Abstract
Purpose of Review
Numerous forms of osteoporosis in childhood are characterized by low bone turnover (for example, osteoporosis due to neuromuscular disorders and glucocorticoid exposure). Anti-resorptive therapy, traditionally used to treat osteoporosis in the young, is associated with further reductions in bone turnover, raising concerns about the long-term safety and efficacy of such therapy. These observations have led to increasing interest in the role of anabolic therapy to treat pediatric osteoporosis.
Recent Findings
While growth hormone and androgens appears to be relatively weak anabolic modulators of bone mass, emerging therapies targeting bone formation pathways (anti-transforming growth factor beta antibody and anti-sclerostin antibody) hold considerable promise. Teriparatide remains an attractive option that merits formal study for patients post-epiphyseal fusion, although it must be considered that adult studies have shown its effect is blunted when administered following bisphosphonate therapy. Mechanical stimulation of bone through whole body vibration therapy appears to be much less effective than bisphosphonate therapy for treating osteoporosis in children.
Summary
New anabolic therapies which target important pathways in skeletal metabolism merit further study in children, including their effects on fracture risk reduction and after treatment discontinuation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
After decades of experience with anti-resorptive therapy to treat pediatric osteoporosis, particularly intravenous bisphosphonates such as pamidronate and zoledronic acid [1, 2], there is now considerable interest in the role of anabolic therapy to treat pediatric osteoporosis. This enthusiasm stems from the finding that several chronic illnesses of childhood such as neuromuscular disorders and glucocorticoid-treated diseases are associated with low bone turnover osteoporosis [3, 4], an observation that is exacerbated by commonly used anti-resorptive agents such as bisphosphonate therapy [4, 5]. Anabolic therapy for the treatment of osteoporosis falls into three main categories: hormone therapy (either as replacement therapy or prescribed in attempts to over-ride the deleterious effects of diseases or their treatment on bone, such as glucocorticoid-induced osteoporosis), mechanical stimulation such as whole body vibration (WBV) therapy, and biologic anabolic (antibody) therapy. The purpose of this review is to discuss the current evidence for the use of these three classes of anabolic therapy in the treatment of osteoporotic conditions of childhood.
Anabolic Hormonal Therapy
Growth Hormone Treatment
Growth hormone (GH) is best known for its growth-promoting effects on growth plate cartilage resulting in endochondral bone formation and thereby longitudinal growth during childhood and adolescence. A number of studies have reported reduced bone mineral density (BMD) in adults and children with GH deficiency (GHD) [6, 7]; studies in adults have also shown a higher risk of fractures [8, 9]. Whether children with GHD have a higher risk of fractures has not been formally studied, though an international group of experts concluded that this was an important step in defining the scope of the problem in pediatric practice [10]. To date, this remains an unresolved question, even though it is pivotal to understand the extent to which bone health outcomes should be monitored in GHD patients during (and after) the pediatric years.
A study of children with GHD during GH replacement therapy provided insight into the muscle-bone response to treatment using a size-independent technique known as peripheral quantitative computed tomography (pQCT) [11]. In this study, pre-pubertal children with GHD receiving 30 mcg/kg/day over 12 months showed a larger increase in cross-sectional muscle and bone size, plus greater increases in strength-strain index, while cortical area and cortical thickness remained unchanged and cortical density decreased (the latter, hypothesized to result from an increase in bone turnover). During the second year, there was additional increase in total bone and marrow areas, bone mineral content (BMC), strength-strain index, and muscle area, while the changes in cortical thickness, cortical density, and cortical area plateaued. The strongest correlation among muscle-bone parameters was between muscle area and strength-strain index, suggesting that improved muscle strength was a key determinant of the positive changes in bone geometry. These observations were in line with others who concluded that GH therapy in GHD largely mediates bone strength through changes in bone geometry as opposed to BMD [12].
Given these observations in children with GHD on replacement therapy, the question then arises whether there are any benefits of GH therapy to treat non-deficient children with osteoporosis (i.e., children with low bone mass and fractures). This has been tested in two clinical situations, both of which are associated with growth failure: patients with osteogenesis imperfecta (OI), where extreme short stature is a feature of the more severe forms, and glucocorticoid-treated patients with inflammatory disorders. In the former, 26 patients with OI types III and IV were treated with GH for 1 year; half of the patients had a 50% increase over their baseline growth rate, whereas the other half did not [13]. The half of children who were “responders” also had increases in lumbar spine BMD by dual energy x-ray absorptiometry (DXA) on the order of 5 to 7% over 6 months, whereas the “non-responders” had more attenuated changes. The impact of GH therapy on final height was not reported, making it difficult to assess the long-term benefit to linear growth. On the other hand, modest gains of 5 to 7% in lumbar spine BMD do not rival the 50% increase in bone mass achieved with intravenous bisphosphonate therapy in the first year of therapy [14]; as such, to date, GH therapy has not supplanted intravenous bisphosphonate therapy in OI.
Among patients with GH-treated inflammatory disorders, the longer-term effect on height has been modest at best, with most studies reporting a positive effect on muscle and bone [15, 16]. However, a number of patients also experienced adverse events potentially linked to GH therapy including reactivation of the underlying disease, glucose intolerance, and osteonecrosis [15, 16].
Boys with Duchenne muscular dystrophy (DMD) treated with glucocorticoid are particularly affected by both short stature and bone fragility, given the high glucocorticoid doses that are used and the fact that they are continued in many patients for many years [17]. The effect of GH on muscle strength in DMD has not been studied; however, since it appears that the main effect of GH on bone strength is via muscle strength, and since muscle damage and fibrosis begin early in life in boys with DMD, it is unlikely that GH would be a major modifier of bone strength in this context. The effect of GH on height in boys with DMD was tested in an uncontrolled pre-post study (39 boys, average age 11.5 years), with results showing an average increase in height velocity from 1.2 cm in the year prior to GH therapy to 5.3 cm in the next year while on growth hormone, effectively preventing a decline in growth velocity that was associated with stabilization at a height z-score of − 2.9 [18]. Growth hormone did not alter the rate of declines in motor or cardiopulmonary function. Three patients experienced side effects (worsening of scoliosis, benign intra-cranial hypertension, and impaired fasting glucose).
Taken together, GH appears to be relatively weak as a bone-targeted anabolic therapy outside of the GH deficiency setting. Given the expense of GH, the burden to children (injections multiple times per week), the potential for side effects, and uncertainties about the longer-term safety, the benefits of therapy to treat or prevent osteoporosis outside of hormone replacement therapy for GH deficiency do not appear to justify the risks, costs, and inconvenience.
Androgen Therapy
Unlike GH therapy, which may be prescribed in children with short stature despite normal GH secretory status (i.e., idiopathic short stature, small-for-gestational-age, chronic glucocorticoid therapy), testosterone is not typically administered except for hormone replacement therapy in boys with delayed puberty (defined as lack of pubertal signs by 14). The effect of testosterone replacement therapy on bone health in boys with delayed puberty has not been reported; however, a few adult studies have examined the benefits of testosterone as replacement therapy in adult men [19,20,21]. A recent large, double-blind placebo-controlled study of testosterone gel over 1 year in older men with low testosterone levels evaluated bone health outcomes by finite element analysis on computed tomography at the spine and the hip [19]. This study showed an increase in volumetric BMD and estimated bone strength at the spine (more so in trabecular than cortical bone) and to a lesser extent at the hip. The effect of testosterone on fracture rates in any population remains unstudied.
Oxandrolone, a non-aromatizable synthetic derivative of 5-alpha dihydrotestosterone, may be more useful as an anabolic agent in children (compared to testosterone), since the non-aromatizable structure prevents conversion to estrogen, which hastens epiphyseal closure and has potential to reduce final adult height. Reeves et al. [22] carried out a study using oxandrolone in children for up to 5 years (average duration 16 months) following severe burns. The rationale for androgen therapy in this context is that severe burns cause hyper-catabolic and metabolic states associated with increases in cardiac work, resting energy expenditure, and muscle protein degradation [23]. In children, this translates into loss of lean mass as well as growth impairment; even several years after the injury, children have reduced growth velocities compared to healthy controls [24], an observation that may be mitigated with GH therapy [25]. This controlled study (approximately 2:1 randomization to placebo versus oxandrolone in both boys and girls) was associated with increases in DXA-based total body and lumbar spine BMC, most notably in children over 7 years of age compared to those who were younger [22]. No significant side effects were reported, suggesting oxandrolone may be an effective method for preventing bone loss due to hypercatabolism-induced burn injury.
Parathyroid Hormone
Teriparatide-recombinant human parathyroid hormone (PTH) (1-34) is approved by the Food and Drug Administration for initial treatment of women with post-menopausal osteoporosis (PMO) who are at high risk of fracture or who have failed prior osteoporosis therapy, and for glucocorticoid-associated osteoporosis [26]. Teriparatide significantly reduces the risk of vertebral and non-vertebral fractures in women with PMO; the effect on hip fractures was inconclusive due to low incidence during a large, randomized controlled trial [27]. Overall, teriparatide appears to have a dramatic effect on spine BMD, without evidence for effect at the hip and forearm [27]. Unfortunately, this anabolic agent has a black box warning against its use in children due to the observation of osteosarcoma in one strain of growing rats treated with doses that were 3 to 50 times higher than the adult human equivalent and for much longer durations [28]. Subsequent studies in the same strain of rats showed no malignant bone changes with doses restricted to three times the human equivalent [29]. Despite the second suite of experiments, which were more appropriate for knowledge translation to the clinical setting, clinicians understandably remain hesitant to use teriparatide in patients with open epiphyses. In adults, it is not recommended for those at increased risk of osteosarcoma, such as adults with Paget disease, history of skeletal radiation, or unexplained elevations in alkaline phosphatase [26]. BMD declines rapidly in the first year following teriparatide discontinuation, although reductions in fracture rates may persist for up to 2 years [30]. Alendronate following teriparatide therapy has been shown to mitigate this loss [31].
Perhaps the most compelling clinical scenario that would theoretically benefit from osteoanabolic therapy such as teriparatide is osteoporosis due to DMD, where both vertebral and non-vertebral fractures are frequent and the clinical sequelae potentially devastating, including premature permanent loss of ambulation [32]. We have previously shown that bone turnover on trabecular surfaces is reduced in boys with DMD even prior to bisphosphonate therapy, falling dramatically to 10% of the healthy average after 2 years of pamidronate or zoledronic acid when given to treat painful vertebral fractures [4, 5]. A recent case report of teriparatide in a 20-year-old man with DMD described complete resolution of back pain due to vertebral fractures following 6 months of therapy, along with increases in lumbar spine BMD, bone biomarkers, and improved quality of life [33]. These findings are consistent with a study using black bear PTH (bbPTH) in the murine model of DMD (the mdx mouse), where microcomputed tomography analyses of long bone metaphyses showed marked increases in bone volume fraction, trabecular number, and osteoblast area compared to wild-type mice [34]. Taken together, these preliminary pre-clinical and human findings support further investigation into the use of PTH as an anabolic treatment for DMD-associated osteoporosis. It should be noted, however, that the effect of PTH on bone appears to be blunted in adults when administered following bisphosphonate therapy [35], an observation which may be a limiting factor in young men with DMD who previously received bisphosphonates in childhood for vertebral or long bone fractures.
Whole-Body Vibration
Apart from pharmacological interventions, bone formation can also be increased by mechanical stimulation, especially in growing children. However, typical physical exercise programs and classical physiotherapy usually require a sophisticated setup, such as the availability of a gym and the presence of a coach or physiotherapist, and therefore are often difficult to implement outside of institutional settings. In contrast, most types of whole body vibration (WBV) require only a vibration device that usually can be used at home without professional supervision. This has stirred interest in WBV as a bone anabolic therapy. WBV might elicit anabolic bone effects either directly through vibrations transmitted to the skeleton, or indirectly through effects on the neuromuscular system [36].
WBV is usually performed with the user standing on a motor-driven vibrating plate. The available devices vary widely in several key aspects of vibration exposure, such as vibration frequency (the number of up-and-down cycles per second, expressed in Hertz, Hz) and the peak-to-peak displacement of the vibration plate (from which the peak acceleration generated by the plate can be derived). The direction of the oscillatory movement also varies between devices [37]. Side-alternating plates oscillate around a pivot, so that the left side of the plate moves upwards while the right side moves downwards and vice versa, whereas synchronous vibration plates oscillate up and down on an angle that is fully parallel to the ground and therefore move the right and the left side up and down simultaneously. Intervention protocols also vary widely with regard to the duration and frequency of treatment sessions and whether users passively stand on the vibration device or perform exercises at the same time. As the skeletal effects of vibration likely depend on these parameters, the results of WBV intervention studies must therefore be interpreted in the context of the device and the settings that were used for a given study.
Over the past 15 years, quite a few pediatric WBV studies have reported bone outcomes, but the skeletal effects of the available WBV modalities remain difficult to judge. Many studies do not allow for conclusions, due to inadequate sample size, lack of control groups, short treatment duration, the administration of several concurrent therapies, or statistical issues in the evaluation of results.
The largest and most detailed controlled studies were performed using a “low-magnitude” vibration device (30 Hz, 0.3 g peak acceleration, where g represents the gravitational acceleration at the surface of Earth, 9.81 ms−2). Leonard et al. evaluated 121 individuals with Crohn’s disease aged 8 to 21 years who received the intervention for 10 min per day over a 12-month period [38•]. For 11 of the 12 reported bone densitometric parameters, no treatment differences were found, whereas one parameter, trabecular volumetric BMD z-score at the lumbar spine, showed a larger increase in the active treatment group (z-score difference: 0.24). Although statistically significant, the clinical relevance of such a treatment effect after 12 months of daily intervention is unclear. In comparison, a placebo-controlled study on adolescents with Crohn’s disease found that a single injection of zoledronic acid increased lumbar spine areal BMD z-score by 0.6 over placebo 6 months later [39]. Thus, it appears that “low-magnitude” vibration is much less effective than bisphosphonate therapy, at least when bone density is considered the main outcome measure and under the conditions (i.e., brief daily duration of vibration using the low-magnitude instrument) set out in this trial.
A similar conclusion can be drawn from a study on 123 girls and young women with idiopathic scoliosis, using the same device as the trial by Leonard et al. [40]. Among 88 reported bone outcomes, 4 outcomes differed significantly (P < 0.05) between the treatment and observation groups, consistent with the predicted effect of chance. In any case, the treatment-associated differences in the four outcomes with statistically significant group differences were very small (2% or less), raising a question about their clinical relevance.
Thus, for most approaches, the efficacy of WBV as a bone anabolic treatment is difficult to judge due to lack of informative data. The WBV modality that has been studied in most detail, “low-magnitude” vibration, seems to have a small bone anabolic effect at best.
Anti-Sclerostin Therapy
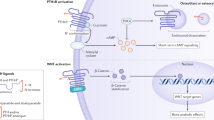
The glycoprotein sclerostin is secreted by osteocytes and inhibits bone formation through its interaction with the LRP5 and LRP6 receptors on the surface of osteoblasts [41]. These receptors contribute to the activation of Wnt signaling in osteoblasts, which is an important pathway to regulate bone formation. When sclerostin interacts with LRP5 or LRP6, Wnt signaling is inhibited and bone formation slows down. Conversely, absence of sclerostin leads to elevated levels of bone formation and high bone mass, as seen in individuals who are homozygous for mutations that decrease or abolish sclerostin production [41, 42].
Sclerostin can be inhibited pharmacologically by systemic application of anti-sclerostin antibodies. In adults, injection of anti-sclerostin antibodies increases bone formation and also decreases bone resorption within a few days, leading to a rapid increase in areal BMD at the spine and hip [43, 44]. A placebo-controlled trial including more than 7000 postmenopausal women with osteoporosis found that sclerostin antibody treatment was associated with a 73% lower incidence of vertebral fractures during the first 12 months of therapy [45•]. At present, the use of anti-sclerostin antibodies has not been reported in children and adolescents but experimental data in animals and information from adult studies highlight some areas that may be relevant for pediatric bone disorders.
Sclerostin antibody appears to be a logical treatment for osteoporosis pseudoglioma syndrome (OPPG), an extremely rare disorder that is caused by homozygous loss-of-function mutations in LRP5, leading to low bone formation. Despite the absence of functional LRP5 in OPPG, sclerostin still inhibits bone formation because LRP6 is not affected [46]. In this context, anti-sclerostin therapy can release the inhibition of bone formation via its action on LRP6 and improve bone mass, at least in an OPPG mouse model [47]. By extension, bone fragility caused by heterozygous LRP5 mutations [48] or by homozygous WNT1 mutations [49], may also represent areas where sclerostin inhibition could be useful. However, data on sclerostin antibody treatment of humans with either LRP5 or WNT1 mutations are not yet available.
There is presently considerable interest in the use of sclerostin antibody to treat OI. OI is a heritable disorder that is usually caused by mutations in one of the two genes coding for collagen type I [2]. There is no indication that Wnt signaling is affected by collagen type I abnormalities, and circulating sclerostin levels are normal in children with such mutations [50]. However, as collagen type I mutations affect osteoblast function, bone anabolic therapy by sclerostin inhibition has an intuitive appeal. Sclerostin antibody treatment increased bone mass and strength in several OI mouse models with mild bone involvement [51,52,53], whereas the beneficial effect of sclerostin inhibition was less obvious in a mouse model of more severe dominant OI [54]. Clinical experience with sclerostin antibody treatment of children with OI has not yet been reported. One potential issue with this approach is that in children with severe OI, bone formation rate is markedly elevated in the absence of any drug therapy [55]. In severe OI, the function of individual osteoblasts is low but this is more than compensated by a markedly increased number of osteoblasts. The elevated pretreatment bone formation rate of severe OI may limit the scope of improvements that can be obtained with bone formation stimulators such as sclerostin antibodies.
Apart from heritable bone fragility disorders, positive effects of sclerostin antibody treatment have been reported in animal models mimicking a range of human bone disorders, such as rheumatoid arthritis [56], inflammatory bowel disease [57], glucocorticoid-induced osteoporosis [58], or orthopedic conditions such as fracture healing [59] and distraction osteogenesis [60]. Sclerostin antibody treatment seems a logical approach for conditions where bone formation is suppressed, such as glucocorticoid-induced osteoporosis. However, controlled studies are required for each potential treatment indication. Even though sclerostin was originally thought to act only on bone cells, recent animal studies indicate that sclerostin antibody treatment could sometimes have detrimental effects in other tissues. For example, sclerostin inhibition accelerated joint destruction in the context of rheumatoid arthritis by interfering with tumor necrosis factor alpha signaling [61•]. This highlights the fact that sclerostin does not only act as an inhibitor of bone formation but may have other functions that may become uncovered with the more widespread use of this treatment approach.
In a large clinical trial, over 4000 women with post-menopausal osteoporosis were randomized 1:1 to monthly sub-cutaneous romosozumab or weekly oral alendronate for 12 months followed by open-label alendronate for another year [62]. There was a 48% lower risk of new vertebral fractures, a 38% reduction in hip fractures, and a 19% reduction in non-vertebral fractures in the romosozumab-to-alendronate group. During year 1, serious cardiovascular adverse events (including cardiac ischemia and cerebrovascular insults) occurred more often with romosozumab than with alendronate (50 of 2040 patients (2.5%) compared to 38 of 2014 patients (1.9%)). However, this imbalance was not seen in an even larger (over 7000 patients) placebo-controlled trial of romosozumab that enrolled a slightly younger post-menopausal population with less advanced osteoporosis [45•]. Whether this observation is a result of a negative effect of romosozumab on cardiovascular health or a protective effect of the comparator, alendronate, remains unsettled.
One issue to consider when contemplating the use of sclerostin antibody therapy in pediatric bone disorders is the fact that this treatment has a short duration of action. In adults, despite ongoing administration, bone formation returns to baseline levels by about 6 months after the first injection of sclerostin antibody and subsequent injections seem to have smaller effects on bone formation [45•]. As bone is a living tissue that adapts to the prevailing environment, it is therefore expected that bone will be rapidly lost once the treatment stimulus wanes, at least when the underlying bone problem persists. This is indeed what has been found both in growing OI mice [63] and in clinical studies on postmenopausal osteoporosis [64]. Consequently, it may be necessary to “lock in” the gains of sclerostin antibody treatment with subsequent long-acting antiresorptive treatment [45•, 63]. The transient nature of the effect of therapy will need to be considered in any future trial of short-acting anabolic therapy in children.
Anti-Transforming Growth Factor-Beta Therapy
Dysregulated transforming growth factor beta (TGF-beta) signaling is associated with a spectrum of heritable disorders that affect the skeleton, such as Camurati-Engelmann disease [65], Marfan syndrome [66], Loeys-Dietz syndrome, and OI [67•]. Mouse studies suggest that injections with anti-TGF-beta antibodies may increase bone mass in OI mouse models [67•], but data in humans are presently not available. Apart from antibody-based approaches, TGF-beta signaling can also be attenuated in some tissues using losartan, an angiotensin II type 1 receptor blocker that lowers the expression of TGF-beta ligands, receptors, and activators [68]. Treatment of a girl with Camurati-Engelmann syndrome with losartan was associated with disappearance of bone pain and a decrease of the (initially elevated) total body areal BMD [69]. A retrospective study in children with Marfan syndrome did not find an effect of losartan on BMD, but more systematic data on the effect of losartan in pediatric bone disorders are lacking [70].
Summary and Conclusions
Hormone replacement therapy in children with growth hormone deficiency and delayed puberty has been shown to at least partially restore indices of bone strength such as BMD and geometry; the effect on fracture rates remains to be studied. On the other hand, hormone therapy to mitigate the adverse effects of glucocorticoids on bone in children without true hormonal deficiencies is a relatively weak modulator of musculoskeletal health compared to bisphosphonates. Furthermore, most of the effect of growth hormone on bone seems to be mediated by its action on muscle size and strength. These observations attenuate enthusiasm for hormone therapy to treat or prevent osteoporosis in chronic illness, particularly when the disease is linked to muscle impairment as in conditions such as DMD. Similarly, whole body vibration therapy appears to be much less effective than bisphosphonates for treating osteoporosis in children, at least when BMD is the main outcome. Teriparatide remains an attractive option that merits formal study for patients’ post-epiphyseal fusion, bearing in mind that its effect may be less following the bone turnover-suppressing effects of bisphosphonate therapy, and that the effect will not be sustained post-discontinuation (thereby warranting longer-activating osteoporosis therapies such as bisphosphonates after teriparatide cessation). The two emerging biologic anabolic therapies are of particular interest for pediatric osteoporosis—anti-TGF-beta antibody and anti-sclerostin antibody. Sclerostin is a potent inhibitor of the Wnt signaling system; pharmacologic inhibition of sclerostin with antibody therapy appears in pre-clinical models to be a potent stimulus of bone formation, making this an attractive agent for pediatric osteoporotic conditions characterized by low bone turnover or osteoblast dysfunction. TGF-beta signaling is dysregulated in a number of pediatric bone disorders, and animal models of OI suggest anti-TGF-beta antibody increases bone mass. On balance, it appears that antibody therapy merits the most attention among the anabolic approaches going forward, recognizing that any positive effects in future human studies will likely only be temporary given the short-acting effects intrinsic to antibody-based therapeutics, and that longer-acting osteoporosis therapy may still be required. To this end, clinical trials are now needed to rigorously assess the safety and efficacy of anabolic therapy in children, including the effects of such therapy not only on surrogates for bone strength such as BMD but also on fracture rates.
Abbreviations
- bb:
-
Black bear
- BMC:
-
Bone mineral content
- BMD:
-
Bone mineral density
- DXA:
-
Dual energy x-ray absorptiometry
- DMD:
-
Duchenne muscular dystrophy
- GH:
-
Growth hormone
- GHD:
-
Growth hormone deficiency
- OPPG:
-
Osteoporosis pseudoglioma syndrome
- PMO:
-
Post-menopausal osteoporosis
- PTH:
-
Parathyroid hormone
- pQCT:
-
Peripheral quantitative computed tomography
- TGF-beta:
-
Transforming growth factor beta
- WBV:
-
Whole body vibration
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Ward LM, Konji VN, Ma J. The management of osteoporosis in children. Osteoporos Int. 2016;27(7):2147–79.
Trejo P, Rauch F. Osteogenesis imperfecta in children and adolescents-new developments in diagnosis and treatment. Osteoporos Int. 2016;27(12):3427–37.
Misof BM, Roschger P, Klaushofer K, Rauch F, Ma J, Mack DR, et al. Increased bone matrix mineralization in treatment-naive children with inflammatory bowel disease. Bone. 2017;105:50–6.
Sbrocchi AM, Rauch F, Jacob P, McCormick A, McMillan HJ, Matzinger MA, et al. The use of intravenous bisphosphonate therapy to treat vertebral fractures due to osteoporosis among boys with Duchenne muscular dystrophy. Osteoporos Int. 2012;23(11):2703–11.
Misof BM, Roschger P, McMillan HJ, Ma J, Klaushofer K, Rauch F, et al. Histomorphometry and bone matrix mineralization before and after bisphosphonate treatment in boys with Duchenne muscular dystrophy: a paired transiliac biopsy study. J Bone Miner Res. 2016;31(5):1060–9.
Rosen T, Hansson T, Granhed H, Szucs J, Bengtsson BA. Reduced bone mineral content in adult patients with growth hormone deficiency. Acta Endocrinol. 1993;129(3):201–6.
Holmes SJ, Economou G, Whitehouse RW, Adams JE, Shalet SM. Reduced bone mineral density in patients with adult onset growth hormone deficiency. J Clin Endocrinol Metab. 1994;78(3):669–74.
Vestergaard P, Jorgensen JO, Hagen C, Hoeck HC, Laurberg P, Rejnmark L, et al. Fracture risk is increased in patients with GH deficiency or untreated prolactinomas—a case-control study. Clin Endocrinol. 2002;56(2):159–67.
Wuster C. Fracture rates in patients with growth hormone deficiency. Horm Res. 2000;54(Suppl 1):31–5.
Cowell CT, Wuster C. The effects of growth hormone deficiency and growth hormone replacement therapy on bone. A meeting report. Horm Res. 2000;54(Suppl 1):68–74.
Schweizer R, Martin DD, Haase M, Roth J, Trebar B, Binder G, et al. Similar effects of long-term exogenous growth hormone (GH) on bone and muscle parameters: a pQCT study of GH-deficient and small-for-gestational-age (SGA) children. Bone. 2007;41(5):875–81.
Hogler W, Briody J, Moore B, Lu PW, Cowell CT. Effect of growth hormone therapy and puberty on bone and body composition in children with idiopathic short stature and growth hormone deficiency. Bone. 2005;37(5):642–50.
Marini JC, Hopkins E, Glorieux FH, Chrousos GP, Reynolds JC, Gundberg CM, et al. Positive linear growth and bone responses to growth hormone treatment in children with types III and IV osteogenesis imperfecta: high predictive value of the carboxyterminal propeptide of type I procollagen. J Bone Miner Res. 2003;18(2):237–43.
Rauch F, Plotkin H, Zeitlin L, Glorieux FH. Bone mass, size, and density in children and adolescents with osteogenesis imperfecta: effect of intravenous pamidronate therapy. J Bone Miner Res. 2003;18(4):610–4.
Frittoli RB, Longhi BS, Silva AM, Filho AAB, Monteiro M, Appenzeller S. Erratum to “Effects of the use of growth hormone in children, adolescents with juvenile idiopathic arthritis: a systematic review” (Rev Bras Reumatol. 2017;57(2):100-106). Rev Bras Reumatol Engl Ed. 2017;57(5):500.
Frittoli RB, Longhi BS, Silva AM, Filho AAB, Monteiro M, Appenzeller S. Effects of the use of growth hormone in children and adolescents with juvenile idiopathic arthritis: a systematic review. Rev Bras Reumatol Engl Ed. 2017;57(2):100–6.
Ward LM, Kinnett K, Bonewald L. Proceedings of a Parent Project Muscular Dystrophy Bone Health Workshop: morbidity due to osteoporosis in DMD: the path forward May 12–13, 2016, Bethesda, Maryland, USA. Neuromuscul Disord. 2017.
Rutter MM, Collins J, Rose SR, Woo JG, Sucharew H, Sawnani H, et al. Growth hormone treatment in boys with Duchenne muscular dystrophy and glucocorticoid-induced growth failure. Neuromuscul Disord. 2012;22(12):1046–56.
Snyder PJ, Kopperdahl DL, Stephens-Shields AJ, Ellenberg SS, Cauley JA, Ensrud KE, et al. Effect of testosterone treatment on volumetric bone density and strength in older men with low testosterone: a controlled clinical trial. JAMA Intern Med. 2017;177(4):471–9.
Benito M, Vasilic B, Wehrli FW, Bunker B, Wald M, Gomberg B, et al. Effect of testosterone replacement on trabecular architecture in hypogonadal men. J Bone Miner Res. 2005;20(10):1785–91.
Tracz MJ, Sideras K, Bolona ER, Haddad RM, Kennedy CC, Uraga MV, et al. Testosterone use in men and its effects on bone health. A systematic review and meta-analysis of randomized placebo-controlled trials. J Clin Endocrinol Metab. 2006;91(6):2011–6.
Reeves PT, Herndon DN, Tanksley JD, Jennings K, Klein GL, Mlcak RP, et al. Five-year outcomes after long-term oxandrolone administration in severely burned children: a randomized clinical trial. Shock. 2016;45(4):367–74.
Jeschke MG, Chinkes DL, Finnerty CC, Kulp G, Suman OE, Norbury WB, et al. Pathophysiologic response to severe burn injury. Ann Surg. 2008;248(3):387–401.
Przkora R, Barrow RE, Jeschke MG, Suman OE, Celis M, Sanford AP, et al. Body composition changes with time in pediatric burn patients. J Trauma. 2006;60(5):968–71. discussion 971
Przkora R, Herndon DN, Suman OE, Jeschke MG, Meyer WJ, Chinkes DL, et al. Beneficial effects of extended growth hormone treatment after hospital discharge in pediatric burn patients. Ann Surg. 2006;243(6):796–801. discussion 801-3
Camacho PM, Petak SM, Binkley N, Clarke BL, Harris ST, Hurley DL, et al. American Association of Clinical Endocrinologists and American College of Endocrinology clinical practice guidelines for the diagnosis and treatment of postmenopausal osteoporosis—2016. Endocr Pract. 2016;22(Suppl 4):1–42.
Neer RM, Arnaud CD, Zanchetta JR, Prince R, Gaich GA, Reginster JY, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med. 2001;344(19):1434–41.
Vahle JL, Sato M, Long GG, Young JK, Francis PC, Engelhardt JA, et al. Skeletal changes in rats given daily subcutaneous injections of recombinant human parathyroid hormone (1-34) for 2 years and relevance to human safety. Toxicol Pathol. 2002;30(3):312–21.
Vahle JL, Long GG, Sandusky G, Westmore M, Ma YL, Sato M. Bone neoplasms in F344 rats given teriparatide [rhPTH(1-34)] are dependent on duration of treatment and dose. Toxicol Pathol. 2004;32(4):426–38.
Lindsay R, Scheele WH, Neer R, Pohl G, Adami S, Mautalen C, et al. Sustained vertebral fracture risk reduction after withdrawal of teriparatide in postmenopausal women with osteoporosis. Arch Intern Med. 2004;164(18):2024–30.
Black DM, Bilezikian JP, Ensrud KE, Greenspan SL, Palermo L, Hue T, et al. One year of alendronate after one year of parathyroid hormone (1-84) for osteoporosis. N Engl J Med. 2005;353(6):555–65.
Ma J, McMillan HJ, Karaguzel G, Goodin C, Wasson J, Matzinger MA, et al. The time to and determinants of first fractures in boys with Duchenne muscular dystrophy. Osteoporos Int. 2017;28(2):597–608.
Catalano A, Vita GL, Russo M, Vita G, Lasco A, Morabito N, et al. Effects of teriparatide on bone mineral density and quality of life in Duchenne muscular dystrophy related osteoporosis: a case report. Osteoporos Int. 2016;27(12):3655–9.
Gray SK, McGee-Lawrence ME, Sanders JL, Condon KW, Tsai CJ, Donahue SW. Black bear parathyroid hormone has greater anabolic effects on trabecular bone in dystrophin-deficient mice than in wild type mice. Bone. 2012;51(3):578–85.
Obermayer-Pietsch BM, Marin F, McCloskey EV, Hadji P, Farrerons J, Boonen S, et al. Effects of two years of daily teriparatide treatment on BMD in postmenopausal women with severe osteoporosis with and without prior antiresorptive treatment. J Bone Miner Res. 2008;23(10):1591–600.
Beck BR. Vibration therapy to prevent bone loss and falls: mechanisms and efficacy. Curr Osteoporos Rep. 2015;13(6):381–9.
Rauch F, Sievanen H, Boonen S, Cardinale M, Degens H, Felsenberg D, et al. Reporting whole-body vibration intervention studies: recommendations of the International Society of Musculoskeletal and Neuronal Interactions. J Musculoskelet Neuronal Interact. 2010;10(3):193–8.
• Leonard MB, Shults J, Long J, Baldassano RN, Brown JK, Hommel K, et al. Effect of low-magnitude mechanical stimuli on bone density and structure in pediatric Crohn's disease: a randomized placebo-controlled trial. J Bone Miner Res. 2016;31(6):1177–88. Even though the study outcome is essentially negative, this report provides an excellent template of how to design, conduct, evaluate and report whole-body vibration studies.
Sbrocchi AM, Forget S, Laforte D, Azouz EM, Rodd C. Zoledronic acid for the treatment of osteopenia in pediatric Crohn's disease. Pediatr Int. 2010;52(5):754–61.
Lam TP, Ng BK, Cheung LW, Lee KM, Qin L, Cheng JC. Effect of whole body vibration (WBV) therapy on bone density and bone quality in osteopenic girls with adolescent idiopathic scoliosis: a randomized, controlled trial. Osteoporos Int. 2013;24(5):1623–36.
Baron R, Kneissel M. WNT signaling in bone homeostasis and disease: from human mutations to treatments. Nat Med. 2013;19(2):179–92.
van Lierop AH, Hamdy NA, van Egmond ME, Bakker E, Dikkers FG, Papapoulos SE. Van Buchem disease: clinical, biochemical, and densitometric features of patients and disease carriers. J Bone Miner Res. 2013;28(4):848–54.
McClung MR, Grauer A, Boonen S, Bolognese MA, Brown JP, Diez-Perez A, et al. Romosozumab in postmenopausal women with low bone mineral density. N Engl J Med. 2014;370(5):412–20.
McColm J, Hu L, Womack T, Tang CC, Chiang AY. Single- and multiple-dose randomized studies of blosozumab, a monoclonal antibody against sclerostin, in healthy postmenopausal women. J Bone Miner Res. 2014;29(4):935–43.
• Cosman F, Crittenden DB, Adachi JD, Binkley N, Czerwinski E, Ferrari S, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med. 2016;375(16):1532–43. This randomized, placebo-controlled study examines the effect of romozosumab on women with post-menopausal osteoporosis, showing a significant reduction in all fractures rates but similar adverse effect frequencies.
Chang MK, Kramer I, Keller H, Gooi JH, Collett C, Jenkins D, et al. Reversing LRP5-dependent osteoporosis and SOST deficiency-induced sclerosing bone disorders by altering WNT signaling activity. J Bone Miner Res. 2014;29(1):29–42.
Kedlaya R, Veera S, Horan DJ, Moss RE, Ayturk UM, Jacobsen CM, et al. Sclerostin inhibition reverses skeletal fragility in an Lrp5-deficient mouse model of OPPG syndrome. Sci Transl Med. 2013;5(211):211ra158.
Korvala J, Juppner H, Makitie O, Sochett E, Schnabel D, Mora S, et al. Mutations in LRP5 cause primary osteoporosis without features of OI by reducing Wnt signaling activity. BMC Med Genet. 2012;13:26.
Fahiminiya S, Majewski J, Mort J, Moffatt P, Glorieux FH, Rauch F. Mutations in WNT1 are a cause of osteogenesis imperfecta. J Med Genet. 2013;50(5):345–8.
Palomo T, Glorieux FH, Rauch F. Circulating sclerostin in children and young adults with heritable bone disorders. J Clin Endocrinol Metab. 2014;99(5):E920–5.
Sinder BP, Eddy MM, Ominsky MS, Caird MS, Marini JC, Kozloff KM. Sclerostin antibody improves skeletal parameters in a Brtl/+ mouse model of osteogenesis imperfecta. J Bone Miner Res. 2013;28(1):73–80.
Jacobsen CM, Barber LA, Ayturk UM, Roberts HJ, Deal LE, Schwartz MA, et al. Targeting the LRP5 pathway improves bone properties in a mouse model of osteogenesis imperfecta. J Bone Miner Res. 2014;29(10):2297–306.
Grafe I, Alexander S, Yang T, Lietman C, Homan EP, Munivez E, et al. Sclerostin antibody treatment improves the bone phenotype of Crtap(−/−) mice, a model of recessive osteogenesis imperfecta. J Bone Miner Res. 2016;31(5):1030–40.
Roschger A, Roschger P, Keplingter P, Klaushofer K, Abdullah S, Kneissel M, et al. Effect of sclerostin antibody treatment in a mouse model of severe osteogenesis imperfecta. Bone. 2014;66:182–8.
Rauch F, Lalic L, Roughley P, Glorieux FH. Relationship between genotype and skeletal phenotype in children and adolescents with osteogenesis imperfecta. J Bone Miner Res. 2010;25(6):1367–74.
Chen XX, Baum W, Dwyer D, Stock M, Schwabe K, Ke HZ, et al. Sclerostin inhibition reverses systemic, periarticular and local bone loss in arthritis. Ann Rheum Dis. 2013;72(10):1732–6.
Eddleston A, Marenzana M, Moore AR, Stephens P, Muzylak M, Marshall D, et al. A short treatment with an antibody to sclerostin can inhibit bone loss in an ongoing model of colitis. J Bone Miner Res. 2009;24(10):1662–71.
Marenzana M, Greenslade K, Eddleston A, Okoye R, Marshall D, Moore A, et al. Sclerostin antibody treatment enhances bone strength but does not prevent growth retardation in young mice treated with dexamethasone. Arthritis Rheum. 2011;63(8):2385–95.
Cui L, Cheng H, Song C, Li C, Simonet WS, Ke HZ, et al. Time-dependent effects of sclerostin antibody on a mouse fracture healing model. J Musculoskelet Neuronal Interact. 2013;13(2):178–84.
Makhdom AM, Rauch F, Lauzier D, Hamdy RC. The effect of systemic administration of sclerostin antibody in a mouse model of distraction osteogenesis. J Musculoskelet Neuronal Interact. 2014;14(1):124–30.
• Wehmeyer C, Frank S, Beckmann D, Bottcher M, Cromme C, Konig U, et al. Sclerostin inhibition promotes TNF-dependent inflammatory joint destruction. Sci Transl Med. 2016;8(330):330ra35. Sclerostin is generally thought to be a bone-specific protein, but this study shows that in pathological situations anti-sclerostin treatments may have unexpected adverse effects. In a mouse model of rheumatoid arthritis, sclerostin inhibition led to increased cartilage destruction.
Saag KG, Petersen J, Brandi ML, Karaplis AC, Lorentzon M, Thomas T, et al. Romosozumab or alendronate for fracture prevention in women with osteoporosis. N Engl J Med. 2017;377(15):1417–27.
Perosky JE, Khoury BM, Jenks TN, Ward FS, Cortright K, Meyer B, et al. Single dose of bisphosphonate preserves gains in bone mass following cessation of sclerostin antibody in Brtl/+ osteogenesis imperfecta model. Bone. 2016;93:79–85.
Recknor CP, Recker RR, Benson CT, Robins DA, Chiang AY, Alam J, et al. The effect of discontinuing treatment with blosozumab: follow-up results of a phase 2 randomized clinical trial in postmenopausal women with low bone mineral density. J Bone Miner Res. 2015;30(9):1717–25.
Janssens K, Vanhoenacker F, Bonduelle M, Verbruggen L, Van Maldergem L, Ralston S, et al. Camurati-Engelmann disease: review of the clinical, radiological, and molecular data of 24 families and implications for diagnosis and treatment. J Med Genet. 2006;43(1):1–11.
Verstraeten A, Alaerts M, Van Laer L, Loeys B. Marfan syndrome and related disorders: 25 years of gene discovery. Hum Mutat. 2016;37(6):524–31.
• Grafe I, Yang T, Alexander S, Homan EP, Lietman C, Jiang MM, et al. Excessive transforming growth factor-beta signaling is a common mechanism in osteogenesis imperfecta. Nat Med. 2014;20(6):670–5. This study shows that altered TGFβ matrix-cell signaling is a primary mechanism in the pathogenesis of OI, and could be a promising target for the treatment of OI.
Loeys BL. Angiotensin receptor blockers: a panacea for Marfan syndrome and related disorders? Drug Discov Today. 2015;20(2):262–6.
Ayyavoo A, Derraik JG, Cutfield WS, Hofman PL. Elimination of pain and improvement of exercise capacity in Camurati-Engelmann disease with losartan. J Clin Endocrinol Metab. 2014;99(11):3978–82.
Trifiro G, Marelli S, Viecca M, Mora S, Pini A. Areal bone mineral density in children and adolescents with Marfan syndrome: evidence of an evolving problem. Bone. 2015;73:176–80.
Acknowledgements
Dr. Ward is supported by a Research Chair Award from the University of Ottawa.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Leanne Ward is participating in clinical trials with AMGEN, outside the submitted work. Frank Rauch declares no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Pediatrics
Rights and permissions
About this article
Cite this article
Ward, L.M., Rauch, F. Anabolic Therapy for the Treatment of Osteoporosis in Childhood. Curr Osteoporos Rep 16, 269–276 (2018). https://doi.org/10.1007/s11914-018-0434-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0434-z