Abstract
Secondary hyperparathyroidism is a frequent complication of chronic kidney disease that begins early in the course of renal insufficiency as an adaptive response to maintain mineral homeostasis. This complex disorder affects the bone, leading to an increase in fracture risk and is associated with increased risks of vascular calcification and mortality.
Purpose of Review
In this review, we examine the different strategies available to manage secondary hyperparathyroidism. Particularly, we focus on the adequate control of serum phosphorus by restricting intake and the use of phosphate binders, correction of hypocalcemia while minimizing calcium burden, and reduction in PTH levels through the use of vitamin D sterols and calcimimetics.
Recent Findings
It was observed that although numerous agents directed at the correction of these abnormalities have demonstrated effectiveness on biochemical markers, there is still a relative scarcity of studies demonstrating treatment effectiveness as measured by hard clinical outcomes. In addition, most agents have side effects that may limit their use, even in patients in which the treatment has demonstrated efficacy in controlling these parameters.
Summary
There is still controversy as to what therapeutic regimens to choose for a particular patient and what parameter should be used to follow their effects, including outcomes, side effects, pill burden, and costs, among others. In the present article, we analyze controversial aspects of the different therapeutic agents available. Although many tools and regimens are available, no one by itself is enough for an adequate management of the patient. But rather, combined therapy and individualization of approaches are recommended for better results. We suggest that new studies analyzing the effectiveness of therapeutic approaches to the management of secondary hyperparathyroidism should be directed not only to controlling parathyroid hormone levels but also to the evaluation of long-term outcomes, based on modification of morbidity, mortality, and end organ impact, while reducing side effects and controlling costs, among others.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Secondary hyperparathyroidism (SHPT) is a frequent complication of CKD that begins early in the course of renal insufficiency as an adaptive response to maintain mineral homeostasis. SHPT is part of the chronic kidney disease-mineral and bone disorder (CKD-MBD) that encompasses alterations in serum calcium, phosphate, PTH, vitamin D, and FGF23; bone abnormalities; and vascular calcification [1]. This complex disorder is associated with increased risks for morbidity and mortality [1, 2].
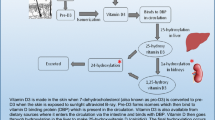
As renal function declines, phosphate levels are maintained by reducing phosphate reabsorption in the remaining nephrons by the action of FGF23. However, the effects of FGF23 are limited due to proximal tubule Klotho deficiency occurring early in the course of CKD [3,4,5,6]. As a consequence, PTH increases to maintain phosphate balance. With the progression of renal insufficiency, FGF23 and PTH are no longer enough to maintain phosphate homeostasis, resulting in hyperphosphatemia. Both phosphate and FGF23 inhibit 1-alpha-hydroxylase in the kidney leading to calcitriol deficiency. Phosphate increases PTH secretion in the parathyroid gland independent of calcium and calcitriol. Calcitriol deficiency decreases intestinal calcium absorption leading to hypocalcemia and diminishes tissue levels of vitamin D receptors (VDR), which in the parathyroid gland results in resistance to calcitriol-mediated regulation of PTH synthesis and elevation of PTH secretion. All these factors lead to SHPT. With time, nodular hyperplasia of the parathyroid gland occurs and the mechanisms controlling PTH secretion become ineffective. The persistent action of PTH increases bone turnover, resorption and frailty, and consequently, elevated fracture risk [3].
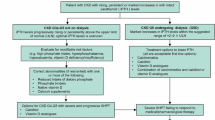
The pathophysiologic mechanism of SHPT summarized above underlines the basis of its prevention and treatment, that is, adequate control of phosphate levels through reduction of intake and the use of phosphate binders, adequate dialysis, and direct inhibition of PTH synthesis and secretion with active vitamin D or calcimimetics (Fig. 1). Guidelines have been developed to help the clinician decide the best approach to this complex problem [7]. Unfortunately, the results are still limited due to both difficulties in their application and scarcity of controlled studies demonstrating treatment effectiveness. As a consequence, there is controversy as to what therapeutic regimens to choose for a particular patient.
Control of Phosphate
Numerous studies have shown that in addition to its central role in SHPT, there is an association between high phosphate levels and cardiovascular and all-cause mortality in patients with advanced CKD [7,8,9,10,11,12]. Although causality has not been demonstrated between phosphate levels and poor clinical outcomes, concern about this association has increased the interest in reducing phosphate levels as a means to control cardiovascular mortality. KDIGO [7] guidelines suggest lowering elevated phosphate levels toward the normal range in CKD 3 to 5D (2C), and that decisions about phosphate-lowering treatment should be based on progressively or persistently elevated serum phosphorus (not graded).
During the early stages of CKD, FGF23 and PTH maintain serum phosphate levels within the normal range [3]. Therefore, a relative reduction in phosphate intake seems logical as a means to decrease the amount of phosphate absorbed in the intestine and, consequently, decrease PTH secretion. The use of phosphate-lowering agents at these stages of CKD is highly controversial as there is no hard evidence of benefit of better outcomes in terms of SHPT [7]. In addition, follow-up of phosphate binders requirement is difficult, as serum phosphate levels at these stages will not express the status of phosphate balance.
In CKD stages 5 and 5D, increases in FGF23 and PTH are not sufficient to control hyperphosphatemia. Therefore, in most patients, management of phosphate load requires the use of phosphate-lowering agents to decrease intestinal absorption and remove phosphate from the circulation. At this point, counseling on nutritional recommendations to reduce phosphate intake by diminishing protein consumption and adequate selection of the protein source (animal vs. vegetable, food additives, medications, etc.) are central to the management of phosphate burden [13, 14]. However, a patient’s compliance frequently limits the effectiveness of protein-restricted diets in reducing phosphate burden and the control of hyperphosphatemia. Furthermore, controversy exists as to the extent of dietary protein restriction in patients with advanced renal disease in which nutritional status may be already compromised by the disease.
Hemodialysis removes phosphorus, but in the usual regimen of 3 times per week in most patients, together with dietary phosphate restriction, is not enough to control serum phosphate levels [15]. Increasing the length and frequency of HD improves phosphate removal and may be enough to control hyperphosphatemia [16,17,18,19], but application of this type of regimen in large scale may be impractical and costly.
Phosphate binders have been the mainstay of phosphate-lowering measures in advanced CKD by their ability to reduce intestinal phosphate absorption. Aluminum-based agents were extensively used given their effectiveness as phosphate binders but have been largely discontinued due to toxicity in bone and the CNS [20, 21]. Calcium-based agents (calcium carbonate and calcium acetate) have been widely used due to their effectiveness as phosphate binders. However, there has been increasing concern about their possible contribution to positive calcium balance and the increase of vascular calcification and cardiovascular mortality in patients with advanced CKD [1, 3, 7, 22,23,24]. Thus, non-calcium-based phosphate binders have been developed and are increasingly used to control phosphate absorption without a calcium burden. These agents include sevelamer, lanthanum carbonate, magnesium, and iron-containing agents. Numerous studies have demonstrated that these agents are as effective as calcium salts in controlling intestinal phosphate absorption. However, controversy exists with regard to their effect on calcium balance, vascular calcification, and mortality.
Several studies have compared calcium- and non-calcium-based phosphate binders in controlling serum PTH, vascular calcification, and cardiovascular morbidity and mortality. Unfortunately, the design of the studies has been heterogeneous with regard to patient selection, duration of follow-up, and outcomes, among others, making comparison difficult to interpret. Some studies comparing calcium-based binders with sevelamer have shown similar effectiveness as phosphate binders and no significant differences in their effects on PTH levels compared to baseline, but higher calcium levels and CAC score have been observed in patients treated with calcium-based binders compared with those receiving sevelamer [25]. In patients new to hemodialysis randomized to receive calcium-based binders or sevelamer over 18 months, calcium-treated patients showed more rapid and severe increase in CAC score compared to those treated with sevelamer [26]. In contrast, in another study comparing calcium acetate treatment with sevelamer, although hypercalcemia was observed more frequently in patients treated with calcium acetate, persistent hypercalcemia did not differ between the two groups. Both compounds were similarly effective in controlling serum phosphate and PTH, and the CAC progression was similar in the two groups [27]. The difference in the results of these and other studies may result from variations in the selection of patients included in the study, baseline CAC, and other factors such as presence of diabetic nephropathy and smoking [28]. Studies comparing mortality are also controversial. Thus, in a randomized study following hemodialysis patients treated with calcium-based binders compared to those treated with sevelamer, all-cause and cause-specific mortality were not different between the two groups, although there was a significant age interaction as only in patients aged more than 65 years was there an effect of sevelamer in lowering the mortality rate [29]. Criticisms to this study include a high rate of loss to follow-up, length of time under dialysis treatment, and high percentage of diabetics [30,31,32,33]. A meta-analysis of several randomized trials comparing non-calcium- vs. calcium-based binders on mortality showed that patients assigned to non-calcium binders had a 22% reduced mortality compared to those assigned to calcium-based phosphate binders [30]. Similarly, in a more recent meta-analysis of randomized controlled trials of patients with CKD stages 3 to 5 and patients on dialysis treated with calcium-based binders compared with sevelamer, patients treated with sevelamer had lower all-cause mortality, but no statistically significant difference on cardiovascular mortality was observed. In addition, patients on sevelamer had a lower risk of hypercalcemia and higher levels of PTH, while serum phosphate levels were not significantly different [31]. In the majority of studies, combined gastrointestinal side effects occurred more often with sevelamer. Thus, the majority of randomized studies suggest that non-calcium binders, while similarly effective at lowering phosphate, are associated with less cardiovascular or all-cause mortality than calcium-based binders. In addition, there seems to be a lower risk of hypercalcemia and CAC in these patients, an aspect that should be considered in choosing the calcium binder in patients with high cardiovascular risk. An additional advantage of sevelamer is its cholesterol-lowering effect that could be of help in reducing CV risk [34, 35].
Lanthanum carbonate has been widely used in more recent years to treat hyperphosphatemia in ESRD. It is as effective as calcium-based binders and sevelamer at controlling phosphate levels, and is associated with less hypercalcemia compared with calcium binders, and its secondary effects seem to be not different [36]. There has been concern about the possible toxicity of lanthanum given the past experience with aluminum-based phosphate binders [37]. However, the pharmacodynamics of lanthanum are different than that of aluminum and several studies have failed to demonstrate the accumulation of lanthanum in the CNS [38]. With respect to bone toxicity, lanthanum can accumulate in the bone in “relatively low quantities” but no alterations in bone histomorphometry have been demonstrated [38]. Side effects of lanthanum carbonate are particularly gastrointestinal, and patients find its pills difficult to swallow, as tablets require crushing or breaking in smaller pieces. Comparative studies of lanthanum carbonate and calcium-based binders and with sevelamer itself are limited [3]. In a recent preliminary study of dialysis patients followed for 3 years, lanthanum carbonate and calcium carbonate yield similar results in terms of effectiveness as phosphate binders and no statistically significant differences were observed in terms of cardiovascular mortality and CVC accumulation [39]. In terms of effects on parathyroid gland function, there was no difference in PTH levels in the two groups.
Comparative studies including more recently released phosphate binders such as iron-based products, and magnesium salts are less available. Ferric citrate binds phosphate in the intestine and reduces serum phosphate levels [40•]. A randomized study comparing ferric citrate with sevelamer and calcium based-binders showed effective phosphate level control. Calcium and PTH levels were not different than in patients receiving calcium binders or sevelamer, with the addition that ferric citrate increased iron stores and decreased requirements of intravenous iron and erythropoietin-stimulating agents while maintaining hemoglobin levels [40•, 41•]. While these properties may give some advantage over other phosphate binders, gastrointestinal adverse effects are frequent and pill burden is similar to calcium-treated patients but less than those receiving sevelamer.
At the present time, the evidence favors control of phosphate levels in CKD and particularly in advances stages. With the exception of aluminum, all phosphate binders currently in use are similarly effective at reducing serum phosphate levels. Unfortunately, despite the advances with the introduction of new phosphate binders, controlling serum phosphorus remains challenging and hyperphosphatemia continues to be extremely common in CKD patients [3, 42]. So, the choice of the binder should be oriented to their potential long-term effect in reducing vascular calcification and limiting other side effects. It seems also clear that single approaches are not enough to maintain phosphate levels within a normal range, and as suggested by KDIGO guidelines [7], phosphate-lowering measures should be used in concert to control hyperphosphatemia in patient-oriented approaches, including diet, phosphate binders, and adequate dialysis. In addition, reduction of PTH levels in patients with SHPT by other means is important as PTH action in the bone results in bone resorption and release of phosphate [3] which in turn will increase serum phosphate levels independent of intestinal absorption.
According to the data available, non-calcium binders should be preferred owing to the data associating calcium-based binders with cardiovascular and all-cause mortality, and vascular calcification, particularly in those patients with higher risk of vascular calcification. In this regard, KDIGO guidelines [7] suggest restricting the dose of calcium-based phosphate binders in patients with CKD G3a-G5D receiving phosphate-lowering treatment (2B). However, non-calcium binders are considerably more expensive, an important factor in medical systems with lower coverage, and are not devoid of side effects. Gastrointestinal side effects and a higher medication burden are more frequent with the non-calcium-containing binders.
Finally, the effectiveness of phosphate binders in reducing serum phosphorus has been well demonstrated; their ultimate effects on clinical outcomes remain controversial. Thus, more placebo-controlled studies are necessary to definitively prove that the reduction of serum phosphorus by phosphate binders improves clinical outcomes.
Calcium Management
The calcium-sensing receptor (CaSR) is central for the regulation of calcium homeostasis. In the parathyroid gland, CaSR regulates PTH secretion. Under normal conditions, low serum calcium levels increase PTH secretion while high calcium acting at the CaSR inhibits the hormone secretion. Many patients with CKD have low serum levels of calcium. This occurs at least in part as a consequence of decreased calcium absorption in the gastrointestinal tract due to vitamin D deficiency [3]. As calcium is the main factor controlling PTH secretion, hypocalcemia in CKD contributes to the development and persistence of SHPT. Interestingly, retrospective analyses in dialysis patients have shown an association between hypocalcemia and mortality risk [28], thus constituting another possible reason to correct hypocalcemia in these patients. However, correction of hypocalcemia is not devoid of risk as the range of normality of serum calcium is narrow, and overzealous correction may lead to an increase in the calcium burden and the risk of vascular calcification [3]. In this regard, KDIGO guidelines [7] suggest avoiding hypercalcemia in adult patients with CKD G3a–G5D (not graded).
In dialysis patients, correction of hypocalcemia is attained mainly via the calcium content in the dialysate, calcium supplements, and vitamin D sterols. However, there is no consensus about the optimal calcium concentration in the dialysate. Several recommendations have been made, taking into account the status of mineral and bone metabolism as well as their impact on the cardiovascular condition. Thus, relatively low calcium concentrations have been suggested for patients with low PTH levels and adynamic bone disease [43, 44] in order to further PTH secretion. Based on expert opinion, the Kidney Disease Outcomes Quality Initiative (KDOQI) [45] suggested a dialysate calcium concentration of 2.5 mEq/l for patients in hemodialysis or peritoneal dialysis. This would allow the use of larger amounts of calcium-based phosphate binders and vitamin D while the risk of hypocalcemia is reduced. However, with the current availability of non-calcium phosphate binders, this concern is no longer critical. More recently, KDIGO [7] suggested using a dialysate calcium concentration between 1.25 and 1.50 mmol/l (2.5 and 3.0 mEq/l). The rationale behind these suggestions is studies showing that a calcium concentration of 2.5 mEq/ml in the dialysate improves bone and mineral parameters as compared with higher calcium concentration [44]. Conversely, while high dialysate calcium concentration helps reduce PTH secretion, it has been associated with all-cause and cardiovascular mortality [46,47,48]. Thus, there is still controversy about the benefits and harms of these calcium concentrations and more studies are needed to clarify this important issue. Furthermore, except in extreme cases of hypercalcemia or hypocalcemia in which it seems reasonable that low and high calcium concentration, respectively, should be used, there are no precise strategies to help the clinician decide which calcium concentration to use in a particular patient. Again, the range of serum calcium is narrow and does not necessarily reflect calcium balance which is tightly regulated. Thus, positive balance may not be reflected in serum calcium levels, and particularly in patients receiving calcium-based phosphate binders, as high calcium concentration in the dialysate may also be a source of positive calcium balance and calcium accumulation [48].
Given the difficulties in assessing the calcium requirements in dialysis patients and the number of calcium sources (diet, calcium-based phosphate binders, increased intestinal calcium absorption in patients receiving vitamin D agonists, and dialysate calcium concentrations), as well as the varied metabolic status of the patients, we agree on individualizing the approach [7, 49] to avoid overcorrection with the possibility of increasing calcium burden or under-correction favoring SHPT.
PTH-Lowering Agents: Vitamin D and Calcimimetics
Vitamin D
Vitamin D sterols and calcimimetics are specific PTH-lowering agents that act directly on the parathyroid gland to inhibit PTH secretion. Their effects are exerted directly through receptors in the parathyroid cell, the vitamin D receptor (VDR) and the CaSR, respectively [50,51,52].
Calcitriol, the active form of vitamin D, has been widely used as one of the main therapies for SHPT. Multiple studies have demonstrated that calcitriol and other active vitamin D sterols, paricalcitol, doxercalciferol, and other analogs, are effective in reducing PTH levels. Since PTH increases bone remodeling, the ultimate goal of the therapy with these agents is to improve bone remodeling and reduce fracture risk. However, treatment with these agents has limitations as excessive inhibition of PTH secretion may lead to a severe decrease in bone turnover leading to adynamic bone disease and vascular calcification [53], whereas insufficient inhibition of PTH will not control SHPT and consequently, high bone turnover persists. Moreover, active vitamin D increases intestinal calcium and phosphate absorption leading to hypercalcemia and hyperphosphatemia, which limit their use. These secondary effects are more severe with calcitriol compared with paricalcitol or doxercalciferol, which act at the parathyroid gland VDR with an affinity similar to calcitriol but have lower affinity at the intestine [53]. Doxercalciferol, a prohormone that requires conversion to the active form in vivo, has similar a effect to paricalcitol albeit larger doses are required for PTH suppression.
As calcitriol deficiency and high PTH levels occur relatively early in the course of CKD, controversy has arisen as to whether treatment with vitamin D should be initiated to prevent or treat SHPT early in the course of the disease. Moreover, since low levels of 25(OH) vitamin D are a frequent finding in CKD patients, there is controversy on when to initiate vitamin D supplementation and whether native or active forms of vitamin D should be utilized in early stages of CKD as well as in patients on dialysis [54]. 25(OH) vitamin D is the substrate for calcitriol; thus, it seems logical to correct vitamin D deficiency or insufficiency by providing cholecalciferol or ergocalciferol, the natural forms of vitamin D, as a means to improve calcitriol levels. However, in a recent study by Batacchi et al. [54], it was found that supplementation of CKD patients with vitamin D, correction of 25(OH) vitamin D levels, was not accompanied by an increase in calcitriol and adequate suppression of SHPT. This suggests that the same defects contributing to impaired 1,25(OH)2D production in the unsupplemented state extend to vitamin D supplementation. Plasma FGF-23 concentration inversely correlated with plasma 1,25(OH)2D concentration at baseline and adjusting for PTH and FGF-23 concentrations attenuated associations of CKD with change in the 24,25(OH)2D3-to-25(OH)D3 metabolic ratio, suggesting that PTH and FGF-23 may mediate, in part, the effects of CKD on vitamin D metabolism. Therefore, suppression of PTH secretion with native vitamin D to control SHPT may not be enough and the use of active vitamin D analogs or other PTH-lowering agents is required [53]. Nevertheless, native vitamin D continues to be used in CKD patients as other actions beyond treatment of SHPT have been considered.
The effectiveness of active vitamin D in controlling SHPT has been demonstrated in multiple studies, particularly with regard to biochemical endpoints in patients with CKD 3 to 5 and ESRD on dialysis. Although biochemical surrogates may be linked to improvement in the end organ effects of PTH levels, relatively scarce studies have analyzed their effect on bone histomorphometry [55, 56]. Therefore, more studies aimed to evaluate the end organ effect of these agents in bone and other organs, particularly as adverse effects of active vitamin D analogs, hypercalcemia, and oversuppression of the parathyroid cell, are relatively frequently observed [57, 58]. Indeed, the KDIGO guidelines update [7] suggests that in adult patients with CKD G3a–G5 not on dialysis, calcitriol and vitamin D analogs should not be routinely used (2C) and that it is reasonable to reserve the use of calcitriol and vitamin D analogs for patients with CKD G4–G5 with severe and progressive hyperparathyroidism (not graded). In a recent analysis, the KDOQI work group [59] agreed with this guideline. However, they claim that the guideline update does not explicitly stipulate when administration of calcitriol and vitamin D analogues for PTH suppression should occur because the term “severe” is not defined. Thus, there is ambiguity facing implementation of the guideline update. So, there is controversy between expert groups as to the time and dose of active vitamin D in patients with SHPT and CKD 4 to 5. In patients with CKD 5D, KDIGO guidelines [7] suggest PTH-lowering therapy with calcitriol or vitamin D analogs (2B). Again, adverse effects such as hypercalcemia and excessive suppression of PTH are the main concerns with this type of therapy.
Among other limitations of active vitamin D analogs is their limited capability of reducing PTH levels to adequate values, particularly in patients with severe SHPT with nodular hypertrophy in whom VDR expression in the parathyroid cells are decreased or downregulated [51]. In these cases, the use of other agents alone or in combination with vitamin D analogs may be required.
Calcimimetics
The CaSR in the parathyroid gland plays an essential role for the treatment of SHPT. This receptor, highly sensitive to changes in calcium concentration, also responds to other substances that can act as modulators of PTH secretion. Natural calcimimetics such as magnesium and other inorganic compounds act directly at the CaSR in the parathyroid gland, decreasing PTH secretion (calcimimetics type 1). In addition, other positive allosteric modulators of the CaSR, classified as type II, bind to a site distinct from the physiological ligand, rendering the CaSR more sensitive to calcium, so that inhibition of PTH secretion is achieved at lower calcium concentration [60]. The calcimimetic most widely used for SHPT treatment is cinacalcet [61,62,63], but more recently, etecalcetide, a new generation of calcimimetics, has been increasingly used [64]. Different from cinacalcet that is used orally on a daily basis, etecalcetide, due to its longer metabolism, is administered intravenously three times a week during dialysis. The efficacy of calcimimetics as a PTH-lowering agent has been demonstrated in several randomized controlled trials [65•, 66]. These compounds also decrease serum calcium and phosphorus levels [61, 62, 64]. The efficacy of cinacalcet has also been demonstrated in patients that did not achieve adequate PTH because of elevated calcium and phosphate levels while on therapy with active vitamin analogs [61]. Indeed, the placebo-controlled EVOLVE study [67], conducted in dialysis patients with SHPT, confirmed a greater achievement of biochemical parameters of CKD-MBD in patients treated with cinacalcet than with standard therapy, including vitamin sterols.
The new calcimimetic agent etelcalcetide has been recently approved for treatment of SHP in patients on dialysis. This agent decreases PTH by acting at the CaSR and induces hypocalcemia, but different from cinacalcet, it may activate the CaSR even in the presence of hypocalcemia and has longer half-life, which confers the advantage that it can be administered three times a week at the end of hemodialysis. Recent trials in hemodialysis patients with moderate or severe SHPT have shown that patients randomized to etelcalcetide for 26 weeks were more likely to achieve a reduction of PTH greater than 30% compared to the placebo group [65•, 66], but also demonstrated non-inferiority compared with cinacalcet, and in fact was superior in achieving control of PTH levels [68]. Thus, significantly more patients in the etelcaletide group achieved more than 50% reduction in PTH levels as compared to 40.2% in the cinacalcet group [65•]. The number of patients receiving calcium-based phosphate binders or higher calcium concentration in the dialysate increased in both groups, and side effects where not different.
Controversy exists about the choice of PTH-lowering agent, active vitamin D sterols or calcimimetics. One of the limitations of vitamin D sterols is the associated increase in calcium and phosphate intestinal absorption that frequently requires a dose reduction in order to avoid or control hypercalcemia or hypophosphatemia. Conversely, calcimimetics, although highly effective in reducing PTH levels, induce hypocalcemia and hypophosphatemia that also require reducing their dose or the use of other measures to control them, more frequently combination with active vitamin D. In addition, other side effects are relatively frequent with calcimimetics, more commonly gastrointestinal events [65•, 66], and also limit their use in a number of patients. Consequently, both vitamin D sterols and calcimimetics are effective at decreasing PTH, but adverse effects limit long-term effectiveness. Cost is another limitation to use calcimimetics in the setting of ESRD [68]. In this context, effectiveness, side effects, adherence, and budget must be considered in a patient-oriented analysis.
One of the criticisms of vitamin D active agents is the scarcity of studies demonstrating, that beyond their biochemical effects (lowering PTH, reducing levels of biochemical bone markers, etc.), they have end organ effectiveness, such as improving bone metabolism, fracture risk, vascular calcification, or reducing cardiac mortality. In contrast, some studies have shown that in patients with ESRD and severe SHPT randomized to cinacalcet in addition to active vitamin D therapy, patients receiving cinacalcet had a reduced risk of fracture, cardiovascular hospitalization, and parathyroidectomy compared with those receiving placebo and conventional therapy [69,70,71].
Prospective histomorphometric studies have shown that in addition to improving biochemical surrogates of bone metabolism in patients with ESRD with SHPT, cinacalcet diminished activation frequency, bone formation rate/bone surface, and fibrosis surface/bone surface after one year of treatment; bone turnover and bone marrow fibrosis are both indicators of parathyroid bone disease [69,70,71]. Interestingly, adynamic bone disease, frequently observed in patients with oversuppression of PTH, was observed in only 2 out of 19 patients receiving cinacalcet in which PTH was persistently low. Similarly, in the BONAFIDE trial [72], cinacalcet effectively decreased PTH and the bone histomorphometric analysis showed a decrease in bone turnover, osteoid perimeter, and eroded perimeter, also indicating improvement in parathyroid-associated bone disease. Two patients with PTH under 150 pg/ml and one patient with overt hypophosphatemia very low PTH had adynamic bone at the end of the study, and one patient with overt hypophosphatemia at baseline that recurred during follow-up developed osteomalacia. These studies demonstrate an end organ effect of cinacalcet in controlling SHPT bone disorder.
In summary, both active vitamin D and calcimimetics decrease effectively PTH levels in patients with SHPT. Cinacalcet also has demonstrated to improve bone metabolism as measured by bone histomorphometry and fracture risk. However, more controlled studies with calcimimetics and active vitamin D agents should be performed to corroborate the end organ effect of these agents and their impact on clinical outcomes.
The availability of an effective PTH-lowering agent has made parathyroidectomy less necessary. However, this method is still considered in some patients, particularly in those with severe SHPT difficult to control by medical means. However, controversies exist about patient selection, surgical expertise, and long-term results as a number of patients may undergo severe hypoparathyroidism, whereas in many others, a very tight control of the mechanisms behind the development of SHPT is required.
Even though there are agents capable of effectively reducing PTH levels in patients with SHPT, controversial questions still need to be addressed. Some of the main doubts are how much control of PTH levels should be achieved, when to start and when to stop treatment with PTH-lowering agents, what agent should be chosen, and what combination of treatment should be recommended. In an effort to help guide clinicians in their selection and application of treatment regimens, expert opinions have been gathered in recommendations or guidelines. One of the main limitations of the guidelines is that the effective diagnosis of metabolic bone disease is based on histomorphometric analysis of the bone. This method, although recognized as the “gold standard” for the diagnosis, is very limited because it is invasive, requires expert analysis of the bone histology, is costly, and hence, is difficult to apply in large scale. Therefore, several studies have attempted to establish a relationship between the type of bone disease with serum biochemical markers. K/DIGO, KDOQI, and other organizations have suggested ranges of PTH levels to be achieved in patients with CKD. Thus, K/DOQI [45] suggested a PTH range of 150 to 300 ng/ml as the optimal limit to avoid low or high turnover. Similarly, since the PTH levels may vary with the different assays used in the clinic, KDIGO [1] in 2009 suggested a range between 2 and 9 times the highest normal value for the PTH assay used, but very few studies have addressed this issue directly. Because bone turnover is a function in large part of the degree of hyperparathyroidism, PTH levels have traditionally been used as a surrogate indicator of bone turnover. Intact PTH (iPTH) has been studied for the diagnosis of bone turnover in dialysis patients, and together with serum calcium, phosphorus, and total alkaline phosphatases or bone-specific alkaline phosphatase (bALP), is frequently used to guide the pharmacologic treatment of CKD-MBD. Additional bone biomarkers have also been evaluated for their predictive value in assessing renal osteodystrophy, but results have been inconclusive. Furthermore, bone turnover cannot be accurately predicted from PTH levels in a high proportion of dialysis patients [73]. In a cross-sectional retrospective study of 492 patients from four different countries, Sprague et al. [74•] studied the accuracy of biochemical markers to predict bone histopathology. PTH and alkaline phosphatase allowed discrimination of low from non-low bone turnover, and high from non-high bone turnover. The combination of these two markers offered a minimal additional discrimination. Furthermore, the best PTH cutoff to discriminate low from non-low bone turnover BFR/BS was 103.8 pg/ml, whereas to discriminate high from non-high, it was 323.0 pg/ml. Similarly, the best cutoff for bALP was 33.1 U/l for low from non-low turnover, and 42.1 U/l for high bone turnover. These results indicate that iPTH, with some certainty, can be used to discriminate low turnover from high turnover with PTH values consistent with the recommendations of both K/DOQI and KDIGO [1, 45]. These results also provide reasonable help with regard to the question of when to start therapy and when to stop to treat high bone turnover disease in dialysis patients. Therefore, PTH, although with limitations, is to the present time the most accurate discriminator between high and low bone turnover disease in patients in dialysis. However, it should be pointed out that bone turnover is not the only indicator of bone disease in these patients and that other alterations important for bone strength have not been evaluated with respect to bone markers.
In conclusion, multiple studies show a tight relationship between SHPT and poor clinical outcomes, including bone fragility, fracture, and cardiovascular morbidity and mortality, as well as all-cause mortality. However, studies demonstrating causality and clinical outcomes are still lacking. Therefore, more randomized controlled trials directed toward answering questions about SHPT and clinical outcomes are necessary in the search of new therapeutic approaches. Indeed, SHPT is complex and requires combined therapies for its management and prevention, which include the control of phosphate and calcium metabolism and the control of PTH levels directly. Although many tools are available, no one by itself is enough for an adequate management of the patient. But rather, combined therapy and individualization of approaches are recommended for better results.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Kidney Disease: Imporoving Global Outcomes (KDIGO) CKD-MBD Word Group. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Kidney Int Suppl. 2009(113):S1–130.
Palmer SC, Hayen A, Macaskill P, Pellegrini F, Craig JC, Elder GJ, et al. Serum levels of phosphorus, parathyroid hormone, and calcium and risks of death and cardiovascular disease in individuals with chronic kidney disease: a systematic review and meta-analysis. JAMA. 2011;305(11):1119–27.
Martin KJ, Floege J, Ketteler M. Bone and mineral disorders in chronic kidney disease. In: Feehally J, J F, R.J. J, editors. Comprehensive clinical nephrology. Sixth Edition ed2019.
Pereira RC, Juppner H, Azucena-Serrano CE, Yadin O, Salusky IB, Wesseling-Perry K. Patterns of FGF-23, DMP1, and MEPE expression in patients with chronic kidney disease. Bone. 2009;45(6):1161–8.
Sakan H, Nakatani K, Asai O, Imura A, Tanaka T, Yoshimoto S, et al. Reduced renal alpha-Klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PLoS One. 2014;9(1):e86301.
Smith RC, O'Bryan LM, Farrow EG, Summers LJ, Clinkenbeard EL, Roberts JL, et al. Circulating alphaKlotho influences phosphate handling by controlling FGF23 production. J Clin Invest. 2012;122(12):4710–5.
KDIGO 2017. Clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease–mineral and bone disorder (CKD-MBD). Kidney International Supplements. 2017;7(1):1–59.
Blacher J, Guerin AP, Pannier B, Marchais SJ, London GM. Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension. 2001;38(4):938–42.
Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–17.
Dhingra R, Sullivan LM, Fox CS, Wang TJ, D'Agostino RB Sr, Gaziano JM, et al. Relations of serum phosphorus and calcium levels to the incidence of cardiovascular disease in the community. Arch Intern Med. 2007;167(9):879–85.
Gutierrez OM, Mannstadt M, Isakova T, Rauh-Hain JA, Tamez H, Shah A, et al. Fibroblast growth factor 23 and mortality among patients undergoing hemodialysis. N Engl J Med. 2008;359(6):584–92.
Isakova T. Comparison of mineral metabolites as risk factors for adverse clinical outcomes in CKD. Semin Nephrol. 2013;33(2):106–17.
de Fornasari ML, Dos Santos Sens YA. Replacing phosphorus-containing food additives with foods without additives reduces phosphatemia in end-stage renal disease patients: a randomized clinical trial. J Ren Nutr. 2017;27(2):97–105.
Nelson SM, Sarabia SR, Christilaw E, Ward EC, Lynch SK, Adams MA, et al. Phosphate-containing prescription medications contribute to the daily phosphate intake in a third of hemodialysis patients. J Ren Nutr. 2017;27(2):91–6.
Elias RM, Alvares VRC, Moyses RMA. Phosphate removal during conventional hemodialysis: a decades-old misconception. Kidney Blood Press Res. 2018;43(1):110–4.
Kjellstrand CM, Ing TS, Kjellstrand PT, Odar-Cederlof I, Lagg CR. Phosphorus dynamics during hemodialysis. Hemodial Int. 2011;15(2):226–33.
Kuhlmann MK. Management of hyperphosphatemia. Hemodial Int. 2006;10(4):338–45.
Mucsi I, Hercz G, Uldall R, Ouwendyk M, Francoeur R, Pierratos A. Control of serum phosphate without any phosphate binders in patients treated with nocturnal hemodialysis. Kidney Int. 1998;53(5):1399–404.
Sampaio MS, Ruzany F, Dorigo DM, Suassuna JH. Phosphate mass removal during hemodialysis: a comparison between eKT/V-matched conventional and extended dialysis. Am J Nephrol. 2012;36(2):121–6.
Malluche HH. Aluminium and bone disease in chronic renal failure. Nephrol Dial Transplant. 2002;17(Suppl 2):21–4.
Rob PM, Niederstadt C, Reusche E. Dementia in patients undergoing long-term dialysis: aetiology, differential diagnoses, epidemiology and management. CNS Drugs. 2001;15(9):691–9.
Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23(8):1407–15.
Guerin AP, London GM, Marchais SJ, Metivier F. Arterial stiffening and vascular calcifications in end-stage renal disease. Nephrol Dial Transplant. 2000;15(7):1014–21.
London GM, Guerin AP, Marchais SJ, Metivier F, Pannier B, Adda H. Arterial media calcification in end-stage renal disease: impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant. 2003;18(9):1731–40.
Chertow GM, Burke SK, Raggi P. Treat to goal working G. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–52.
Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68(4):1815–24.
Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51(6):952–65.
Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–55.
Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72(9):1130–7.
Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382(9900):1268–77.
Patel L, Bernard LM, Elder GJ. Sevelamer versus calcium-based binders for treatment of hyperphosphatemia in CKD: a meta-analysis of randomized controlled trials. Clin J Am Soc Nephrol. 2016;11(2):232–44.
Vervloet MG, van Ballegooijen AJ. Prevention and treatment of hyperphosphatemia in chronic kidney disease. Kidney Int. 2018;93(5):1060–72.
Wald R, Rabbat CG, Girard L, Garg AX, Tennankore K, Tyrwhitt J, et al. Two phosphAte taRGets in end-stage renal disease trial (TARGET): a randomized controlled trial. Clin J Am Soc Nephrol. 2017;12(6):965–73.
Chertow GM, Burke SK, Dillon MA, Slatopolsky E. Long-term effects of sevelamer hydrochloride on the calcium x phosphate product and lipid profile of haemodialysis patients. Nephrol Dial Transplant. 1999;14(12):2907–14.
Wilkes BM, Reiner D, Kern M, Burke S. Simultaneous lowering of serum phosphate and LDL-cholesterol by sevelamer hydrochloride (RenaGel) in dialysis patients. Clin Nephrol. 1998;50(6):381–6.
Sprague SM. A comparative review of the efficacy and safety of established phosphate binders: calcium, sevelamer, and lanthanum carbonate. Curr Med Res Opin. 2007;23(12):3167–75.
Slatopolsky E, Liapis H, Finch J. Progressive accumulation of lanthanum in the liver of normal and uremic rats. Kidney Int. 2005;68(6):2809–13.
Laville M. Efficacy and safety of lanthanum carbonate in chronic kidney disease patients with hyperphosphataemia. Nephrol Ther. 2011;7(3):154–61.
Ogata H, Fukagawa M, Hirakabata H, Kagimura T. Group atLS. ASN National Meeting: Comparison of lanthanum carbonate and calcium carbonate in the cardiovascular mortality and morbidity in hemofialysis patients; 2018.
• Lewis JB, Sika M, Koury MJ, Chuang P, Schulman G, Smith MT, et al. Ferric citrate controls phosphorus and delivers iron in patients on dialysis. J Am Soc Nephrol. 2015;26(2):493–503. Control of phosphate levels and correction of anemia are important factors in the treatment of CKD patients. This study shows that ferric citrate is an effective phosphate binder, which at the same time increases iron stores, thus reducing the requirements of IV iron and the use of erythropoiesis-stimulating agents to correct anemia. Limitations of the study and of possible use of this type of agent to treat high phosphate levels include patients with high ferritin or TSAT levels as well as those with phosphate > 6.0 as they were not included in the study.
• Umanath K, Jalal DI, Greco BA, Umeukeje EM, Reisin E, Manley J, et al. Ferric citrate reduces intravenous iron and erythropoiesis-stimulating agent use in ESRD. J Am Soc Nephrol. 2015;26(10):2578–87. This study adds evidence about the effect of ferric citrate on ferritin levels and transferrin saturation which reduces the requirements for i.v. iron and ESA use in patients with ESRD.
Cannata-Andia JB, Martin KJ. The challenge of controlling phosphorus in chronic kidney disease. Nephrol Dial Transplant. 2016;31(4):541–7.
Ok E, Asci G, Bayraktaroglu S, Toz H, Ozkahya M, Yilmaz M, et al. Reduction of dialysate calcium level reduces progression of coronary artery calcification and improves low bone turnover in patients on hemodialysis. J Am Soc Nephrol. 2016;27(8):2475–86.
Spasovski G, Gelev S, Masin-Spasovska J, Selim G, Sikole A, Vanholder R. Improvement of bone and mineral parameters related to adynamic bone disease by diminishing dialysate calcium. Bone. 2007;41(4):698–703.
National Kidney Foundation. K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis. 2003;42(4 Suppl 3):S1–201.
Kim HW, Kim SH, Kim YO, Jin DC, Song HC, Choi EJ, et al. Impact of dialysate calcium concentration on clinical outcomes in incident hemodialysis patients. Medicine (Baltimore). 2015;94(40):e1694.
Miller JE, Kovesdy CP, Norris KC, Mehrotra R, Nissenson AR, Kopple JD, et al. Association of cumulatively low or high serum calcium levels with mortality in long-term hemodialysis patients. Am J Nephrol. 2010;32(5):403–13.
Spiegel DM. Brady K. Kidney Int: Calcium balance in normal individuals and in patients with chronic kidney disease on low- and high-calcium diets; 2012.
Jean G, Chazot C. Individualizing the dialysate calcium concentration. Curr Opin Nephrol Hypertens. 2015;24(6):538–45.
Brown EM, Gamba G, Riccardi D, Lombardi M, Butters R, Kifor O, et al. Cloning and characterization of an extracellular Ca(2+)-sensing receptor from bovine parathyroid. Nature. 1993;366(6455):575–80.
Rodriguez M, Nemeth E, Martin D. The calcium-sensing receptor: a key factor in the pathogenesis of secondary hyperparathyroidism. Am J Physiol Renal Physiol. 2005;288(2):F253–64.
Yano S, Sugimoto T, Tsukamoto T, Chihara K, Kobayashi A, Kitazawa S, et al. Decrease in vitamin D receptor and calcium-sensing receptor in highly proliferative parathyroid adenomas. Eur J Endocrinol. 2003;148(4):403–11.
Sprague SM, Coyne D. Control of secondary hyperparathyroidism by vitamin D receptor agonists in chronic kidney disease. Clin J Am Soc Nephrol. 2010;5(3):512–8.
Batacchi Z, Robinson-Cohen C, Hoofnagle AN, Isakova T, Kestenbaum B, Martin KJ, et al. Effects of vitamin D2 supplementation on vitamin D3 metabolism in health and CKD. Clin J Am Soc Nephrol. 2017;12(9):1498–506.
Coen G, Mazzaferro S, Bonucci E, Ballanti P, Massimetti C, Donato G, et al. Treatment of secondary hyperparathyroidism of predialysis chronic renal failure with low doses of 1,25(OH)2D3: humoral and histomorphometric results. Miner Electrolyte Metab. 1986;12(5–6):375–82.
Sperschneider H, Humbsch K, Abendroth K. Oral calcitriol pulse therapy in hemodialysis patients. Effects on histomorphometry of bone in renal hyperparathyroidism. Med Klin (Munich). 1997;92(10):597–603.
Thadhani R, Appelbaum E, Pritchett Y, Chang Y, Wenger J, Tamez H, et al. Vitamin D therapy and cardiac structure and function in patients with chronic kidney disease: the PRIMO randomized controlled trial. JAMA. 2012;307(7):674–84.
Wang AY, Fang F, Chan J, Wen YY, Qing S, Chan IH, et al. Effect of paricalcitol on left ventricular mass and function in CKD--the OPERA trial. J Am Soc Nephrol. 2014;25(1):175–86.
Isakova T, Nickolas TL, Denburg M, Yarlagadda S, Weiner DE, Gutierrez OM, et al. KDOQI US commentary on the 2017 KDIGO clinical practice guideline update for the diagnosis, evaluation, prevention, and treatment of chronic kidney disease-mineral and bone disorder (CKD-MBD). Am J Kidney Dis. 2017;70(6):737–51.
Harrington PE, Fotsch C. Calcium sensing receptor activators: calcimimetics. Curr Med Chem. 2007;14(28):3027–34.
Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–25.
Lindberg JS, Culleton B, Wong G, Borah MF, Clark RV, Shapiro WB, et al. Cinacalcet HCl, an oral calcimimetic agent for the treatment of secondary hyperparathyroidism in hemodialysis and peritoneal dialysis: a randomized, double-blind, multicenter study. J Am Soc Nephrol. 2005;16(3):800–7.
Pereira L, Meng C, Marques D, Frazao JM. Old and new calcimimetics for treatment of secondary hyperparathyroidism: impact on biochemical and relevant clinical outcomes. Clin Kidney J. 2018;11(1):80–8.
Eidman KE, Wetmore JB. Treatment of secondary hyperparathyroidism: how do cinacalcet and etelcalcetide differ? Semin Dial. 2018;31(5):440–4.
• Block GA, Bushinsky DA, Cheng S, Cunningham J, Dehmel B, Drueke TB, et al. Effect of etelcalcetide vs cinacalcet on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: a randomized clinical trial. JAMA. 2017;317(2):156–64. The study shows that both calcimimetics are effective in lowering PTH levels in CKD patients on dialysis with moderate to severe secondary hyperparathyroidism. Etelcalcitide was not inferior to cinacalcet and even demonstrated superiority in lowering PTH, but also was more frequently associated with hypocalcemia. Since the patients included in the study were relatively young, no extrapolation of the results should be done to older patients.
Block GA, Bushinsky DA, Cunningham J, Drueke TB, Ketteler M, Kewalramani R, et al. Effect of etelcalcetide vs placebo on serum parathyroid hormone in patients receiving hemodialysis with secondary hyperparathyroidism: two randomized clinical trials. JAMA. 2017;317(2):146–55.
Pun PH, Abdalla S, Block GA, Chertow GM, Correa-Rotter R, Dehmel B, et al. Cinacalcet, dialysate calcium concentration, and cardiovascular events in the EVOLVE trial. Hemodial Int. 2016;20(3):421–31.
Stollenwerk B, Iannazzo S, Cooper K, Belozeroff V. Exploring the potential value of improved care for secondary hyperparathyroidism with a novel calcimimetic therapy. J Med Econ. 2017;20(10):1110–5.
Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68(4):1793–800.
Investigators ET, Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–94.
Malluche HH, Monier-Faugere MC, Wang G, Fraza OJ, Charytan C, Coburn JW, et al. An assessment of cinacalcet HCl effects on bone histology in dialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2008;69(4):269–78.
Behets GJ, Spasovski G, Sterling LR, Goodman WG, Spiegel DM, De Broe ME, et al. Bone histomorphometry before and after long-term treatment with cinacalcet in dialysis patients with secondary hyperparathyroidism. Kidney Int. 2015;87(4):846–56.
Barreto FC, Barreto DV, Moyses RM, Neves KR, Canziani ME, Draibe SA, et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73(6):771–7.
• Sprague SM, Bellorin-Font E, Jorgetti V, Carvalho AB, Malluche HH, Ferreira A, et al. Diagnostic accuracy of bone turnover markers and bone histology in patients with CKD treated by dialysis. Am J Kidney Dis. 2016;67(4):559–66. This study, one of the largest bone biopsy studies evaluating serum bone biomarkers (PTH, bone alkaline phosphatase bALP, PINP) for the prediction of bone histomorphometry, shows that iPTH and bALP had the best discriminating ability (although suboptimal) to predict high or low bone turnover, and that iPTH continues to be the best available tool to discriminate bone turnover in CKD patients on dialysis. In addition, iPTH had relatively high specificity, although lower sensitivity, to detect low turnover and high turnover utilizing the KDIGO-recommended cutoff of less than 2 times the upper limit of normal and more than 9 times the upper limit of normal in differentiating high bone turnover.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Ezequiel Bellorin-Font and George Vasquez- Rios declare no conflict of interest.
Kevin Martin reports personal fees from Amgen, outside the submitted work.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Kidney and Bone
Rights and permissions
About this article
Cite this article
Bellorin-Font, E., Vasquez-Rios, G. & Martin, K.J. Controversies in the Management of Secondary Hyperparathyroidism in Chronic Kidney Disease. Curr Osteoporos Rep 17, 333–342 (2019). https://doi.org/10.1007/s11914-019-00533-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-019-00533-x