Abstract
-
Lyme disease (also known as Lyme borreliosis) is the most common vector-borne illness in the United States.
-
Lyme disease is now epidemic in certain geographic areas in the northeastern United States and is estimated to cause nearly half a million infections annually (Kugeler et al., Emerg Infect Dis. 27:616–19, 2021).
-
The causative agent is the spirochete Borrelia burgdorferi, which is transmitted by the bite of Ixodes scapularis or Ixodes pacificus ticks.
-
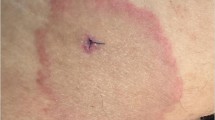
The initial disease manifestation in many patients is an expanding erythema migrans (EM) rash (Fig. 43.1) at the site of the tick bite.
-
Within weeks, if untreated, the spirochete may disseminate throughout the body and cause systemic symptoms and neurologic or cardiac manifestations.
-
Arthritis is a late manifestation of Lyme disease, which occurs a mean of 6 months, but as long as 2 years following the tick bite.
-
Lyme arthritis was first recognized following an outbreak of monoarticular and oligoarticular arthritis in children in Lyme, Connecticut in the 1970s (Steere et al., Arthritis Rheum. 20:7–17, 1977).
-
Prior to the use of antibiotic therapy, about 60% of untreated patients developed Lyme arthritis (Steere et al., Ann Intern Med. 107:725–31, 1987). Although arthritis may now be prevented by antibiotic treatment of early infection, joint involvement is still reported in approximately one-third of the cases reported to the Centers for Disease Control and Prevention (CDC) (Schwartz et al., MMWR Surveill Summ. 66:1–12, 2017).
Access provided by Autonomous University of Puebla. Download chapter PDF
Similar content being viewed by others
Keywords
- Lyme disease
- Borrelia burgdorferei
- Spirochete
- Ixodes scapularis
- Ixodes pacificus
- Lyme arthritis
- Neuroborreliosis
- Babesiosis
- Enzyme immunoassay
- Western blot immunoassay
- Erythema migrans
- Baker’s cyst
- Bell’s palsy
- Doxycycline
- Ceftriaxone
- Post-antibiotic Lyme arthritis
- Post-treatment Lyme disease syndrome
-
Lyme disease (also known as Lyme borreliosis) is the most common vector-borne illness in the United States.
-
Lyme disease is now epidemic in certain geographic areas in the northeastern United States and is estimated to cause nearly half a million infections annually (Kugeler et al. 2021).
-
The causative agent is the spirochete Borrelia burgdorferi, which is transmitted by the bite of Ixodes scapularis or Ixodes pacificus ticks.
-
The initial disease manifestation in many patients is an expanding erythema migrans (EM) rash (Fig. 43.1) at the site of the tick bite.
-
Within weeks, if untreated, the spirochete may disseminate throughout the body and cause systemic symptoms and either neurologic or cardiac manifestations.
-
Arthritis is a late manifestation of Lyme disease, which occurs a mean of 6 months, but as long as 2 years following the tick bite.
-
Lyme arthritis was first recognized following an outbreak of monoarticular and oligoarticular arthritis in children in Lyme, Connecticut in the 1970s (Steere et al. 1977).
-
Prior to the use of antibiotic therapy, about 60% of untreated patients developed Lyme arthritis (Steere et al. 1987). Although arthritis may now be prevented by antibiotic treatment of early infection, joint involvement is still reported in approximately one-third of the cases reported to the Centers for Disease Control and Prevention (CDC) (Schwartz et al. 2017).
Epidemiology/Prevention
Myth
Lyme disease can be acquired anywhere in the United States.
Reality: The majority of Lyme disease cases come from high-incidence areas and their surrounding regions, particularly in the Northeastern, Mid-Atlantic, and Upper Midwest areas of the United States (Fig. 43.2). During 2008–2015, 14 states accounted for approximately 95% of all reported cases in the United States (Schwartz et al. 2017).
Reported cases of Lyme disease in the United States, 2019. (Source: CDC, https://www.cdc.gov/lyme/datasurveillance/maps-recent.html)
Myth
Lyme disease is usually accompanied by co-infections.
Reality: Early Lyme disease may be accompanied by co-infections such as babesiosis, anaplasmosis, or Borrelia myiamotoi infection, but the reported incidence of such infections has ranged from 4% to 20% of patients with erythema migrans (Wormser et al. 2019). Thus, the majority of cases of Lyme disease do not involve co-infections. Co-infection generally occurs in early Lyme disease and is almost never present in the setting of Lyme arthritis. Babesiosis is the most common infection detected concurrently with Lyme disease.
It is also a misconception that Bartonella infection is often observed simultaneously in Lyme disease patients. Bartonella infection is not transmitted by ticks and is not anticipated to be a co-infection in patients with Lyme disease.
Clues to presence of co-infection include:
-
High fever or fever that persists despite treatment of presumed or confirmed Lyme disease with doxycycline
-
Laboratory abnormalities not expected with Lyme disease alone (Examples: cytopenias, abnormalities of liver function tests, and findings compatible with hemolysis, such as anemia or elevated concentrations indirect bilirubin or lactate dehydrogenase)
-
Acquisition of Lyme disease in certain geographic areas known to be “hot spots” for pathogens causing concomitant infections
Myth
All patients should receive preventative antibiotics following a tick bite.
Reality: Patients may be offered prophylaxis for high-risk tick bites (one-time 200 mg dose of oral doxycycline), occurring within 72 h of removal (Lantos et al. 2021). In children, including those <8 years of age, doxycycline 4.4 mg/kg up to the adult dose of 200 mg may be given in a single dose. Patients should monitor the site for the development of erythema migrans.
A high-risk tick bite consists of the following features:
-
Exposure in a highly endemic area
-
Tick attachment for ≥36 h
-
An engorged nymphal Ixodes scapularis tick
Individual measures to avoid tick exposures, including repellants containing DEET or permethrin and the wearing of protective clothing, are recommended for individuals at high-risk of encountering ticks.
Diagnosis
Pearl
The gold-standard for the diagnosis of Lyme disease is two-tier serologic testing in blood.
Comment: A two-test method for detecting IgM and IgG antibodies in serum is recommended by the CDC for support of the diagnosis of Lyme disease (Fig. 43.3). Generally, the first test is an enzyme immunoassay (EIAs). For samples with a positive or equivocal result, a second test—the Western blot immunoassay—is performed. Recently, the FDA cleared a two-test approach involving two EIAs as an acceptable alternative for the second test rather than a Western blot. This is referred to now as “modified” two-tier testing (Mead et al. 2019). For Lyme arthritis, however, contrasted with the detection of Lyme disease infections in general, the authors prefer the standard method: an EIA followed by a Western blot.
During the first several weeks of infection, when most patients have erythema migrans, the incidence of false-negative tests is high. In such patients, the diagnosis can often be made on a clinical basis. By the time of convalescence, however, the majority of patients who had erythema migrans rashes have positive IgM tests for Lyme disease. In patients with systemic organ involvement, a positive IgG result is universally found by the 4- to 8-week time point after infection.
After 4–8 weeks, the diagnosis of Lyme disease should not be based on IgM positivity alone: a positive IgG test is required to support the diagnosis. A positive IgM response occurring in the absence of IgG weeks to months after the onset of symptoms is either a false-positive result or indicates past infection.
Finally, it is important to recognize that many non-approved tests exist for Lyme disease. Methods that are not recommended include serologic testing of synovial fluid, urine testing, NK cell assays, and alternative criteria for interpretation of Western blot, among others.
Pearl
The diagnosis of Lyme arthritis is based on a positive IgG antibody response in blood.
Comment: Although patients with early Lyme disease may initially be seronegative, patients with Lyme arthritis universally are seropositive for IgG antibodies to Lyme disease. IgM positivity may also be present, but it is not required for the diagnosis of Lyme arthritis. IgM positivity alone without the presence of IgG antibodies may be seen in early Lyme disease but does not support of a diagnosis of Lyme arthritis. Given that Lyme arthritis is a late disease manifestation of the disease, Lyme arthritis patients usually have highly expanded IgG antibody responses, generally featuring seven to ten IgG bands on Western blot (Fig. 43.4). It is not recommended to check antibody responses in synovial fluid.
Highly expanded IgG responses via Western Blot in Lyme arthritis. The above figure demonstrates that IgG antibody responses are highly expanded on Western blot testing. A typical Lyme arthritis patient (third row) with reactivity to nine of ten IgG bands is shown, along with positive and negative controls, on a ViraStripe blot
Myth
A positive polymerase chain reaction (PCR) test in synovial fluid is required for the diagnosis of Lyme arthritis.
Reality: PCR testing for B. burgdorferi DNA in synovial fluid is often positive (~70% of patients) prior to antibiotic treatment (Li et al. 2011). This testing may be helpful to support the diagnosis, especially if the joint in question is being aspirated. Since a positive serologic test result typically persists for years after Lyme arthritis, a positive PCR result may be helpful in establishing whether the patient has a recurrent episode of Lyme arthritis. However, PCR testing is not standardized for routine clinical use and is not required for the diagnosis. Moreover, PCR testing may remain positive for weeks to several months after spirochetal killing. Therefore, PCR testing is not a reliable surrogate marker for active infection.
Myth
The preferred diagnostic test to diagnose central nervous system Lyme disease is PCR assay for the organism on cerebrospinal fluid.
Comment: In neuroborreliosis caused by Lyme disease, only 10–15% of cerebrospinal samples are positive in patients known to have Lyme meningitis. PCR testing of the cerebrospinal fluid is, therefore, not recommended if Lyme neuroborreliosis is suspected. On the other hand, the finding of an elevated cerebrospinal fluid protein in the proper clinical setting (compatible neurological symptoms or signs and positive Lyme assays on peripheral blood) can provide compelling evidence that B. burgdorferi is the etiology of a patient’s neurological presentation.
Myth
Antibody responses to Borrelia burgdorferi become negative with successful treatment.
Reality: Although the quantities of antibodies as determined by ELISA decline into low-positive ranges after spirochetal killing, Western blot results—which represent a nonquantitative test—do not change much after successful treatment (or they change very slowly. In fact, in patients with previous Lyme arthritis, positive serologic responses with reactivity with many spirochetal proteins often persist for years and even decades after successful treatment and clearance of infection (Kalish et al. 2001). Thus, resolution of the serologic response cannot be used as criteria for successful treatment of the infection.
Lyme Arthritis
Myth
Lyme arthritis presents most commonly in summer months.
Reality: Early Lyme disease typically occurs in summer months, with a peak of new cases in July (Schwartz et al. 2017). However, Lyme arthritis is a late manifestation of the disease and can, therefore, present at any time of the year.
Myth
Lyme arthritis is unlikely in the absence of either a history of a tick bite or an erythema migrans rash.
Reality: Because the recognition and appropriate treatment of early Lyme disease prevents subsequent arthritis, Lyme arthritis is often the presenting manifestation of the illness. In such patients, early disease is asymptomatic, and a history of tick bite or EM rash is often lacking. These patients have the expanded antibody response associated with Lyme arthritis, suggesting that they were infected months prior to the onset of arthritis. Thus, the diagnosis of Lyme arthritis should be considered in patients from endemic areas presenting with a monoarticular or oligoarticular arthritis, whether or not there is any history of an antecedent tick bite or EM rash.
Pearl
Lyme arthritis is most commonly a mono- or oligo-arthritis affecting one or both knees.
Comment: Patients with Lyme arthritis have swelling and pain in one or a few large joints, most commonly one or both knees (Fig. 43.5), though a few other joints may be involved. The affected knee is typically very swollen, but the swelling is usually out-of-proportion to the pain. Ruptured Baker’s cysts are common. Lyme arthritis does not present as a symmetrical polyarthritis (as rheumatoid arthritis does). Lyme arthritis may be preceded by brief attacks of arthritis lasting only days or several weeks, but is typically followed by longer attacks. Patients with early Lyme disease may have arthralgias, but frank arthritis is rare during early disease.
Myth
Patients with Lyme arthritis may have numerous systemic symptoms and other manifestations of Lyme disease such as carditis and Bell’s palsy.
Reality: Patients with Lyme arthritis rarely have prominent systemic symptoms such as fever. Furthermore, because manifestations of acute neurologic involvement such as meningitis, facial palsy, sensory polyneuropathy, or radiculoneuropathy occur during early disseminated infection, patients with those complications rarely have concurrent Lyme arthritis. Patients with Lyme carditis also seldom have Lyme arthritis, for the same reason.
Myth
A patient with Lyme arthritis who describes fatigue and cognitive slowing must have neuroborreliosis.
Reality: As in other infections (e.g., pneumonia, influenza) or inflammatory states (e.g., active rheumatoid arthritis, inflammatory bowel disease), patients with systemic inflammation often feel tired and may perceive cognitive slowing. This seldom reflects brain inflammation or infection caused by the spirochete.
Pearl
The initial recommended treatment for Lyme arthritis is a 28-day course of oral antibiotic.
Comment: The recommended oral antibiotic choices are oral doxycycline, 100 mg twice daily, or amoxicillin, 500 mg three times daily (Lantos et al. 2021). Cefuroxime axetil, 500 mg twice daily, is an alternative for patients unable to take doxycycline or amoxicillin. A longer duration (28 days) of therapy is recommended for Lyme arthritis, in contrast to a 10- to 14-day course of treatment for erythema migrans.
Myth
If a patient with Lyme arthritis responds minimally to 1 month of doxycycline, it is recommended to treat with a longer course of doxycycline.
Reality: If a patient has a partial response to oral doxycycline, it is reasonable to treat with a second month. If the patient’s response to a 1-month course of doxycycline is minimal, however, the recommended course is to treat with intravenous (IV) ceftriaxone, rather than further courses of doxycycline (Lantos et al. 2021). The course of IV ceftriaxone recommended is 2 g daily for 28 days.
Myth
Most patients with Lyme arthritis do not respond to antibiotic therapy and require treatment with disease-modifying antirheumatic drugs (DMARDs).
Reality: Most patients with Lyme arthritis DO respond to oral antibiotics or, if necessary, to intravenous antibiotics administered according to recommended regimens. A small subset of patients, however, may have persistent synovitis despite 2–3 months of oral and IV antibiotics. This situation is termed post-antibiotic (post-infectious or antibiotic-refractory) Lyme arthritis (Steere and Angelis 2006; Steere and Arvikar 2022).
Post-antibiotic Lyme arthritis is an immune-mediated process in which the histopathology is similar to that of other forms of chronic inflammatory arthritis, such as rheumatoid arthritis. Post-antibiotic Lyme arthritis patients usually respond well to DMARD therapy. The choice for DMARD is usually methotrexate, but hydroxychloroquine may be used for milder cases (Steere and Arvikar 2022). TNF inhibitors are prescribed for those who have methotrexate contraindications or do not respond completely to methotrexate. Synovectomy is an option for synovitis limited to one joint. An algorithm for treatment of Lyme arthritis is shown in Fig. 43.6. Risk factors for post-antibiotic Lyme arthritis may include (Lochhead et al. 2021):
-
Infection with highly inflammatory strains of B. burgdorferi (e.g., OspC type A), which are more common in the northeastern United States
-
Retained inflammatory spirochetal antigens
-
Host genetic factors such as TLR1 polymorphism (1805GG) and certain HLA-DR alleles
-
An imbalance of CD4+ T effector and regulatory cells
-
T and B cell responses to Lyme disease-associated autoantigens
-
Some combination of the above factors leading to an excessive, dysregulated pro-inflammatory response characterized by high levels of IFN-gamma and inadequate levels of IL-10, an anti-inflammatory cytokine.
Algorithm for the treatment of Lyme arthritis. Patients with Lyme arthritis should be treated initially with a 28-day course of oral doxycycline, 100 mg twice daily, or amoxicillin, 500 mg three times daily. For those with partial but not full improvement, an additional month of doxycycline could be considered. If there is minimal or no response to doxycycline, a 1-month course of IV ceftriaxone may be given. For those with moderate–severe arthritis following IV antibiotic, disease-modifying antirheumatic drug treatment is recommended
Myth
Treatment with DMARDs may cause reactivation of infection in patients with Lyme arthritis.
Reality: Patients who have been treated with recommended oral and IV antibiotic regimens rarely have evidence of persistent infection. PCR testing of synovial fluid for B. burgdorferi DNA is usually negative after antibiotic treatment, and both culture and PCR testing of synovial tissue have been uniformly negative from synovectomy specimens obtained months to several years after antibiotic therapy (Li et al. 2011). Patients with post-antibiotic Lyme arthritis usually respond well to DMARD therapy without recurrence of infection (Steere and Angelis 2006).
Myth
Patients with post-infectious Lyme arthritis may need life-long treatment with DMARDs.
Reality: Unlike rheumatoid arthritis, patients with Lyme arthritis do not require chronic treatment with DMARDs. Arthritis usually resolves in 6–12 months with DMARD treatment.
Pearl
Adjunctive therapies such as physical therapy are beneficial for Lyme arthritis patients.
Comment: The combination of inflammation in a knee joint with inability to exercise commonly leads to quadriceps atrophy, particularly in adult patients with Lyme arthritis. Therefore, following the completion of antibiotic treatment and resolution of joint inflammation, formal physical therapy that focuses upon quadriceps strengthening is often necessary and can hasten recovery.
Myth
Other tick-borne diseases may be the cause of arthritis.
Reality: Other Ixodes-borne illnesses such as anaplasmosis, babesiosis, Borrelia miyamotoi disease, and the Powassan virus generally cause an acute febrile illness. Although painful arthralgias and myalgias may accompany some of these conditions, the frank arthritis that is observed in Lyme arthritis is not seen. If present, this would raise concern for co-infection with Lyme disease.
Post-Lyme Disease Symptoms
Myth
Most patients have persistent, disabling symptoms after Lyme disease.
Reality: The majority of patients with Lyme disease have resolution of their symptoms following treatment. Approximately 10% have persistent pain, fatigue, or neurocognitive symptoms following treated Lyme disease. Although these symptoms often resolve within months, they sometimes persist for years. Symptoms that lead to functional impairment are known as posttreatment Lyme disease syndrome (PTLDS) (Aucott 2015; Rebman and Aucott 2020).
Myth
Persistent symptoms after Lyme disease are due to infection and require chronic antibiotic treatment.
Reality: Mechanisms of PTLDS are not well understood and may include central sensitization and immune-mediated mechanisms. However, there is no clear evidence that infection persists in humans after appropriate antibiotic regimens. Five double-blind, placebo-controlled trials of patients with PTLDS, conducted in both the United States and Europe, have shown no sustained benefit from additional oral or IV antibiotics given for 1–3 months.
There is no approved treatment for PTLDS. Unproven and potentially harmful therapies are often marketed to patients (Lantos et al. 2015). Approaches for widespread pain can be borrowed from fibromyalgia treatment (Steere and Arvikar 2015). These include:
-
Tricylic antidepressants
-
Dual-reuptake inhibitors (duloxetine, milnacipran)
-
Alpha 2 ligands (gabapentin, pregabalin)
-
Exercise
-
Cognitive behavioral therapy
-
Mind-body medicine
Pearl
Lyme disease may trigger other autoimmune conditions.
Comment: Systemic rheumatic diseases such as rheumatoid arthritis, psoriatic arthritis, and other spondyloarthropathies and autoimmune neurologic conditions have been described following months after treated Lyme disease, most commonly after treatment of early Lyme disease (Arvikar et al. 2017; Rupprecht et al. 2008). Lyme disease may serve as a trigger for onset of a systemic autoimmune disease, as is true for other infections. Even if the occurrence of a systemic rheumatic disease within months after Lyme disease is coincidental, it is important to consider this possibility when evaluating inflammatory symptoms that occur soon after antibiotic-treated Lyme disease, since the appropriate treatment for an autoimmune condition is not further antibiotic therapy.
References
Arvikar SL, Steere AC. Diagnosis and treatment of Lyme arthritis. Infect Dis Clin N Am. 2022;36:563–77.
Arvikar SL, Crowley JT, Sulka KB, et al. Autoimmune arthritides, rheumatoid arthritis, psoriatic arthritis, or peripheral spondyloarthropathy, following Lyme disease. Arthritis Rheumatol. 2017;69:194–202.
Aucott JN. Posttreatment Lyme disease syndrome. Infect Dis Clin N Am. 2015;29:309–23.
Kalish RA, McHugh G, Granquist J, et al. Persistence of immunoglobulin M or immunoglobulin G antibody responses to Borrelia burgdorferi 10-20 years after active Lyme disease. Clin Infect Dis. 2001;33:780–5.
Kugeler KJ, Schwartz AM, Delory M, et al. Estimating the frequency of Lyme disease diagnoses—United States, 2010-2018. Emerg Infect Dis. 2021;27:616–9.
Lantos PM, Shapiro ED, Auwaerter PG, et al. Unorthodox alternative therapies marketed to treat Lyme disease. Clin Infect Dis. 2015;60:1776–82.
Lantos PM, Rumbaugh J, Bockenstedt LK, et al. Clinical practice guidelines by the Infectious Diseases Society of America (IDSA), American Academy of Neurology (AAN), and American College of Rheumatology (ACR): 2020 guidelines for the prevention, diagnosis and treatment of Lyme disease. Arthritis Care Res. 2021;73:1–9.
Li X, McHugh G, Damle N, et al. Burden and viability of Borrelia burgdorferi in skin or joints of patients with erythema migrans or Lyme arthritis. Arthritis Rheum. 2011;63:2238–47.
Lochhead RB, Strle K, Arvikar SL, et al. Lyme arthritis: linking infection, inflammation and autoimmunity. Nat Rev Rheumatol. 2021;17:449–61.
Mead P, Petersen J, Hinckley A. Updated CDC recommendation for serologic diagnosis of Lyme disease. MMWR Morb Mortal Wkly Rep. 2019;68:703.
Rebman AW, Aucott JN. Post-treatment Lyme disease as a model for persistent symptoms in Lyme disease. Front Med. 2020;25(7):57.
Rupprecht TA, Elstner M, Weil S, et al. Autoimmune-mediated polyneuropathy triggered by borrelial infection? Muscle Nerve. 2008;37:781–5.
Schwartz AM, Hickley AF, Mead PS, et al. Surveillance for Lyme disease—United States, 2008-2015. MMWR Surveill Summ. 2017;66:1–12.
Steere AC, Angelis SM. Therapy for Lyme arthritis: strategies for the treatment of antibiotic-refractory arthritis. Arthritis Rheum. 2006;54:3079–86.
Steere AC, Arvikar SL. Editorial commentary: what constitutes appropriate treatment of post-Lyme disease symptoms and other pain and fatigue syndromes? Clin Infect Dis. 2015;60:1783–5.
Steere AC, Malawista SE, Snydman DR, et al. Lyme arthritis: an epidemic of oligoarticular arthritis in children and adults in three Connecticut communities. Arthritis Rheum. 1977;20:7–17.
Steere AC, Schoen RT, Taylor E. The clinical evolution of Lyme arthritis. Ann Intern Med. 1987;107:725–31.
Wormser GP, McKenna D, Scavarda C, et al. Co-infections in persons with early Lyme disease, New York, USA. Emerg Infect Dis. 2019;25:748–52.
Author information
Authors and Affiliations
Corresponding author
Editor information
Editors and Affiliations
Rights and permissions
Copyright information
© 2023 The Author(s), under exclusive license to Springer Nature Switzerland AG
About this chapter
Cite this chapter
Arvikar, S.L., Halperin, J.J., Steere, A.C. (2023). Lyme. In: Stone, J.H. (eds) A Clinician's Pearls & Myths in Rheumatology. Springer, Cham. https://doi.org/10.1007/978-3-031-23488-0_43
Download citation
DOI: https://doi.org/10.1007/978-3-031-23488-0_43
Published:
Publisher Name: Springer, Cham
Print ISBN: 978-3-031-23487-3
Online ISBN: 978-3-031-23488-0
eBook Packages: MedicineMedicine (R0)