Abstract
Reactive arthritis (ReA) has traditionally been described as a nonseptic arthritis occurring in the joint following an extra-articular bacterial infection. This concept became clinically associated with antecedent infections of either the gastrointestinal or genitourinary tract. Yet this operational definition of ReA has led to diagnostic uncertainty in different clinical settings. There are several scenarios in which the ReA has been complex. One is in the SAPHO syndrome, which shares many features with ReA. Another is the development of arthritis after infection with atypical organisms such as Clostridium difficile and Giardia lamblia. Treatment of ReA remains an area of ongoing investigation. There has been a randomized controlled trial of combination antibiotics in Chlamydia-induced ReA, which reported a positive result. There are several uncontrolled reports of anti-TNF agents being used successfully in refractory ReA. These studies in treatment modalities require validation on larger samples but do provide some encouraging preliminary findings from which to develop new therapeutic approaches.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
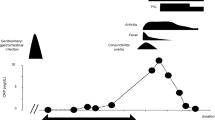
Reactive arthritis (ReA) has traditionally been described as a nonseptic arthritis occurring in the joint following an extra-articular bacterial infection [1]. This concept became clinically associated with antecedent infections of either the gastrointestinal (GI) or genitourinary (GU) tract. Yet this operational definition of ReA has led to diagnostic uncertainty in different clinical settings (Fig. 1).
Proposed classification of the spondylarthropathies—notably, SAPHO—on a spectrum with ReA. PsA, psoriatic arthritis; AS, ankylosing spondylitis; ReA, reactive arthritis; EA, enteropathic arthritis; USpA, undifferentiated spondylarthropathy; SA, SAPHO. (Reprinted from Rohekar and Inman [8], with permission)
Several attempts have been made to create classification criteria; however, lack of consensus has led to a failure to achieve any universally validated diagnostic criteria. At the last International Workshop on Reactive Arthritis, a consensus opinion determined that ReA should include only patients with clinical features of ReA and cases where a pathogen known to cause ReA is implicated [2]. However, there have been several isolated case reports of atypical infections purportedly triggering ReA, such as Clostridium difficile, Giardia lamblia, Propionobacterium acnes, and Group G streptococci. If these patients should be included under the diagnostic entity of ReA, the concept needs to be broadened in terms of the range of arthritogenic pathogens.
Chlamydia trachomatis represents the most common single cause of ReA. Approximately 4 %–15 % of those with symptomatic genital C. trachomatis infections subsequently develop arthritis. Those with asymptomatic infections are not accounted for, and it remains unknown to what extent this could be playing a causal role in many patients with undifferentiated spondylarthropathy (SpA). Given the high prevalence of chlamydial genital tract infections, it has been proposed that the incidence of chlamydia-induced ReA (CiReA) might rival that of rheumatoid arthritis (RA). Taken together with GI-related ReA and the less common triggering pathogens, ReA represents an underrecognized disease with a significant physical and economic burden.
In light of this burden, a firm yet encompassing definition of ReA will be beneficial in providing directed care and improved allocation of resources. This review will address the traditional definition of ReA by evaluating atypical causes of ReA that are not from a GI or GU source and variants of ReA such as SAPHO, as well as review advancements in CiReA that call into question the belief that ReA is an aseptic arthritis.
SAPHO
One ongoing controversy in the SpA field is whether SpA should include the clinical syndrome called SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis). The acronym of SAPHO accurately describes its clinical features. In addition to synovitis, both hyperostosis and osteitis are seen. The most common site of involvement is the anterior chest wall—in particular, the clavicles, sternum, and sternoclavicular joints. Symptoms consistent with an SpA include a lower extremity oligoarthritis involving the knees, hips, and ankles. Furthermore, sacroiliitis is present in 52 % of patients with SAPHO [3]. Its classical dermatologic features include both severe acne and palmoplantar pustulosis. On both histopathology and radiography, SAPHO can initially be indistinguishable from osteomyelitis. Radiographic lesions are typically seen in the sternoclavicular joints.
Because of its overlap with SpA, it has been suggested that SAPHO is not a unique entity but, rather, one component on a spectrum of disease [4]. Not only does it have clinical and radiographic features similar to SpA, but also infection may play a role in its pathogenesis. Govoni et al. [5] recently reviewed several smaller studies that have proposed a possible link between P. acnes and SAPHO. Among 69 investigated patients by means of bone biopsies, P. acnes was isolated from 24 specimens. Furthermore, intra-articular injection of inactivated P. acnes in the knees of laboratory rats can cause joint lesions, including bony erosions and proliferation of the synovial lining [6]. Also suggestive for an infectious etiology is the observation that SAPHO may be responsive to antibiotics. Assman [7•] showed improvement in MRI findings and activity of skin disease and osteitis after completion of a 4-month course of azithromycin. Three months after the completion of antibiotic treatment, however, the benefits had disappeared.
These findings raise the possibility that SAPHO is a reactive osteitis that is mechanistically on the same spectrum of ReA. We have previously proposed a classification system to address this issue [8]. If the traditional definition of ReA is utilized, SAPHO does not meet its diagnostic criteria, but there are several features that suggest that SAPHO has an infectious trigger and may be a variant of ReA.
Clostridium Difficile and Giardia Lamblia
Classically, Salmonella, Shigella, Campylobacter, and Yersinia have been implicated as the pathogens proceeding ReA. However, there are a number of reported cases of Clostridium-difficile-associated ReA. In these cases, knees and wrists are the most common sites of involvement. Although the temporal relationship is clear, Clostridium difficile has not been isolated from the joint itself and is not associated with C. difficile bacteremia.
A retrospective analysis in Canada demonstrated that the incidence of C. difficile had increased fourfold from 1991 to 2003, with a 10-fold increase in individuals over 65 years old [9]. Given this marked increase in incidence, rheumatologists should expect to encounter an increasing number of patients with C. difficile-associated ReA. Perhaps with more clinical exposure, a more uniform approach to treatment will evolve. In the 40 documented cases, a variety of treatment modalities were used; however, on average, cases resolved in 68 days with treatment of the underlying C. difficile colitis [10].
Like C. difficile, Giardia is a GI pathogen that has been associated with ReA, albeit rarely. Giardia is a parasite that colonizes the GI tract. It is typically contracted by drinking water in rural areas. Cantey et al. [11] performed a case series on patients with nonoutbreak Giardia infections and demonstrated that 11.7 % of the cases experienced joint pain, most commonly affecting the knee, and 13.6 % experienced ocular symptoms. Although this study did not include sacroiliac involvement in its analysis, previous case reports have documented its occurrence [12].
It is clear that atypical pathogens can lead to a clinical presentation consistent with ReA. For this reason, the traditional definition of ReA needs to be revisited to include these and other atypical pathogens that have yet to be identified.
Streptococcal Infections
To be congruent with the current definition of ReA, only pathogens causing GI or GU infections lead to a diagnosis of ReA. However, group A Streptococci and Chlamydia pneumoniae, both implicated in respiratory tract infections, have been associated with ReA. Although C. pneumoniae is far less commonly linked to ReA, PCR analysis has recovered its DNA from the synovial tissue of patients with ReA [13].
Poststreptococaal ReA (PSRA) can present as a predominantly lower extremity oligoarthritis and may have an associated enthesitis or tendonitis [14]. A review by Mackie [15] concluded that PSRA is a heterogeneous group of disorders, some of which share features of SpA and some of which are more like acute rheumatic fever (ARF). The consensus is that ARF and PSRA are distinct clinical entities. PSRA has a longer duration between infection and symptom onset and does not respond as readily to salicylates. The pericardial disease and valvular heart disease characteristic of ARF are not recognized to be features of PSRA.
Chlamydia-Induced ReA
Chlamydia trachomatis represents the most common pathogen to trigger ReA, with 4 %–15 % of infected individuals developing ReA. C. pneumoniae has also been implicated in ReA, albeit far less frequently than C. trachomatis. Recent studies have shown that both C. trachomatis and C. pneumoniae are able to disseminate from their site of primary infection to distant sites, such as the synovium, and establish residence in the tissues. At these distant sites, the pathogens enter into a persistent yet aberrant state, at which point the organisms are unable to be cultured; however, they are detectable by electron microscopy and nucleic acid detection [1].
To further support the concept of ReA as a septic arthritis, a clinical trial in 2010 demonstrated that combination antibiotics could alter the course of CiReA [16•]. Patients were randomized to receive placebo, doxycycline + rifampin, or azithromycin + rifampin. After 6 months of treatment, 63 % of the patients randomized to combination antibiotic therapy versus 20 % of the patients assigned to the placebo group had clinical improvement, as measured by swollen joint count. Furthermore, 70 % of the patients who received antibiotics demonstrated clearance of Chlamydia as measured by PCR of peripheral blood mononuclear cells. Although several studies have previously been conducted using antibiotics in treating ReA, this is the first that has demonstrated clinical improvement and examined clearance of Chlamydia. This trial was unique in that it enrolled only patients with blood or synovial tissue that was positive for Chlamydia by PCR. Other studies using antibiotics have enrolled patients with heterogeneous etiologies of ReA. Furthermore, they have often employed antibiotic monotherapy, which may not be effective in treating aberrant pathogens. These findings lead to a reconsideration of the definition of ReA as being a nonseptic arthritis.
Biologic Agents in the Treatment of ReA
Traditionally, the treatment of ReA has involved an initial trial of NSAIDs and local corticosteroid injections with the addition of a DMARD should the individual continue to be symptomatic. Limited evidence exists about the use of DMARDs, in part because of the relative infrequency of their use; approximately 50 % of patients recover from ReA within the initial 6 months, and only 4 %–19 % of patients go on to develop a protracted course of ReA lasting more than 1 year [17]. Unlike the other clinical subsets of SpA, no guidelines exist for ReA with respect to more aggressive treatment should the initial therapy fail. Because there are no guidelines and most patients respond to NSAIDs, there are a limited number of case reports in which patients were placed on biologics.
There is limited experience with the use of anti-TNF agents in ReA (Table 1). The largest reported experience with biologic therapy in ReA is from Flagg et al. [18]. In this study, the efficacy and safety of etanercept (25 mg subcutaneous twice weekly) was examined in 16 patients with reactive arthritis in a 6-month open-label trial. Synovial biopsies were performed before and after treatment with etanercept. PCR analysis was performed on the synovial biopsy samples to evaluate for the presence of nucleic acid material of bacterial organisms. Ten of 16 patients completed the trial. Six patients withdrew, but none had a worsening of arthritis or infection. Of the 10 completers, 9 could be classified as treatment responders, despite the evidence of bacterial organisms on PCR analysis prior to initiating etanercept in 3 patients; 2 patients became PCR negative on etanercept. Five of 6 patients with adequate synovial biopsy specimens showed improvement but not normalization of histology.
The most recent study to examine the efficacy of anti-TNFs in ReA patients was a retrospective analysis of 10 patients [19•]. Ten patients with ReA previously refractory to NSAIDs and DMARDs received anti-TNF therapy within a median of 6 months (range 2–12 months) between the onset of ReA and the initiation of the treatment. The median follow-up was 20.6 months. There were no severe adverse events observed. Anti-TNF therapy was rapidly effective in 9 patients (90 %), as shown by the rapid effect on a visual analog scale pain score, tender joint count, swollen joint count, and extraarticular manifestations and by the corticosteroid-sparing effect. The relatively short disease duration in most patients in this series raises the issues of generalizability and cost effectiveness. Given the self-limited nature of ReA in most cases, it is unclear whether the resolution of disease is secondary to the anti-TNF agent or simply its spontaneous resolution. Understandably, there is a theoretical concern in administering an anti-TNF agent to someone who is harboring a potential pathogen. Fortunately, in Mayer’s study [19•], no adverse events, including severe infection, were documented. Only mild infections were documented, none of which were associated with the triggering infection.
Conclusions and Future Directions in ReA
With the advent of the improved methods of detecting pathogens in the synovium and the recognition of an expanding number of pathogens implicated in ReA, there remains a challenge for creating a practical and more generalized definition of ReA. Presently, inclusion and exclusion criteria are not well defined, and clinicians are often left to treat ReA on the basis of clinical experience. Current evidence supports the notion that ReA is a variant of septic arthritis in which the pathogen cannot be cultured. This would direct future research efforts toward therapies targeted at eradication of the intra-articular pathogens. But prior to such initiatives, more reliable detection of these pathogens in the joints needs to be developed. With increased utilization of biologic agents for the treatment of the SpA, defined guidelines need to be elucidated that balance both the risk and cost of these agents in patients with ReA. Hopefully, the use of these biologic agents will be curtailed with the development of directed antibiotic therapy or, perhaps, the discovery of effective vaccines for arthritogenic pathogens.
References
Papers of particular interest, published recently, have been highlighted as:• Of importance
Sieper J. Pathogenesis of reactive arthritis. Curr Rheumatol Rep. 2001;3(5):412.
Braun J, Kingsley G, van der Heijde D, Sieper J. On the difficulties of establishing a consensus on the definition of and diagnostic investigations for reactive arthritis. Results and discussion of a questionnaire prepared for the 4th International Workshop on Reactive Arthritis, Berlin, Germany, July 3. J Rheumatol. 2000;27(9):2185.
Earwaker JW, Cotton A. SAPHO: syndrome or concept? Imaging findings. Skeletal Radiol. 2003;32(6):311–27. Epub 2003 Apr 29.
Rosner I. SAPHO: disease, syndrome, or category? J Clin Rheumatol. 2002;8(1):3.
Govoni M, Colina M, Massara A, Trotta F. SAPHO syndrome and infections. Autoimmun Rev. 2009;8(3):256–9. Epub 2008 Aug 20.
Trimble BS, Evers CJ, Ballaron SA, Young JM. Intraarticular injection of Propionibacterium acnes causes an erosive arthritis in rats. Agent Actions. 1987;21(3–4):281–3.
• Assmann G, Kueck O, Kirchhoff T, Rosenthal H, Voswinkel J, Pfreundschuh M, Zeidler H, Wagner AD. Efficacy of antibiotic therapy for SAPHO syndrome is lost after its discontinuation: an interventional study. Arthritis Res Ther. 2009;11:R140. This study examines the evidence for the use of antibiotics in SAPHO syndrome and follow-up after discontinuance.
Rohekar G, Inman RD. Conundrums in nosology: synovitis, acne, pustulosis, hyperostosis, and osteitis syndrome and spondylarthritis. Arthritis Rheum. 2006;55(4):665–9.
Pépin J, Valiquette L, Alary ME, Villemure P, Pelletier A, Forget K, Pépin K, Chouinard D. Clostridium difficile-associated diarrhea in a region of Quebec from 1991 to 2003: a changing pattern of disease severity. CMAJ. 2004;171(5):466–7.
Jacobs A, Barnard K, Fishel R, Gradon JD. Extracolonic manifestations of Clostridium difficile infections. Presentation of 2 cases and review of the literature. Medicine (Baltimore). 2001;80(2):88–101.
Cantey PT, Roy S, Lee B, Cronquist A, Smith K, Liang J, Beach MJ. Study of nonoutbreak giardiasis: novel findings and implications for research. Am J Med. 2011;124(12):1175.e1-8. Epub 2011 Oct 18.
Layton MA, Dziedzic K, Dawes PT. Sacroiliitis in an HLA B27-negative patient following giardiasis. Br J Rheumatol. 1998;37(5):581–3.
Gérard HC, Schumacher HR, El-Gabalawy H, Goldbach-Mansky R, Hudson AP. Chlamydia pneumoniae present in the human synovium are viable and metabolically active. Microb Pathog. 2000;29(1):17–24.
Sarakbi HA, Hammoudeh M, Kanjar I, Al-Emadi S, Mahdy S, Siam A. Poststreptococcal reactive arthritis and the association with tendonitis, tenosynovitis, and enthesitis. J Clin Rheumatol. 2010;16(1):3–6.
Mackie SL, Keat A. Poststreptococcal reactive arthritis: what is it and how do we know? Rheumatology (Oxford). 2004;43:949–54.
• Carter JD, Espinoza LR, Inman RD, Sneed KB, Ricca LR, Vasey FB, Valeriano J, Stanich JA, Oszust C, Gerard HC, Hudson AP. Combination antibiotics as a treatment for chronic Chlamydia-induced reactive arthritis: a double-blind, placebo-controlled, prospective trial. Arthritis Rheum. 2010;62(5):1298–307. This is the first randomized, placebo-controlled trial of combination antibiotics in Chlamydia-induced ReA and provides supportive evidence for antibiotic efficacy.
Leirisalo-Repo M. Prognosis, course of disease, and treatment of the spondyloarthropathies. Rheum Dis Clin N Am. 1998;24(4):737–51. viii.
Flagg SD, Meador R, Hsia E, Kitumnuaypong T, Schumacher Jr HR. Decreased pain and synovial inflammation after etanercept therapy in patients with reactive and undifferentiated arthritis: an open-label trial. Arthritis Rheum. 2005;53(4):613–7.
• Meyer A, Chatelus E, Wendling D, Berthelot JM, Dernis E, Houvenagel E, Morel J, Richer O, Schaeverbeke T, Gottenberg JE, Sibilia J, Club Rhumatisme et Inflammation. Safety and efficacy of anti-tumor necrosis factor α therapy in ten patients with recent-onset refractory reactive arthritis. Arthritis Rheum. 2011;63(5):1274–80. This is the largest recent experience with anti-TNF agents in ReA and supports the concept of the safety and efficacy of these agents in refractory ReA.
Oili KS, Niinisalo H, Korpilähde T, Virolainen J. Treatment of reactive arthritis with infliximab. Scand J Rheumatol. 2003;32(2):122–4.
Gaylis N. Infliximab in the treatment of an HIV positive patient with Reiter’s syndrome. J Rheumatol. 2003;30(2):407–11.
Gill H, Majithia V. Successful use of infliximab in the treatment of Reiter’s syndrome: a case report and discussion. Clin Rheumatol. 2008;27(1):121–3.
Abdelmoula LC, Yahia CB, Testouri N, Tekaya R, Ben M’barek R, Chaabouni L, Zouari R. Treatment of reactive arthritis with infliximab. Tunis Med. 2008;86(12):1095–7.
Schafranski MD. Infliximab for reactive arthritis secondary to Chlamydia trachomatis infection. Rheumatol Int. 2010;30(5):679–80.
Disclosure
Dr. Morris has no disclosures.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morris, D., Inman, R.D. Reactive Arthritis: Developments and Challenges in Diagnosis and Treatment. Curr Rheumatol Rep 14, 390–394 (2012). https://doi.org/10.1007/s11926-012-0280-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11926-012-0280-4