreliosis - a systematic review. Eur J Neurol. 2015;22(9):1249–59.
Jowett N, Gaudin RA, Banks CA, Hadlock TA. Steroid use in Lyme disease-associated facial palsy is associated with worse long-term outcomes. Laryngoscope. 2017;127(6):1451–8.
Wormser GP, McKenna D, Scavarda C, Karmen C. Outcome of facial palsy from Lyme disease in prospectively followed patients who had received corticosteroids. Diagn Microbiol Infect Dis. 2018;91(4):336–8.
Lantos PM. Chronic Lyme disease. Infect Dis Clin N Am. 2015;29(2):325–40.
Aguero-Rosenfeld ME, Wormser GP. Lyme disease: diagnostic issues and controversies. Expert Rev Mol Diagn. 2015;15(1):1–4.
Dersch R, Sarnes AA, Maul M, Hottenrott T, Baumgartner A, Rauer S, et al. Quality of life, fatigue, depression and cognitive impairment in Lyme neuroborreliosis. J Neurol. 2015;262(11):2572–7.
Weitzner E, McKenna D, Nowakowski J, Scavarda C, Dornbush R, Bittker S, et al. Long-term assessment of post-treatment symptoms in patients with culture-confirmed early Lyme disease. Clin Infect Dis. 2015;61(12):1800–6.
Wills AB, Spaulding AB, Adjemian J, Prevots DR, Turk SP, Williams C, et al. Long-term follow-up of patients with Lyme disease: longitudinal analysis of clinical and quality-of-life measures. Clin Infect Dis. 2016;62(12):1546–51.
Berende A, Nieuwenhuis L, Ter Hofstede HJM, Vos FJ, Vogelaar ML, Tromp M, et al. Cost-effectiveness of longer-term versus shorter-term provision of antibiotics in patients with persistent symptoms attributed to Lyme disease. PLoS One. 2018;13(4):e0195260.
• Berende A, ter Hofstede HJ, Vos FJ, van Middendorp H, Vogelaar ML, Tromp M, et al. Randomized trial of longer-term therapy for symptoms attributed to Lyme disease. N Engl J Med. 2016;374(13):1209–20. A study demonstrating lack of benefit for long-term antibiotic therapy.
Aucott JN. Posttreatment Lyme disease syndrome. Infect Dis Clin N Am. 2015;29(2):309–23.
Rebman AW, Bechtold KT, Yang T, Mihm EA, Soloski MJ, Novak CB, et al. The clinical, symptom, and quality-of-life characterization of a well-defined group of patients with posttreatment Lyme disease syndrome. Front Med. 2017;4:224.
Wormser GP, Weitzner E, McKenna D, Nadelman RB, Scavarda C, Nowakowski J. Long-term assessment of fatigue in patients with culture-confirmed Lyme disease. Am J Med. 2015;128(2):181–4.
Wormser GP, Weitzner E, McKenna D, Nadelman RB, Scavarda C, Molla I, et al. Long-term assessment of health-related quality of life in patients with culture-confirmed early Lyme disease. Clin Infect Dis. 2015;61(2):244–7.
Adrion ER, Aucott J, Lemke KW, Weiner JP. Health care costs, utilization and patterns of care following Lyme disease. PLoS One. 2015;10(2):e0116767.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Dr. Schoen has no conflicts of interest to disclose.
Human and Animal Rights and Informed Consent
All reported studies/experiments with human or animal subjects performed by the authors have been previously published and complied with all applicable ethical standards (including the Helsinki declaration and its amendments, institutional/national research committee standards, and international/national/ institutional guidelines).
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Infection and Arthritis
Rights and permissions
About this article
Cite this article
Schoen, R.T. Challenges in the Diagnosis and Treatment of Lyme Disease. Curr Rheumatol Rep 22, 3 (2020). https://doi.org/10.1007/s11926-019-0857-2
Published:
DOI: https://doi.org/10.1007/s11926-019-0857-2