Abstract
Secondary hyperparathyroidism (SHPT) is a common complication of chronic kidney disease (CKD) and is part of the CKD-mineral bone disorder (CKD-MBD). SHPT is associated with increased risk of fracture and mortality; thus, SHPT control is recommended as kidney function declines. Effective SHPT management becomes more difficult once skeletal and cardiovascular adverse effects associated with severe SHPT have become established. However, interventional studies to lower parathyroid hormone (PTH) have so far shown inconsistent results in improving patient-centred outcomes such as mortality, cardiovascular events and fracture. Pharmacological treatment effect on PTH level is also inconsistent between pre-dialysis CKD and dialysis patients, which adds to the complexity of SHPT management. This review aims to give an overview on the pathophysiology, pharmacological and non-pharmacological treatment for SHPT in CKD including some of the limitations of current therapeutic options.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
SHPT is associated with adverse outcomes in CKD and dialysis patients. |
Optimum PTH level is unclear in pre-dialysis CKD and the recommended range is wide for dialysis patients. |
Management of SHPT is largely pharmacological focussing on lowering PTH. |
PTH lowering treatment has not shown improved cardiovascular risk, fractures and mortality. |
1 Introduction
Secondary hyperparathyroidism (SHPT) is a common complication of chronic kidney disease (CKD), affecting almost all CKD patients by the time they reach end-stage renal disease requiring dialysis. Biochemical [calcium, phosphate, parathyroid hormone (PTH) and vitamin D] and bone abnormalities (renal osteodystrophy) complicating advanced CKD and dialysis are associated with significant morbidity and mortality [1–6], probably due to greater risk of fracture and extra-skeletal calcification leading to cardiovascular disease [7–9]. Severe SHPT in dialysis patients is associated with up to 20 % increased mortality [4, 5] and dialysis patients also have up to a four-fold increase in fracture incidence compared to non-dialysis patients [10] due to impaired bone quality [11, 12]. Vascular calcification is highly prevalent in CKD and is only weakly related to traditional risk factors of cardiovascular disease in the general population such as hypertension and hyperlipidaemia [13]. In 2006, this triad of biochemical abnormalities in mineral metabolism, vascular calcification and renal osteodystrophy was described as chronic kidney disease-mineral bone disorder (CKD-MBD) by the Kidney Disease: Improving Global Outcomes (KDIGO) group [14].
Interventional studies to lower PTH have so far shown inconsistent results in improving patient-centred outcomes such as mortality, cardiovascular events and fracture [15, 16]. Furthermore, the healthcare cost associated with uncontrolled SHPT treatment have been estimated at an additional US$5600 per patient annually [17]. This review aims to give an overview on the pathophysiology, pharmacological and non-pharmacological treatment for SHPT in CKD including some of the limitations of current therapeutic options.
2 Pathophysiology of SHPT in CKD
High serum phosphate can directly increase PTH secretion by parathyroid gland [18] but SHPT onset in CKD often precedes high serum phosphate. Fibroblast growth factor-23 (FGF-23) is a phosphaturic hormone which is released through an as yet uncertain mechanism by osteocytes and osteoblasts. FGF-23 levels start to rise in early CKD stage 2 while serum phosphate, calcium and PTH are still within the normal range and bone mineral metabolism is considered to be normal [19, 20]. FGF-23 maintains normal serum phosphate via two main mechanisms. Firstly, by binding to its co-receptor, klotho, in the distal tubule in the kidney [21] which down-regulates the sodium-phosphate co-transporter in the proximal tubule resulting in reduced phosphate reabsorption and thereby, promotes phosphaturia [22]. Secondly, FGF-23 affects the vitamin D pathway by inhibiting 1α-hydroxylase activity in the kidney [22]. At the same time, it increases the activity of 24-hydroxylase which metabolises 1,25-dihydroxy and 25-hydroxyvitamin D [23]. The net effect is a reduction in total 1,25-dihydroxyvitamin D level which reduces calcium and phosphate absorption from the gut (see Fig. 1). Serum phosphate level is maintained in the normal range until the phosphaturic effect of FGF-23 is blunted due to declining nephron number with progressive CKD.
The effect of high FGF-23 on vitamin D metabolism is detected by two other receptors on parathyroid gland, namely calcium sensing receptor (CaSR) and vitamin D receptor (VDR). Hypocalcaemia due to low 1,25-dihydroxyvitamin D is detected by CaSR leading to increased PTH gene expression and PTH release in order to restore normal serum calcium. Similarly, reduced 1,25-dihydroxyvitamin D levels are sensed by VDR, also stimulating PTH release. Persistent stimulation of parathyroid gland in CKD leads to diffuse (polyclonal) hyperplasia which transforms into nodular (monoclonal) hyperplasia [24]. These monoclonal parathyroid cells have reduced expression of CaSR and VDR [25]; thus, they have reduced sensitivity to the change in serum calcium and 1,25-dihydroxyvitamin D levels. PTH release becomes autonomous which often results in hypercalcaemia and is termed tertiary hyperparathyroidism.
Elevated levels of FGF-23, often a 1000-fold higher than in healthy individuals, are found in dialysis patients. Experimental study in rats with normal kidney function or early CKD showed that high circulating FGF-23 lowers PTH secretion. However, there is downregulation of Klotho-FGFR1c receptor complex in parathyroid gland of rats with advanced CKD. Therefore, high level of FGF-23 failed to decrease PTH level [26].
3 Management of SHPT in CKD
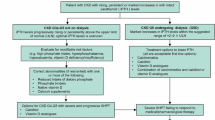
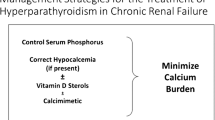
An approach to managing SHPT is summarised in Fig. 2. It is important to follow the trend in the rise/fall of PTH rather than the absolute value when deciding on treatment at any level of CKD. This is because PTH level is influenced by serum calcium, vitamin D level and circadian rhythm [27]. Furthermore, variation in sample stability, accuracy of assay calibration, standardisation and antibody specificity contribute to the variability between assays [28, 29]. The frequently used assays are the second generation assays, so called ‘intact’ PTH (iPTH], which measure both the whole PTH (1-84) and PTH (7-84) molecules. The third generation ‘whole’ PTH assays, which only measure PTH (1-84) molecule, are available but not currently recommended for routine clinical use. The ratio of PTH (1–84)/PTH (7–84) has been assessed in the context of discriminating renal osteodystrophy subtypes, for which the findings have been inconsistent [30–33]. There is limited evidence for its role in predicting clinical outcomes such as mortality and cardiovascular events [34–36].
The KDIGO CKD-MBD guideline recommends keeping the PTH level 2- 9-times the upper limit of normal for the assay in dialysis patients [37] based on a number of bone biopsy studies which demonstrated that PTH poorly predicts underlying bone turnover within this range [38–40]. Whilst very low or very high PTH levels can predict low-turnover and high-turnover bone disease, PTH remains a poor marker of underlying bone histomorphometry within the target range. Alkaline phosphatase (ALP) may improve the sensitivity of PTH in predicting high/low bone turnover [41, 42] and mortality in dialysis patients [43, 44]. The guideline also recommended simultaneous measurement of total ALP although no clear management target was set. The optimum PTH level is unknown for pre-dialysis CKD patients but consistently rising levels above the upper limit of normal should prompt an assessment of bone mineral parameters.
Hyperphosphataemia is usually found in CKD stage 5 or on dialysis; it worsens SHPT and is associated with increased morbidity and mortality [3, 4, 6, 45–47]. An observational study in haemodialysis patients by Streja et al. showed that the lowest mortality was associated with concurrently controlled PTH and phosphate levels [2].
3.1 Dietary Phosphate Restriction
Dietary phosphate restriction has been shown to reduce 24-h urinary phosphate excretion resulting from reduction of dietary phosphate absorption [48]. Two studies have compared strict dietary phosphate restriction (500–900 mg of phosphate daily) against normal or high phosphate diet (1500–2500 mg of phosphate daily). Firstly, a study by Burnett et al. in healthy subjects showed that dietary phosphate restriction reduced urinary phosphate excretion, lowered serum phosphate and FGF-23 although PTH level was unchanged [49]. In contrast, a pilot study by Isakova et al. in normophosphataemic CKD stage 3–4 showed that dietary phosphate restriction reduced urinary phosphate excretion but serum phosphate, PTH and FGF-23 remained unchanged [48]. Unchanged PTH level in both studies may be due to the short period of intervention and a longer period may be required in CKD to show the true overall effect on phosphate balance, PTH and FGF-23 control. However, there is no interventional study to show that phosphate restriction improves clinical outcomes and it is widely accepted that dietary phosphate restriction is unlikely to be sustained and extreme protein restriction to lower dietary phosphate can also be harmful [50].
3.2 Pharmacological
3.2.1 Phosphate Binders
The main reason for using phosphate binders is to lower intestinal absorption of phosphate by binding with dietary phosphate in the gut; thus lowering serum phosphate, which may indirectly attenuate worsening SHPT. Specific instruction on and adherence to taking it before or with meals is crucial for its effectiveness. There is little doubt that all types of phosphate binders are effective in lowering serum phosphate in dialysis patients when compared to placebo as shown in the systematic review carried out by Navaneethan et al. [51]. PTH reduction has also been observed with the use of phosphate binders in dialysis patients in some studies [52–55].
The KDIGO CKD-MBD guideline recommends using phosphate binders only in hyperphosphataemic pre-dialysis CKD [37]. This is because the optimum PTH level and the benefit of using phosphate binders in moderate CKD patients who are normophosphataemic to control SHPT remain unclear. Block et al. randomised 148 patients with CKD stage 3–4 who had serum phosphate in the upper normal range (mean 4.2 mg/dL) to calcium acetate, lanthanum carbonate, sevelamer carbonate and placebo. Results showed that all the phosphate binders modestly reduced serum phosphate despite significant reduction in urinary phosphate when compared to placebo [56]. PTH level was unchanged in the treated group but worsened in the placebo group whereas FGF-23 level was unchanged in both groups. The treated group had an improvement in their bone mineral density but there was worsening of coronary artery calcification when compared to placebo, which raises the question on safety of using phosphate binders in moderate CKD patients. In contrast, another study found that the progression of vascular calcification in patients randomised to a low phosphate diet were similar to those on calcium-based phosphate binder [57]. Thus, treating SHPT in pre-dialysis CKD remains controversial.
Calcium-based binders (acetate and carbonate) are cheap and in current practice, modest dose of calcium (<1.5 g of elemental calcium daily) is widely accepted. However, there have been concerns regarding calcium loading and the risk of extra-skeletal calcification. This led to the development of non-calcium-based binders, namely sevelamer (hydrochloride and carbonate) and lanthanum (carbonate). These non-calcium-based phosphate binders have not shown superior phosphate or PTH reduction compared to calcium-based phosphate binders in head-to-head studies [51, 58, 59] but the studies have also focussed on three other outcomes; incidence of hypercalcaemia, mortality [60] and progression of vascular calcification [52, 53, 56, 61–63]. Three systematic reviews have been published by Navaneethan et al. [51] and Jamal et al. [64, 65] with different conclusions on whether there is a survival benefit when using non-calcium-based compared to calcium-based phosphate binders. It is unlikely that a sufficiently large study will be done to resolve this question.
Aluminium-based phosphate binder is inexpensive and effective. Concerns regarding aluminium toxicity led to almost complete discontinuation of its use in the 1980s although the primary source of aluminium exposure at the time came from dialysis fluid rather than aluminium-based binder [66]. Reverse osmosis and stringent testing of dialysis water have almost completely removed aluminium in current practice. Thus, 6–14 % of dialysis patients in Australia, New Zealand, Germany, Italy and Spain use this binder, whereas its use in the UK is minimal (2 %) and often short term [67]. Regular monitoring of serum aluminium is required.
A recently published phase 3 clinical trial of PA21 compound (sucroferric oxyhydroxide) showed comparable efficacy to sevelamer in dialysis patients and PTH was reduced in both groups but more so with PA21 [68]. Its use has been approved in the USA and Europe in 2014. Small clinical trials of SBR759 iron-based compound have also shown its safety and efficacy in phosphate lowering but its effect on PTH was inconsistent [69–71].
3.2.2 Vitamin D
Vitamin D deficiency is highly prevalent in CKD, haemodialysis and peritoneal dialysis patients [72–74] and is associated with increased mortality [75, 76]. The KDIGO CKD-MBD guideline suggests that the level is measured in CKD stages 3–5 and dialysis, and replaced if deficiency or insufficiency is detected [37]. It is a weak recommendation given the lack of conclusive evidence on patient level outcome from randomised controlled trials (RCTs) although a number of large observational studies showed a survival benefit associated with vitamin D treatment in CKD and dialysis patients [46, 77–82]. The dose of vitamin D that should be used is also unclear in the guideline, so the dose in our treatment algorithm (Fig. 2) is based on our local guideline.
Vitamin D replacement in CKD patients can cause increased gut absorption of calcium and phosphate [83, 84]. Given the importance of lowering serum phosphate to control SHPT and the risk of vascular calcification with hypercalcaemia, vitamin D replacement may seem counter-productive. However, not all vitamin D trials have shown significant hypercalcaemia and hyperphosphataemia as one would expect [85–87]; nonetheless, the development of hypercalcaemia requires vitamin D dose reduction or cessation.
The broad term of vitamin D has caused a lot of confusion in terms of investigations and treatment. The 25-hydroxyvitamin D level is the metabolite routinely measured in clinical practice whilst 1,25-dihydroxyvitamin D is often measured in research setting only. Treatment options for vitamin D deficiency comes in several forms; either as vitamin D2 or D3, or in its native form or analogues. Vitamin D receptor activator or agonist (VDRA) describes the vitamin D compounds which directly activate VDR. VDR are polymorphic and most VDRAs are non-selective (calcitriol, alfacalcidol, and doxercalciferol) but the selective VDRA (paricalcitol) has the theoretical advantage of targeting the VDR on parathyroid gland with minimal effect on VDR elsewhere such as in the gut and bone. Table 1 lists the commonly used vitamin D preparations.
3.2.2.1 Native Vitamin D
It was previously thought that CKD and dialysis patients should only be given alfacalcidol (1-alpha hydroxyvitamin D3) or doxercalciferol (1-alpha hydroxyvitamin D2) which will then undergo 25-hydroxylation in the liver to form the active 1,25-dihydroxyvitamin D. However, other sites in the body, such as skin, lymph nodes and macrophages, also have 1α-hydroxylation activity [88, 89]. To confirm this, several studies have shown that native vitamin D3 (cholecalciferol) supplementation improves 25-hydroxyvitamin D3 and 1,25-dihydroxyvitamin D3 levels in dialysis patients [85–87]. PTH level was significantly reduced by 20–35 % from baseline in two studies although there was no placebo group for comparison [85, 86]. One RCT on 42 haemodialysis patients over 15 weeks did not show significant change in PTH with cholecalciferol compared to placebo [87], whereas a more recent RCT on 43 haemodialysis patients by Delanaye et al. showed statistically significant reduction in PTH with cholecalciferol whilst PTH was increased in the placebo group over a 1-year period [90]. An RCT using ergocalciferol (native vitamin D2) or placebo over 12 weeks in 105 dialysis patients did not show any difference in PTH level between the groups [91]. The difference in PTH lowering effect from these three RCTs using native vitamin D in dialysis patients may be due to the different study duration but it is worth noting that there were no significant changes in serum calcium and phosphate.
Despite the beneficial effect of native vitamin D replacement in controlling SHPT in dialysis patients, the effect in pre-dialysis CKD is inconsistent. In a study of elderly women, where over half had CKD stage 3, calcium (1.2 g elemental calcium) and 800 IU cholecalciferol supplementation resulted in >30 % decrease in PTH compared to placebo [92]. However, this study cannot differentiate the individual effect from either calcium or vitamin D supplementation. Two small RCTs in pre-dialysis CKD using cholecalciferol did not show significant reduction in PTH compared to placebo but both studies had short intervention and follow-up periods [93, 94]. Two observational studies assessing ergocalciferol in CKD stages 3–4 consistently showed significant PTH reduction by up to 20 % in CKD stage 3 but not in the more advanced CKD stage 4 [95, 96].
3.2.2.2 Non-Selective VDRA
The active 1,25-dihydroxyvitamin D3, calcitriol, is a non-selective VDRA. A retrospective study showed that oral calcitriol significantly lowered PTH in pre-dialysis CKD but at the expense of higher risk of hypercalcaemia compared to non-users [82]. In the 1990s, intravenous (IV) calcitriol was recommended as an alternative to oral calcitriol in dialysis patients with SHPT. Thrice weekly IV calcitriol on haemodialysis significantly lowered PTH but majority of patients also developed hypercalcaemia [97].
Vitamin D analogues are non-selective synthetic VDRA of vitamin D3 (alfacalcidol) or vitamin D2 (doxercalciferol). A study using alfacalcidol in 176 mild-moderate CKD for 2 years showed attenuation of PTH rise compared to placebo, but with increased incidence of hypercalcaemia, which was likely to be dose-dependent as it resolved with dose reduction [98]. In contrast, two other studies using alfacalcidol in pre-dialysis CKD showed a significant PTH reduction from baseline by 15–50 % but no hypercalcaemia [99, 100]. A number of small studies assessed different preparations and dosing schedules of alfacalcidol in dialysis patients [101–103] but none compared it with placebo. These studies consistently showed that alfacalcidol is equally effective in reducing PTH across all forms (oral or IV) and dosing schedules (intermittent or daily). Doxercalciferol showed >40 % PTH reduction from baseline with no significant change in serum calcium or phosphate in CKD [104] and dialysis patients [105]. In contrast, another two studies in dialysis patients showed similar PTH reduction but with elevation of serum calcium and phosphate with doxercalciferol [106, 107].
3.2.2.3 Selective VDRA
Paricalcitol, a selective synthetic VDRA, is effective in lowering PTH in CKD [108, 109] and dialysis patients [110–112]. Both RCTs in CKD stages 3–4 by Coyne et al. [108] and the PRIMO study [109] showed that 85–90 % of paricalcitol-treated group achieved >30 % PTH reduction compared to only around 15 % in the placebo group achieving the same endpoint. However, there was a significantly higher incidence of hypercalcaemia when compared to placebo in the PRIMO study but no difference was found in the Coyne et al. study [108]. This difference may be due to the different dosing regimens but it is hard to compare because only one study reported the average dose used throughout the study [108].
A small head-to-head study in 66 dialysis patients demonstrated that paricalcitol was as effective as calcitriol in reducing PTH and had similar safety profile [111]. An earlier direct comparison study using their respective IV forms, which also included a dose adjustment protocol, showed that paricalcitol may achieve quicker PTH reduction with less sustained hypercalcaemia compared to calcitriol [112]. Furthermore, an observational study on 38,000 dialysis patients showed a survival advantage associated with paricalcitol use compared to calcitriol [113]. Despite this favourable evidence for using paricalcitol, it is uncertain whether it is cost effective compared to non-selective VDRAs, which are much cheaper. An investigator-led randomised crossover trial comparing IV paricalcitol with alfacalcidol over a 16-week period in dialysis patients demonstrated equal effectiveness in PTH reduction whilst maintaining calcium and phosphate in the desired range for dialysis population [114].
The IMPACT SHPT (Improved Management of iPTH with Paricalcitol-centred Therapy versus Cinacalcet Therapy with Low-dose Vitamin D in Haemodialysis Patients with Secondary Hyperparathyroidism) study was an open-label study comparing paricalcitol to calcimimetic (cinacalcet) plus low-dose vitamin D in 268 dialysis patients over a 28-week period. The mean baseline iPTH in this study was around 500 pg/mL and the primary efficacy end point was the proportion of patients achieving iPTH value of 150–300 pg/mL from week 21 onwards. A greater proportion of patients achieved the primary end point in paricalcitol group compared to cinacalcet group [115, 116]. However, FGF-23 level was increased significantly with paricalcitol, which was likely due to the significant increase in serum phosphate. Around 8–17 % of patients on paricalcitol developed hypercalcaemia whilst 14–26 % of patients on cinacalcet developed hypocalcaemia. This study highlights the difficulty in reducing PTH with VDRA at the expense of rising serum phosphate and FGF-23, and furthermore, paricalcitol still causes significant rise in serum phosphate despite being a selective VDRA.
3.2.3 Calcimimetics
Calcimimetics increase the sensitivity of CaSR in parathyroid glands to extracellular calcium concentrations, leading to a reduction in circulating PTH concentrations within 1–2 h after dosing. In the UK, cinacalcet is not part of routine treatment for SHPT largely due to cost [117]. The National Institute of Health and Care Excellence (NICE) guidelines recommend its use in patients with uncontrolled SHPT (iPTH >800 pg/mL) refractory to standard therapy with normal or high adjusted serum calcium or in whom the risk of surgical parathyroidectomy outweighs the benefit [118].
Phase 3 clinical study of cinacalcet in dialysis patients demonstrated its effectiveness in PTH reduction, safety and tolerability [119]. Vitamin D is often used to counteract hypocalcaemia related to cinacalcet. Thus, the combination use of native vitamin D or its analogues with cinacalcet allows calcium, phosphate and PTH target levels to be achieved simultaneously and greater PTH reduction has been observed using this combination therapy compared to either agent alone [120, 121]. The OPTIMA study was an open-label study which used an algorithm-based adjustment of VDRAs during the dose titration of cinacalcet in patients with moderate to severe SHPT (iPTH 300–800 pg/mL). The analysis suggested that VDRAs can be used in conjunction with cinacalcet in order to achieve greater PTH reduction but there is a trade-off with respect to higher serum phosphate and calcium relating to VDRA use [122]. In addition to PTH reduction, the combination therapy also attenuated vascular calcification in moderate-to-severe SHPT as shown in the ADVANCE study [123].
A pooled analysis of phase 3 clinical trials’ data suggested benefit of using cinacalcet on a number of important end points including parathyroidectomy rates, fracture, cardiovascular hospitalisation and quality of life [15]. This led to the design of EVOLVE (Evaluation of Cinacalcet HCl Therapy to Lower Cardiovascular Events) trial, an ambitious RCT aimed at a primary composite end point comprising time until death, myocardial infarction, hospitalization for unstable angina, heart failure or peripheral vascular events [16]. There were 3883 haemodialysis patients with moderate to severe SHPT (median iPTH of 693 pg/mL) who were randomised to cinacalcet or placebo while continuing conventional therapy including phosphate binders and vitamin D analogues as required. After a follow-up of up to 63 months, the unadjusted intention-to-treat analysis showed a non-significant 7 % reduction in primary outcome measure (i.e. a negative study). However, in the prespecified subgroup analysis, a 30 % reduction in primary outcome was found in the older (≥65 years) patients who also had more cardiovascular co-morbidities [124]. Secondary end point analysis showed no difference in fracture rate but parathyroidectomy rate was half in the intervention group compared to that in the control group. Despite significantly higher adverse events of hypocalcaemia in the cinacalcet group, it did not seem to have adverse impact on fractures or cardiovascular mortality.
Gastrointestinal symptoms such as nausea and vomiting are known side effects of oral cinacalcet, which may be overcome by IV calcimimetics. IV velcalcetide or AMG416 is currently in phase 3 clinical trials in haemodialysis patients with SHPT. It is a novel, long-acting selective peptide agonist of the CaSR, which has been well tolerated and has shown rapid and sustained PTH reduction after a single dose in haemodialysis patients [125].
3.3 Surgical
Parathyroidectomy is considered for patients with uncontrolled SHPT when pharmacological treatment options have been exhausted. Evidence of potential survival benefit after parathyroidectomy came from retrospective studies [77, 126, 127] but one retrospective study suggested that patients with persistently very low PTH levels post-parathyroidectomy had a higher 5-year mortality [128]. Furthermore, there is concern relating to bone turnover as parathyroidectomy is likely to turn high turnover bone disease to adynamic bone disease [129], which is also associated with worse vascular calcification [130]. An improvement of around 10 % in bone mineral density (BMD) at 6 months after parathyroidectomy was reported [131] but another study found only modest improvement in BMD at 5 years after parathyroidectomy, although no patient suffered any fractures during the follow-up period [132]. An RCT to compare the benefit of parathyroidectomy versus pharmacological treatment in uncontrolled SHPT is unlikely to ever be carried out and with increasing age and co-morbidities amongst dialysis patients, surgical parathyroidectomy is becoming rare. Parathyroidectomy may be largely replaced by pharmacological treatment in the future if the cost of calcimimetics is reduced.
4 Summary
SHPT is associated with adverse outcomes in CKD and dialysis patients, thus there is a recommendation to control SHPT as renal function declines. Effective SHPT management becomes more difficult once skeletal and cardiovascular adverse effects associated with severe SHPT are established. Interventional strategies largely comprise dietary phosphate restriction and pharmacotherapy, although it remains very difficult to extrapolate biochemical end point to meaningful patient-centred outcomes. Management of SHPT in pre-dialysis CKD remains inconclusive, partly because optimum target PTH level is still unknown and there is such heterogeneity in PTH level and CKD stages between clinical trials. The wide PTH range in dialysis patients as recommended by the KDIGO CKD-MBD guideline is sensible given that PTH is a poor marker of bone turnover and quality. Future studies in controlling SHPT requires multiple pharmacological and non-pharmacological approach reflecting real clinical practice, inclusion of patient-centred outcomes, and better assessment of bone and vascular calcification, which are also part of CKD-MBD.
References
Floege J, Kim J, Ireland E, Chazot C, Drueke T, de Francisco A, et al. Serum iPTH, calcium and phosphate, and the risk of mortality in a European haemodialysis population. Nephrol Dial Transplant. 2011;26(6):1948–55.
Streja E, Wang HY, Lau WL, Molnar MZ, Kovesdy CP, Kalantar-Zadeh K, et al. Mortality of combined serum phosphorus and parathyroid hormone concentrations and their changes over time in hemodialysis patients. Bone. 2014;61:201–7.
Block GA, Hulbert-Shearon TE, Levin NW, Port FK. Association of serum phosphorus and calcium x phosphate product with mortality risk in chronic hemodialysis patients: a national study. Am J Kidney Dis. 1998;31(4):607–17.
Block GA, Klassen PS, Lazarus JM, Ofsthun N, Lowrie EG, Chertow GM. Mineral metabolism, mortality, and morbidity in maintenance hemodialysis. J Am Soc Nephrol. 2004;15(8):2208–18.
Tentori F, Blayney MJ, Albert JM, Gillespie BW, Kerr PG, Bommer J, et al. Mortality risk for dialysis patients with different levels of serum calcium, phosphorus, and PTH: the Dialysis Outcomes and Practice Patterns Study (DOPPS). Am J Kidney Dis. 2008;52(3):519–30.
Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, et al. Predictors and consequences of altered mineral metabolism: the Dialysis Outcomes and Practice Patterns Study. Kidney Int. 2005;67(3):1179–87.
Adragao T, Herberth J, Monier-Faugere MC, Branscum AJ, Ferreira A, Frazao JM, et al. Low bone volume—a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol. 2009;4(2):450–5.
Aoki A, Kojima F, Uchida K, Tanaka Y, Nitta K. Associations between vascular calcification, arterial stiffness and bone mineral density in chronic hemodialysis patients. Geriatr Gerontol Int. 2009;9(3):246–52.
Cejka D, Weber M, Diarra D, Reiter T, Kainberger F, Haas M. Inverse association between bone microarchitecture assessed by HR-pQCT and coronary artery calcification in patients with end-stage renal disease. Bone. 2014;64:33–8.
Maravic M, Ostertag A, Torres PU, Cohen-Solal M. Incidence and risk factors for hip fractures in dialysis patients. Osteoporos Int. 2014;25(1):159–65.
Negri AL, Del Valle EE, Zanchetta MB, Nobaru M, Silveira F, Puddu M, et al. Evaluation of bone microarchitecture by high-resolution peripheral quantitative computed tomography (HR-pQCT) in hemodialysis patients. Osteoporos Int. 2012;23(10):2543–50.
Nickolas TL, Stein EM, Dworakowski E, Nishiyama KK, Komandah-Kosseh M, Zhang CA, et al. Rapid cortical bone loss in patients with chronic kidney disease. J Bone Miner Res Off J Am Soc Bone Miner Res. 2013;28(8):1811–20.
Rodriguez-Garcia M, Gomez-Alonso C, Naves-Diaz M, Diaz-Lopez JB, Diaz-Corte C, Cannata-Andia JB, et al. Vascular calcifications, vertebral fractures and mortality in haemodialysis patients. Nephrol Dial Transplant. 2009;24(1):239–46.
Moe S, Drueke T, Cunningham J, Goodman W, Martin K, Olgaard K, et al. Definition, evaluation, and classification of renal osteodystrophy: a position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2006;69(11):1945–53.
Cunningham J, Danese M, Olson K, Klassen P, Chertow GM. Effects of the calcimimetic cinacalcet HCl on cardiovascular disease, fracture, and health-related quality of life in secondary hyperparathyroidism. Kidney Int. 2005;68(4):1793–800.
Investigators ET, Chertow GM, Block GA, Correa-Rotter R, Drueke TB, Floege J, et al. Effect of cinacalcet on cardiovascular disease in patients undergoing dialysis. N Engl J Med. 2012;367(26):2482–94.
Lee A, Belozeroff V, Song X, Diakun D, Goodman W. Cost of treatment and clinical events for secondary hyperparathyroidism. Am J Pharm Benefits. 2013;5(2):24–35.
Almaden Y, Canalejo A, Hernandez A, Ballesteros E, Garcia-Navarro S, Torres A, et al. Direct effect of phosphorus on PTH secretion from whole rat parathyroid glands in vitro. J Bone Miner Res Off J Am Soc Bone Miner Res. 1996;11(7):970–6.
Gutierrez O, Isakova T, Rhee E, Shah A, Holmes J, Collerone G, et al. Fibroblast growth factor-23 mitigates hyperphosphatemia but accentuates calcitriol deficiency in chronic kidney disease. J Am Soc Nephrol. 2005;16(7):2205–15.
Isakova T, Wahl P, Vargas GS, Gutierrez OM, Scialla J, Xie H, et al. Fibroblast growth factor 23 is elevated before parathyroid hormone and phosphate in chronic kidney disease. Kidney Int. 2011;79(12):1370–8.
Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem. 2006;281(10):6120–3.
Saito H, Kusano K, Kinosaki M, Ito H, Hirata M, Segawa H, et al. Human fibroblast growth factor-23 mutants suppress Na+-dependent phosphate co-transport activity and 1alpha,25-dihydroxyvitamin D3 production. J Biol Chem. 2003;278(4):2206–11.
Jones G, Prosser DE, Kaufmann M. 25-Hydroxyvitamin D-24-hydroxylase (CYP24A1): its important role in the degradation of vitamin D. Arch Biochem Biophys. 2012;523(1):9–18.
Lewin E, Huan J, Olgaard K. Parathyroid growth and suppression in renal failure. Semin Dial. 2006;19(3):238–45.
Valimaki S, Farnebo F, Forsberg L, Larsson C, Farnebo LO. Heterogeneous expression of receptor mRNAs in parathyroid glands of secondary hyperparathyroidism. Kidney Int. 2001;60(5):1666–75.
Galitzer H, Ben-Dov IZ, Silver J, Naveh-Many T. Parathyroid cell resistance to fibroblast growth factor 23 in secondary hyperparathyroidism of chronic kidney disease. Kidney Int. 2010;77(3):211–8.
Trivedi H, Szabo A, Zhao S, Cantor T, Raff H. Circadian variation of mineral and bone parameters in end-stage renal disease. J Nephrol. 2014;28(3):351–9.
Sturgeon CM, Sprague SM, Metcalfe W. Variation in parathyroid hormone immunoassay results—a critical governance issue in the management of chronic kidney disease. Nephrol Dial Transplant. 2011;26(11):3440–5.
Garrett G, Sardiwal S, Lamb EJ, Goldsmith DJ. PTH—a particularly tricky hormone: why measure it at all in kidney patients? Clin J Am Soc Nephrol. 2013;8(2):299–312.
Monier-Faugere MC, Geng Z, Mawad H, Friedler RM, Gao P, Cantor TL, et al. Improved assessment of bone turnover by the PTH-(1-84)/large C-PTH fragments ratio in ESRD patients. Kidney Int. 2001;60(4):1460–8.
Coen G, Bonucci E, Ballanti P, Balducci A, Calabria S, Nicolai GA, et al. PTH 1-84 and PTH “7-84” in the noninvasive diagnosis of renal bone disease. Am J Kidney Dis. 2002;40(2):348–54.
Lehmann G, Stein G, Huller M, Schemer R, Ramakrishnan K, Goodman WG. Specific measurement of PTH (1–84) in various forms of renal osteodystrophy (ROD) as assessed by bone histomorphometry. Kidney Int. 2005;68(3):1206–14.
Herberth J, Branscum AJ, Mawad H, Cantor T, Monier-Faugere MC, Malluche HH. Intact PTH combined with the PTH ratio for diagnosis of bone turnover in dialysis patients: a diagnostic test study. Am J Kidney Dis. 2010;55(5):897–906.
Inaba M, Okuno S, Imanishi Y, Ishimura E, Yamakawa T, Shoji S. Increased active PTH (1–84) fraction as a predictor of poor mortality in male hemodialysis patients. Osteoporos Int. 2013;24(11):2863–70.
Zitt E, Kirsch AH, Haueis M, Strasak A, Neyer U, Mayer G, et al. The PTH (1–84)/non-PTH (1–84) ratio is a risk factor for cardiovascular events in hemodialysis patients. Clin Nephrol. 2011;75(4):309–18.
Melamed ML, Eustace JA, Plantinga LC, Jaar BG, Fink NE, Parekh RS, et al. Third-generation parathyroid hormone assays and all-cause mortality in incident dialysis patients: the CHOICE study. Nephrol Dial Transplant. 2008;23(5):1650–8.
Kidney Disease: Improving Global Outcomes CKDMBDWG. KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl. 2009;113:S1–130.
Barreto FC, Barreto DV, Moyses RM, Neves KR, Canziani ME, Draibe SA, et al. K/DOQI-recommended intact PTH levels do not prevent low-turnover bone disease in hemodialysis patients. Kidney Int. 2008;73(6):771–7.
Coen G, Ballanti P, Bonucci E, Calabria S, Costantini S, Ferrannini M, et al. Renal osteodystrophy in predialysis and hemodialysis patients: comparison of histologic patterns and diagnostic predictivity of intact PTH. Nephron. 2002;91(1):103–11.
Coen G, Mazzaferro S, Ballanti P, Sardella D, Chicca S, Manni M, et al. Renal bone disease in 76 patients with varying degrees of predialysis chronic renal failure: a cross-sectional study. Nephrol Dial Transplant. 1996;11(5):813–9.
Coen G, Ballanti P, Bonucci E, Calabria S, Centorrino M, Fassino V, et al. Bone markers in the diagnosis of low turnover osteodystrophy in haemodialysis patients. Nephrol Dial Transplant. 1998;13(9):2294–302.
Lehmann G, Ott U, Kaemmerer D, Schuetze J, Wolf G. Bone histomorphometry and biochemical markers of bone turnover in patients with chronic kidney disease stages 3–5. Clin Nephrol. 2008;70(4):296–305.
Beddhu S, Baird B, Ma X, Cheung AK, Greene T. Serum alkaline phosphatase and mortality in hemodialysis patients. Clin Nephrol. 2010;74(2):91–6.
Rhee CM, Molnar MZ, Lau WL, Ravel V, Kovesdy CP, Mehrotra R, et al. Comparative mortality-predictability using alkaline phosphatase and parathyroid hormone in patients on peritoneal dialysis and hemodialysis. Perit Dial Int. 2014;34(7):732–48.
Noordzij M, Korevaar JC, Boeschoten EW, Dekker FW, Bos WJ, Krediet RT, et al. The kidney disease outcomes quality initiative (K/DOQI) guideline for bone metabolism and disease in CKD: association with mortality in dialysis patients. Am J Kidney Dis. 2005;46(5):925–32.
Kalantar-Zadeh K, Kuwae N, Regidor DL, Kovesdy CP, Kilpatrick RD, Shinaberger CS, et al. Survival predictability of time-varying indicators of bone disease in maintenance hemodialysis patients. Kidney Int. 2006;70(4):771–80.
Kimata N, Albert JM, Akiba T, Yamazaki S, Kawaguchi T, Fukuhara S, et al. Association of mineral metabolism factors with all-cause and cardiovascular mortality in hemodialysis patients: the Japan dialysis outcomes and practice patterns study. Hemodial Int Int Symp Home Hemodial. 2007;11(3):340–8.
Isakova T, Gutierrez OM, Smith K, Epstein M, Keating LK, Juppner H, et al. Pilot study of dietary phosphorus restriction and phosphorus binders to target fibroblast growth factor 23 in patients with chronic kidney disease. Nephrol Dial Transplant. 2011;26(2):584–91.
Burnett SM, Gunawardene SC, Bringhurst FR, Juppner H, Lee H, Finkelstein JS. Regulation of C-terminal and intact FGF-23 by dietary phosphate in men and women. J Bone Miner Res Off J Am Soc Bone Miner Res. 2006;21(8):1187–96.
Shinaberger CS, Greenland S, Kopple JD, Van Wyck D, Mehrotra R, Kovesdy CP, et al. Is controlling phosphorus by decreasing dietary protein intake beneficial or harmful in persons with chronic kidney disease? Am J Clin Nutr. 2008;88(6):1511–8.
Navaneethan SD, Palmer SC, Craig JC, Elder GJ, Strippoli GF. Benefits and harms of phosphate binders in CKD: a systematic review of randomized controlled trials. Am J Kidney Dis. 2009;54(4):619–37.
Chertow GM, Burke SK, Raggi P, Treat to Goal Working G. Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int. 2002;62(1):245–52.
Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, et al. Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int. 2005;68(4):1815–24.
Joy MS, Finn WF, Group LAMS. Randomized, double-blind, placebo-controlled, dose-titration, phase III study assessing the efficacy and tolerability of lanthanum carbonate: a new phosphate binder for the treatment of hyperphosphatemia. Am J Kidney Dis. 2003;42(1):96–107.
Goldberg DI, Dillon MA, Slatopolsky EA, Garrett B, Gray JR, Marbury T, et al. Effect of RenaGel, a non-absorbed, calcium- and aluminium-free phosphate binder, on serum phosphorus, calcium, and intact parathyroid hormone in end-stage renal disease patients. Nephrol Dial Transplant. 1998;13(9):2303–10.
Block GA, Wheeler DC, Persky MS, Kestenbaum B, Ketteler M, Spiegel DM, et al. Effects of phosphate binders in moderate CKD. J Am Soc Nephrol. 2012;23(8):1407–15.
Russo D, Corrao S, Miranda I, Ruocco C, Manzi S, Elefante R, et al. Progression of coronary artery calcification in predialysis patients. Am J Nephrol. 2007;27(2):152–8.
Zhang C, Wen J, Li Z, Fan J. Efficacy and safety of lanthanum carbonate on chronic kidney disease-mineral and bone disorder in dialysis patients: a systematic review. BMC Nephrol. 2013;14:226.
Oliveira RB, Cancela AL, Graciolli FG, Dos Reis LM, Draibe SA, Cuppari L, et al. Early control of PTH and FGF23 in normophosphatemic CKD patients: a new target in CKD-MBD therapy? Clin J Am Soc Nephrol. 2010;5(2):286–91.
Suki WN, Zabaneh R, Cangiano JL, Reed J, Fischer D, Garrett L, et al. Effects of sevelamer and calcium-based phosphate binders on mortality in hemodialysis patients. Kidney Int. 2007;72(9):1130–7.
Toussaint ND, Lau KK, Polkinghorne KR, Kerr PG. Attenuation of aortic calcification with lanthanum carbonate versus calcium-based phosphate binders in haemodialysis: a pilot randomized controlled trial. Nephrology (Carlton). 2011;16(3):290–8.
Barreto DV, Barreto Fde C, de Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, et al. Phosphate binder impact on bone remodeling and coronary calcification—results from the BRiC study. Nephron Clin Pract. 2008;110(4):c273–83.
Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, et al. A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: the Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis. 2008;51(6):952–65.
Jamal SA, Fitchett D, Lok CE, Mendelssohn DC, Tsuyuki RT. The effects of calcium-based versus non-calcium-based phosphate binders on mortality among patients with chronic kidney disease: a meta-analysis. Nephrol Dial Transplant. 2009;24(10):3168–74.
Jamal SA, Vandermeer B, Raggi P, Mendelssohn DC, Chatterley T, Dorgan M, et al. Effect of calcium-based versus non-calcium-based phosphate binders on mortality in patients with chronic kidney disease: an updated systematic review and meta-analysis. Lancet. 2013;382(9900):1268–77.
Mudge DW, Johnson DW, Hawley CM, Campbell SB, Isbel NM, van Eps CL, et al. Do aluminium-based phosphate binders continue to have a role in contemporary nephrology practice? BMC Nephrol. 2011;12:20.
2012 annual report of the dialysis outcomes and practice patterns study: hemodialysis data 1997–2011. Ann Arbor: Arbor Research Collaborative for Health; 2012.
Floege J, Covic AC, Ketteler M, Rastogi A, Chong EM, Gaillard S, et al. A phase III study of the efficacy and safety of a novel iron-based phosphate binder in dialysis patients. Kidney Int. 2014;86(3):638–47.
Block GA, Brillhart SL, Persky MS, Amer A, Slade AJ. Efficacy and safety of SBR759, a new iron-based phosphate binder. Kidney Int. 2010;77(10):897–903.
Fukagawa M, Kasuga H, Joseph D, Sawata H, Junge G, Moore A, et al. Efficacy and safety of SBR759, a novel calcium-free, iron (III)-based phosphate binder, versus placebo in chronic kidney disease stage V Japanese patients on maintenance renal replacement therapy. Clin Exp Nephrol. 2014;18(1):135–43.
Chen JB, Chiang SS, Chen HC, Obayashi S, Nagasawa M, Hexham JM, et al. Efficacy and safety of SBR759, a novel calcium-free, iron(III)-based phosphate binder, in Asian patients undergoing hemodialysis: a 12-week, randomized, open-label, dose-titration study versus sevelamer hydrochloride. Nephrology (Carlton). 2011;16(8):743–50.
Gonzalez EA, Sachdeva A, Oliver DA, Martin KJ. Vitamin D insufficiency and deficiency in chronic kidney disease. A single center observational study. Am J Nephrol. 2004;24(5):503–10.
Levin A, Bakris GL, Molitch M, Smulders M, Tian J, Williams LA, et al. Prevalence of abnormal serum vitamin D, PTH, calcium, and phosphorus in patients with chronic kidney disease: results of the study to evaluate early kidney disease. Kidney Int. 2007;71(1):31–8.
de Oliveira RA, Barreto FC, Mendes M, Dos Reis LM, Castro JH, Britto ZM, et al. Peritoneal dialysis per se is a risk factor for sclerostin-associated adynamic bone disease. Kidney Int. 2014;87(5):1039–45.
Wolf M, Shah A, Gutierrez O, Ankers E, Monroy M, Tamez H, et al. Vitamin D levels and early mortality among incident hemodialysis patients. Kidney Int. 2007;72(8):1004–13.
Ravani P, Malberti F, Tripepi G, Pecchini P, Cutrupi S, Pizzini P, et al. Vitamin D levels and patient outcome in chronic kidney disease. Kidney Int. 2009;75(1):88–95.
Teng M, Wolf M, Ofsthun MN, Lazarus JM, Hernan MA, Camargo CA Jr, et al. Activated injectable vitamin D and hemodialysis survival: a historical cohort study. J Am Soc Nephrol. 2005;16(4):1115–25.
Tentori F, Hunt WC, Stidley CA, Rohrscheib MR, Bedrick EJ, Meyer KB, et al. Mortality risk among hemodialysis patients receiving different vitamin D analogs. Kidney Int. 2006;70(10):1858–65.
Cozzolino M, Brancaccio D, Cannella G, Messa P, Gesualdo L, Marangella M, et al. VDRA therapy is associated with improved survival in dialysis patients with serum intact PTH ≤150 pg/mL: results of the Italian FARO Survey. Nephrol Dial Transplant. 2012;27(9):3588–94.
Kovesdy CP, Ahmadzadeh S, Anderson JE, Kalantar-Zadeh K. Association of activated vitamin D treatment and mortality in chronic kidney disease. Arch Intern Med. 2008;168(4):397–403.
Naves-Diaz M, Alvarez-Hernandez D, Passlick-Deetjen J, Guinsburg A, Marelli C, Rodriguez-Puyol D, et al. Oral active vitamin D is associated with improved survival in hemodialysis patients. Kidney Int. 2008;74(8):1070–8.
Shoben AB, Rudser KD, de Boer IH, Young B, Kestenbaum B. Association of oral calcitriol with improved survival in nondialyzed CKD. J Am Soc Nephrol. 2008;19(8):1613–9.
Palmer SC, McGregor DO, Macaskill P, Craig JC, Elder GJ, Strippoli GF. Meta-analysis: vitamin D compounds in chronic kidney disease. Ann Intern Med. 2007;147(12):840–53.
Kilpatrick RD, Danese MD, Belozeroff V, Smirnakis K, Goodman WG, Rothman KJ. The association of vitamin D use with hypercalcemia and hyperphosphatemia in hemodialysis patients: a case-crossover study. Pharmacoepidemiol Drug Saf. 2011;20(9):914–21.
Jean G, Terrat JC, Vanel T, Hurot JM, Lorriaux C, Mayor B, et al. Daily oral 25-hydroxycholecalciferol supplementation for vitamin D deficiency in haemodialysis patients: effects on mineral metabolism and bone markers. Nephrol Dial Transplant. 2008;23(11):3670–6.
Jean G, Souberbielle JC, Chazot C. Monthly cholecalciferol administration in haemodialysis patients: a simple and efficient strategy for vitamin D supplementation. Nephrol Dial Transplant. 2009;24(12):3799–805.
Armas LA, Andukuri R, Barger-Lux J, Heaney RP, Lund R. 25-Hydroxyvitamin D response to cholecalciferol supplementation in hemodialysis. Clin J Am Soc Nephrol. 2012;7(9):1428–34.
Dusso AS, Finch J, Brown A, Ritter C, Delmez J, Schreiner G, et al. Extrarenal production of calcitriol in normal and uremic humans. J Clin Endocrinol Metab. 1991;72(1):157–64.
Hewison M, Zehnder D, Bland R, Stewart PM. 1alpha-Hydroxylase and the action of vitamin D. J Mol Endocrinol. 2000;25(2):141–8.
Delanaye P, Weekers L, Warling X, Moonen M, Smelten N, Medart L, et al. Cholecalciferol in haemodialysis patients: a randomized, double-blind, proof-of-concept and safety study. Nephrol Dial Transplant. 2013;28(7):1779–86.
Bhan I, Dobens D, Tamez H, Deferio JJ, Li YC, Warren HS, et al. Nutritional vitamin D supplementation in dialysis: a randomized trial. Clin J Am Soc Nephrol. 2015;10(4):611–9.
Kooienga L, Fried L, Scragg R, Kendrick J, Smits G, Chonchol M. The effect of combined calcium and vitamin D3 supplementation on serum intact parathyroid hormone in moderate CKD. Am J Kidney Dis. 2009;53(3):408–16.
Chandra P, Binongo JN, Ziegler TR, Schlanger LE, Wang W, Someren JT, et al. Cholecalciferol (vitamin D3) therapy and vitamin D insufficiency in patients with chronic kidney disease: a randomized controlled pilot study. Endocr Pract Off J Am Coll Endocrinol Am Assoc Clin Endocrinol. 2008;14(1):10–7.
Alvarez JA, Law J, Coakley KE, Zughaier SM, Hao L, Shahid Salles K, et al. High-dose cholecalciferol reduces parathyroid hormone in patients with early chronic kidney disease: a pilot, randomized, double-blind, placebo-controlled trial. Am J Clin Nutr. 2012;96(3):672–9.
Zisman AL, Hristova M, Ho LT, Sprague SM. Impact of ergocalciferol treatment of vitamin D deficiency on serum parathyroid hormone concentrations in chronic kidney disease. Am J Nephrol. 2007;27(1):36–43.
Al-Aly Z, Qazi RA, Gonzalez EA, Zeringue A, Martin KJ. Changes in serum 25-hydroxyvitamin D and plasma intact PTH levels following treatment with ergocalciferol in patients with CKD. Am J Kidney Dis. 2007;50(1):59–68.
Andress DL, Norris KC, Coburn JW, Slatopolsky EA, Sherrard DJ. Intravenous calcitriol in the treatment of refractory osteitis fibrosa of chronic renal failure. N Engl J Med. 1989;321(5):274–9.
Hamdy NA, Kanis JA, Beneton MN, Brown CB, Juttmann JR, Jordans JG, et al. Effect of alfacalcidol on natural course of renal bone disease in mild to moderate renal failure. BMJ. 1995;310(6976):358–63.
Rix M, Eskildsen P, Olgaard K. Effect of 18 months of treatment with alfacalcidol on bone in patients with mild to moderate chronic renal failure. Nephrol Dial Transplant. 2004;19(4):870–6.
Reichel H. Low-dose alfacalcidol controls secondary hyperparathyroidism in predialysis chronic kidney disease. Nephron Clin Pract. 2010;114(4):c268–76.
Lessard M, Ouimet D, Leblanc M, Nadeau-Fredette AC, Bell R, Lafrance JP, et al. Comparison of oral and intravenous alfacalcidol in chronic hemodialysis patients. BMC Nephrol. 2014;15:27.
Alghareeb A, Sabry A, Bawadekji H, Alsaran K. Intravenous alfacalcidol once versus twice or thrice weekly in hemodialysis patients. Ther Apheresis Dial Off Peer Rev J Int Soc Apheresis Jpn Soc Apheresis Jpn Soc Dial Therapy. 2013;17(1):30–4.
Tarrass F, Yazidi A, Sif H, Zamd M, Benghanem MG, Ramdani B. A randomized trial of intermittent versus continuous oral alfacalcidol treatment of hyperparathyroidism in end-stage renal disease. Clin Nephrol. 2006;65(6):415–8.
Coburn JW, Maung HM, Elangovan L, Germain MJ, Lindberg JS, Sprague SM, et al. Doxercalciferol safely suppresses PTH levels in patients with secondary hyperparathyroidism associated with chronic kidney disease stages 3 and 4. Am J Kidney Dis. 2004;43(5):877–90.
Tan AU Jr, Levine BS, Mazess RB, Kyllo DM, Bishop CW, Knutson JC, et al. Effective suppression of parathyroid hormone by 1 alpha-hydroxy-vitamin D2 in hemodialysis patients with moderate to severe secondary hyperparathyroidism. Kidney Int. 1997;51(1):317–23.
Maung HM, Elangovan L, Frazao JM, Bower JD, Kelley BJ, Acchiardo SR, et al. Efficacy and side effects of intermittent intravenous and oral doxercalciferol (1alpha-hydroxyvitamin D(2)) in dialysis patients with secondary hyperparathyroidism: a sequential comparison. Am J Kidney Dis. 2001;37(3):532–43.
Frazao JM, Elangovan L, Maung HM, Chesney RW, Acchiardo SR, Bower JD, et al. Intermittent doxercalciferol (1alpha-hydroxyvitamin D(2)) therapy for secondary hyperparathyroidism. Am J Kidney Dis. 2000;36(3):550–61.
Coyne D, Acharya M, Qiu P, Abboud H, Batlle D, Rosansky S, et al. Paricalcitol capsule for the treatment of secondary hyperparathyroidism in stages 3 and 4 CKD. Am J Kidney Dis. 2006;47(2):263–76.
Thadhani R, Tamez H, Solomon SD. Vitamin D therapy and cardiac function in chronic kidney disease-reply. JAMA J Am Med Assoc. 2012;307(21):2253.
Ross EA, Tian J, Abboud H, Hippensteel R, Melnick JZ, Pradhan RS, et al. Oral paricalcitol for the treatment of secondary hyperparathyroidism in patients on hemodialysis or peritoneal dialysis. Am J Nephrol. 2008;28(1):97–106.
Ong LM, Narayanan P, Goh HK, Manocha AB, Ghazali A, Omar M, et al. Randomized controlled trial to compare the efficacy and safety of oral paricalcitol with oral calcitriol in dialysis patients with secondary hyperparathyroidism. Nephrology (Carlton). 2013;18(3):194–200.
Sprague SM, Llach F, Amdahl M, Taccetta C, Batlle D. Paricalcitol versus calcitriol in the treatment of secondary hyperparathyroidism. Kidney Int. 2003;63(4):1483–90.
Teng M, Wolf M, Lowrie E, Ofsthun N, Lazarus JM, Thadhani R. Survival of patients undergoing hemodialysis with paricalcitol or calcitriol therapy. N Engl J Med. 2003;349(5):446–56.
Hansen D, Rasmussen K, Danielsen H, Meyer-Hofmann H, Bacevicius E, Lauridsen TG, et al. No difference between alfacalcidol and paricalcitol in the treatment of secondary hyperparathyroidism in hemodialysis patients: a randomized crossover trial. Kidney Int. 2011;80(8):841–50.
Ketteler M, Martin KJ, Wolf M, Amdahl M, Cozzolino M, Goldsmith D, et al. Paricalcitol versus cinacalcet plus low-dose vitamin D therapy for the treatment of secondary hyperparathyroidism in patients receiving haemodialysis: results of the IMPACT SHPT study. Nephrol Dial Transplant. 2012;27(8):3270–8.
Cozzolino M, Ketteler M, Martin KJ, Sharma A, Goldsmith D, Khan S. Paricalcitol- or cinacalcet-centred therapy affects markers of bone mineral disease in patients with secondary hyperparathyroidism receiving haemodialysis: results of the IMPACT-SHPT study. Nephrol Dial Transplant. 2014;29(4):899–905.
Garside R, Pitt M, Anderson R, Mealing S, D’Souza R, Stein K. The cost-utility of cinacalcet in addition to standard care compared to standard care alone for secondary hyperparathyroidism in end-stage renal disease: a UK perspective. Nephrol Dial Transplant Off Publ Eur Dial Transplant Assoc Eur Renal Assoc. 2007;22(5):1428–36.
NICE. Cinacalcet for the treatment of secondary hyperparathyroidism in patients with end-stage renal disease on maintenance dialysis therapy [TA117]. London: National Institute for Health and Care Excellence; 2007.
Block GA, Martin KJ, de Francisco AL, Turner SA, Avram MM, Suranyi MG, et al. Cinacalcet for secondary hyperparathyroidism in patients receiving hemodialysis. N Engl J Med. 2004;350(15):1516–25.
Lee YT, Ng HY, Kuo CC, Chen TC, Wu CS, Chiu TT, et al. Comparison between calcitriol and calcitriol plus low-dose cinacalcet for the treatment of moderate to severe secondary hyperparathyroidism in chronic dialysis patients. Nutrients. 2013;5(4):1336–48.
Fukagawa M, Fukuma S, Onishi Y, Yamaguchi T, Hasegawa T, Akizawa T, et al. Prescription patterns and mineral metabolism abnormalities in the cinacalcet era: results from the MBD-5D study. Clin J Am Soc Nephrol. 2012;7(9):1473–80.
Wilkie M, Pontoriero G, Macario F, Yaqoob M, Bouman K, Braun J, et al. Impact of vitamin D dose on biochemical parameters in patients with secondary hyperparathyroidism receiving cinacalcet. Nephron Clin Pract. 2009;112(1):c41–50.
Raggi P, Chertow GM, Torres PU, Csiky B, Naso A, Nossuli K, et al. The ADVANCE study: a randomized study to evaluate the effects of cinacalcet plus low-dose vitamin D on vascular calcification in patients on hemodialysis. Nephrol Dial Transplant. 2011;26(4):1327–39.
Parfrey PS, Drueke TB, Block GA, Correa-Rotter R, Floege J, Herzog CA, et al. The effects of cinacalcet in older and younger patients on hemodialysis: the evaluation of cinacalcet HCl therapy to lower cardiovascular events (EVOLVE) trial. Clin J Am Soc Nephrol. 2015;10(5):791–9.
Martin KJ, Pickthorn K, Huang S, Block GA, Vick A, Mount PF, et al. AMG 416 (velcalcetide) is a novel peptide for the treatment of secondary hyperparathyroidism in a single-dose study in hemodialysis patients. Kidney Int. 2014;85(1):191–7.
Iwamoto N, Sato N, Nishida M, Hashimoto T, Kobayashi H, Yamasaki S, et al. Total parathyroidectomy improves survival of hemodialysis patients with secondary hyperparathyroidism. J Nephrol. 2012;25(5):755–63.
Sharma J, Raggi P, Kutner N, Bailey J, Zhang R, Huang Y, et al. Improved long-term survival of dialysis patients after near-total parathyroidectomy. J Am Coll Surg. 2012;214(4):400–7 (discussion 7–8).
Fotheringham J, Balasubramanian SP, Harrison B, Wilkie M. Post-parathyroidectomy parathyroid hormone levels: the impact on patient survival—a single-centre study in a stage 5 chronic kidney disease population. Nephron Clin Pract. 2011;119(2):c113–20.
Santos FR, Moyses RM, Montenegro FL, Jorgetti V, Noronha IL. IL-1beta, TNF-alpha, TGF-beta, and bFGF expression in bone biopsies before and after parathyroidectomy. Kidney Int. 2003;63(3):899–907.
London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC. Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol. 2004;15(7):1943–51.
Chou FF, Chen JB, Lee CH, Chen SH, Sheen-Chen SM. Parathyroidectomy can improve bone mineral density in patients with symptomatic secondary hyperparathyroidism. Arch Surg. 2001;136(9):1064–8.
Yamanouchi M, Ubara Y, Hayami N, Suwabe T, Hiramatsu R, Sumida K, et al. Bone mineral density 5 years after parathyroidectomy in hemodialysis patients with secondary hyperparathyroidism. Clin Nephrol. 2013;79(5):380–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding and conflict of interest
S. N. Salam—none to declare. A. Khwaja–has received lecturing fees from Amgen. M. E. Wilkie—has received lecturing fees and a research Grant from Baxter. No funding was received for the preparation of this manuscript.
Rights and permissions
About this article
Cite this article
Salam, S.N., Khwaja, A. & Wilkie, M.E. Pharmacological Management of Secondary Hyperparathyroidism in Patients with Chronic Kidney Disease. Drugs 76, 841–852 (2016). https://doi.org/10.1007/s40265-016-0575-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s40265-016-0575-2