Abstract
Purpose of Review
Exosomes are membrane vesicles that are released by most cell types into the extracellular environment. The purpose of this article is to discuss the main morphological features and contents of bone-derived exosomes, as well as their major isolation and physical characterization techniques. Furthermore, we present various scenarios and discuss potential clinical applications of bone-derived exosomes in bone repair and regeneration.
Recent Findings
Exosomes were believed to be nanosized vesicles derived from the multivesicular body. Reports now suggest that nanovesicles could bud directly from the plasma membrane. However, the exosome cargo is cell-type specific and is derived from the parent cell. In the bone matrix, several intracellular proteins lacking a signal peptide are transported to the ECM by exosomes. Besides proteins, several mRNA, miRNA, and lipids are exported to the ECM by bone cells and bone marrow stromal cells. Recent evidence suggests that several of the functional components in the cargo could regulate processes of bone formation, inhibit osteoclast activity, and promote fracture repair.
Summary
Exosomes are powerful cellular molecular machines produced without human intervention and packaged with physiological cargo that could be utilized for molecular therapy in several skeletal disorders such as osteoporosis, osteogenesis imperfecta, and fracture healing. Although much work has been done, there is a lot of information that is still unknown, as exosomes contain a multitude of molecules whose identity and function have yet to be identified.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
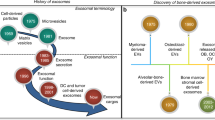
Most eukaryotic cells secrete extracellular vesicles which are associated with intercellular communication. These highly heterogeneous membrane enclosed structures can have an impact on both neighboring and distant cells. Extracellular vesicles can be classified according to their cellular origin or biogenesis. The three main classes are apoptotic bodies, microvesicles, and exosomes [1•, 2]. Specific extracellular vesicles called matrix vesicles were initially identified as unique extracellular membrane-bound microparticles and served as sites for mineral formation in the cartilage growth plate and in other vertebrate mineralizing tissues [3]. Besides being carriers of mineral foci, matrix vesicles were thought to provide cell-cell communication within complex tissues like the avascular growth cartilage.
A subclass of extracellular vesicles have been identified as secreted bodies in several tissues and based on their size (30–120 nm) have been termed as “exosomes”. They were identified in most body fluids (e.g., blood, breast milk, saliva, semen, urine) and were purified from nearly all mammalian cell types (e.g., endothelial cells, neuronal cells, muscle cells, stem cells) [2, 4, 5]. These bioactive cell-derived vesicles are characterized by different sets of lipids, cytosolic and membrane proteins, as well as functionally active ribonucleic acids (e.g., mRNA, miRNA) [6,7,8]. It is now well established that these nanoparticles are an important means for healthy cells to function in several biological events like intercellular communication, transport of proteins and nucleic acids, tumorigenesis, and metabolism, and they also have numerous applications in diagnosis and therapies of different pathologies [4, 8, 9].
During the past few years, these nanosized vesicles have received significant attention based on their involvement in cancer progression, host immune response, and as carriers of prions. Recently, a number of published studies revealed that bone-related cells, like osteoclasts, osteoblasts, osteocytes, and bone marrow mesenchymal stem cells, also release exosomes [10]. Such bone-derived exosomes play pivotal roles during the bone remodeling process by transferring biologically active molecules to target cells, favoring osteoclast and osteoblast differentiation.
Defining Exosomes
Based on their intracellular origin and extracellular function, exosomes have been described as “the carrier pigeons of the cell” [11], “sending the message without the messenger” [12], and “hijacking the cellular mail” [13]. These descriptions indicate that exosomes are able to transport intracellular material out of the cell where their contents could have an autocrine or paracrine effect thereby eliciting a physiological response during tissue growth and development.
Isolation of Bone-Derived Exosomes
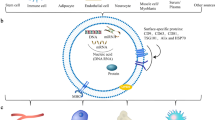
In order to isolate these unique structures from various biological fluids and from the supernatants of cultured cells, several strategies have been developed. Each isolation technique exploits a certain characteristic property of exosomes, such as their size, shape, density, and enriched surface proteins to facilitate their isolation. Commonly applied techniques include centrifugation, chromatography, filtration, polymer-based precipitation, and affinity chromatography [14,15,16,17].
Ultracentrifugation is the most widely used and reported separation technique for the isolation of bone-derived exosome [18,19,20,21,22]. Specifically, it can be grouped into two categories: analytical and preparative ultracentrifugation. Analytical ultracentrifugation is mainly used for the quantitative analysis of macromolecules, while the preparative method is utilized for fractionation of small bioparticles such as viruses, bacteria, subcellular organelles, and exosomes [14]. The latter can be divided into two types: differential ultracentrifugation and density gradient ultracentrifugation.
Differential ultracentrifugation fractionates exosomes from other components based on their size and density differences. Usually, it consists of repeated centrifugations, including (1) a low-speed spin (300–500 RCF) to remove cells and apoptotic debris; (2) a higher speed spin (1000–20,000 RCF) to eliminate larger vesicles; and finally, (3) high speed centrifugation (100,000–150,000 RCF) to isolate exosomes [23, 24•, 25]. Since the viscosity of biological fluids have a remarkable correlation with the purity of isolated exosomes, viscous samples require longer and higher speed of centrifugation [17].
Density gradient ultracentrifugation is a widely accepted technique for isolation of exosomes [21, 26]. It has two types, namely rate-zonal ultracentrifugation and isopycnic ultracentrifugation. In the former, the components are separated primarily on their size and mass, while in the latter the components are separated solely on the basis of their density. Both techniques employ a gradient of varying concentrations of small molecules (e.g., sucrose) distributed along the axis of the centrifugally generated force [21, 27]. The lowest concentration is at the solution meniscus and the highest at the base. When the components are subjected to centrifugation in a density gradient, they sediment toward the bottom if they have a greater buoyant density than the solution, or they will float toward the top if they have a lower density.
Precipitation is an effective and proven alternative to time-consuming ultracentrifugation [13, 28, 29, 30•, 31]. By modulating the solubility or dispersibility of exosomes, they can be settled out of biological fluids. Currently, several biofluid compatible exosome precipitation kits (e.g., ExoQuick kit, Total Exosome Isolation kit) are commercially available enabling simple and reliable concentration of intact exosomes. Such kits contain volume-excluding proprietary polymers like PEG that gently precipitate the exosomes. Finally, the precipitated exosomes can be isolated easily by using either low-speed centrifugation or filtration.
Physical Characterization of Bone-Derived Exosomes
The most relevant properties of exosomes are size, morphology, concentration, biochemical composition, and cellular origin. Optical methods are extensively used to accurately obtain clinically relevant properties of bone-derived exosomes at a high speed. The most commonly used optical method is nanoparticle tracking analysis (NTA) [20, 29], which measures the size distribution and concentration of samples in liquid suspensions. NTA utilizes the properties of both light scattering and Brownian motion to calculate an exosome’s diameter using statistical methods. This method has the advantage of being a fast and simple way of analyzing large numbers of particles simultaneously, and at a relatively cheap price (as compared with sophisticated fluorescence or electron microscopes). However, the method does not differentiate a vesicle from a protein aggregate of similar size.
Dynamic light scattering (DLS), also known as photon correlation spectroscopy, is also used for the determination of size distribution of different bone-derived exosomes in solution [25, 28]. DLS measures Brownian motion and relates this to the size of the exosomes. It does this by illuminating the suspended particles with a laser and analyzing the intensity fluctuations of the scattered light.
Transmission electron microscopy (TEM) is a widely used non-optical method to characterize exosomes [13, 18, 22, 27, 28, 31,32,33]. TEM operates on the same optical principles as light microscopy but uses electrons instead of photons to create an image. However, since TEM is performed in a vacuum, exosomes require fixation and dehydration, which could affect both size and morphology (Fig. 1). Nonetheless, with immunogold labeling, it is possible to provide biochemical information of the contents of exosomes [34].
The presence of classical bone-derived exosomal marker proteins has been studied extensively by techniques such as antibody-based detection techniques of specific proteins like western blotting (WB), immuno-EM, or by bead-based fluorescence-activated cell sorting (FACS) [21, 25, 28, 30•, 33]. In several studies, RT-PCR and qRT-PCR were used for the RNA analysis of bone-derived exosomes [19, 25].
Composition of Exosomes
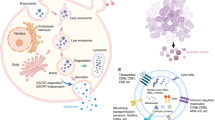
The compositions of extracellular vesicles vary according to their cellular origin. In contrast to microvesicles, which bud directly from the plasma membrane, exosomes are small vesicles of different sizes that are derived from the endolysosomal pathway (Fig. 2). Even though they are heavily enriched in phosphatidylserine, the membrane composition of microvesicles resembles that of the parent cell more closely than the membrane composition of exosomes [35, 36].
Interestingly, the protein composition analysis of the exosomes has demonstrated that the diversity of proteins is rather limited—exosomes do not contain any proteins of nuclear, mitochondrial, endoplasmic-reticulum, or Golgi apparatus origin. Moreover, all exosomal proteins that have been identified are found in the cytosol, in the membrane of endocytic compartments, or at the plasma membrane [37]. During endochondral and intramembranous bone development, we have observed the translocation of several intracellular resident proteins lacking a signal peptide to the extracellular matrix. Notable among these are two ER resident proteins namely GRP-78 (glucose regulatory protin-78) [38] and TRIP-1 (TGF-beta receptor II interacting protein 1) [39] which do not contain a signal peptide but are localized in the ECM. Their transport and secretion to the ECM was highly enigmatic. It is now known that unconventional transport mechanisms such as exosomal carriers are responsible for this event.
Recent studies have revealed that bone marrow MSC (mesenchymal stem cell)-derived exosomes express well-established transmembrane marker proteins such as CD9, CD63, and CD81 [18, 20, 27, 28]. Wang and co-workers also detected transmembrane marker proteins and other exosomal markers including heat shock protein 90 (Hsp90), Hsp70, and flotillin-1 in bone marrow stromal cell-derived exosomes [29]. Very recently, Sun et al. detected 1536 proteins by mass spectrometry in osteoblast-secreted exosomes and found 172 proteins overlap with proteins in the bone database [22]. Moreover, through proteomic analysis, they detected proteins including ephrinB1 (EFNB1), transforming growth factor beta receptor 3 (TGFBR3), lipoprotein receptor-related protein (LRP6), bone morphogenetic protein receptor type-1 (BMPR1), and Smad ubiquitylation regulatory factor-1 (SMURF1) that have important functions in bone disease.
In addition to proteins, various nucleic acids have been identified in bone-derived exosomal lumen [25, 40]. Xu et al. characterized the differences in exosomal miRNA during osteogenic differentiation of human bone marrow-derived MSCs [21]. They verified the presence of miRNA in exosomes during osteogenic differentiation of bone marrow MSCs and found that let-7a, miR-199b, miR-218, miR-148a, miR-135b, miR-203, miR-219, miR-299-5p, and miR-302b were significantly increased. In contrast, miR-221, miR-155, miR-885-5p, miR-181a, and miR-320c were significantly under expressed in individual exosomal samples. Furthermore, seven mRNAs (RPS2, DGKA, ACIN1, DKK2, Xsox17, DDX6, and Lsm2) were found to be differentially expressed over time in differentiated bone marrow MSC-derived exosomes.
Exosomes and Bone Regeneration
Bone defects associated with complex skeletal disorders such as osteoporosis, infections, injuries, and surgical removal of carcinomas are major challenges in bone regeneration therapy. Therefore, developing new effective treatments with stronger osteogenic potential and a lower incidence of complications is desirable.
Regenerative medicine has shown great promise by utilizing embryonic, adult, and induced pluripotent stem cells to repair and regenerate damaged tissues. Recently, exosomes secreted by stem cells were used in several regenerative applications [41, 42]. Published reports have shown that specific proteins, mRNA and miRNA, can be laterally transferred through exosomal machinery into neighboring or distant cells to activate regenerative programs in diseased tissues [43].
Over the last few years, exosomes have received significant attention in the field of bone tissue repair and regeneration which is strongly associated with the controlled application of cells, scaffolds, or biologically active molecules in order to restore, or improve damaged, malfunctioning, or missing tissues [9, 10, 44, 45].
Furuta et al. showed that MSC exosomes are a novel factor for MSC paracrine signaling [46]. This was demonstrated in a fracture healing model in CD9−/− mice, a strain that produces reduced levels of exosomes. Injection of MSC exosomes enhanced fracture healing when compared with exosome-free media. The study of Zhang et al. showed that tricalcium phosphate scaffold containing MSC-derived exosomes could enhance osteogenesis when compared to pure tricalcium phosphate scaffolds [26]. Additionally, the internalization of exosomes in human bone marrow-derived MSCs could profoundly enhance their proliferation, migration, and osteogenic differentiation.
Recently, mineralizing pre-osteoblast MC3T3-E1 cell-derived exosomes were used to promote the differentiation of bone marrow stromal cell to osteoblasts [30•]. They revealed that exosomes significantly influence miRNA profiles in recipient stromal cells, and such changes tend to activate the Wnt signaling pathway by inhibiting Axin1 expression and increasing β-catenin expression.
Recent research has shown the involvement of a number of bone-derived exosomal miRNAs in bone formation [10]. Of the many miRNAs identified from mineralized osteoblast-derived exosomes, four were highly expressed namely miR-677-3p, miR 680, miR-3084-3p, and miR-5000. Let-7 was found in exosomes from both osteoblast precursors and differentiated osteoblasts and shown to enhance osteogenesis by regulating high-mobility group AT-hook 2 (HMGA2) and Axin 2 [47]. Reports also suggest that the bone master transcription factor Runx2 is regulated by miR-30d-5p, miR-133b-3p, miR-199b, mir221, and miR-133b-3p, while miR-30d-5p and miR-885-5p negatively regulates osteoblast differentiation [48]. Li et al. have shown that osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Therefore, inhibition of miR-214-3p in osteoclasts could be exploited as a strategy for treating skeletal disorders where low bone formation is observed [19]. Thus, bone-derived exosomal miRNAs could be utilized for targeting osteoblast differentiation and matrix formation.
Exosomes derived from stem cells and osteoblast precursors can also activate osteoclast formation. Exosomes derived from bone marrow stromal cells have been shown to influence pre-osteoclasts leading to osteoclastogenesis, and therefore, these exosomes could be utilized in tissues where osteolastogenesis is required, such as in orthodontic treatment and bisphosphonate-related osteonecrosis of the jaw [28]. Overall, these findings indicate the potential of mineralizing osteoblast- and stem cell-derived exosomes in bone tissue regeneration.
Huang et al. evaluated the potential of exosomes derived from dental pulp cells to induce odontogenic differentiation of naïve human dental pulp stem cells and human bone marrow-derived stromal cells in vitro and in vivo [31]. Their results indicated that exosomes were endocytosed by both cell types and stimulated increased expression of genes required for odontogenic differentiation. Moreover, when tested in vivo in a tooth root slice model with dental pulp stem cells, the exosomes triggered regeneration of dental pulp-like tissue.
In addition, Qi and co-workers used stem cell-derived exosomes to repair critical-sized bone defects in osteoporotic rats [49]. They demonstrated that exosomes effectively stimulate the proliferation and osteogenic differentiation of bone marrow MSCs, and the effect was enhanced with increasing exosome concentration. In vivo application of these exosomes promoted bone regeneration in critical-sized calvarial defects through enhanced angiogenesis and osteogenesis in an ovariectomized rat model [49].
Very recently, human embryonic MSC-derived exosomes were used in the treatment of rats with osteochondral defects [50]. The exosome-treated defects showed enhanced gross appearance and improved histological scores. By 12 weeks, exosome-treated defects displayed complete restoration of cartilage and subchondral bone with characteristic features including a hyaline cartilage with good surface regularity, complete bonding to adjacent cartilage, and extracellular matrix deposition that closely resemble that of age-matched unoperated control.
Conclusions
Exosomes are naturally occurring nanospheres with a cell-specific composition that can efficiently transport proteins, miRNA, mRNA, and other cellular material to near and distant cells. The specific cargo within bone exosomes provides a cell-specific mechanism to dock and unload their cellular contents. Therefore, bone cell-derived exosomes could potentially be tailored for use in bone regeneration and repair by promoting osteoblast differentiation and downregulating osteoclast formation and differentiation. Exosomes contain a multitude of molecules, and future research analyzing their properties and function could lead to the development of therapeutic exosomes in a multitargeted systems biology approach for bone repair and regeneration.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
• Yáñez-Mó M, Siljander PR-M, Andreu Z, Zavec AB, Borràs FE, Buzas EI, et al. Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles. 2015;4:27066. https://doi.org/10.3402/jev.v4.27066. This study is a good overview of the characterization and identification of the contents of extracellular vesicles and the biological function of these vesicles.
Raposo G, Stoorvogel W. Extracellular vesicles: exosomes, microvesicles, and friends. J Cell Biol. 2013;200(4):373–83. https://doi.org/10.1083/jcb.201211138.
Shapiro IM, Landis WJ, Risbud MV. Matrix vesicles: are they anchored exosomes? Bone. 2015;79:29–36. https://doi.org/10.1016/j.bone.2015.05.013.
Keller S, Ridinger J, Rupp A-K, Janssen JW, Altevogt P. Body fluid derived exosomes as a novel template for clinical diagnostics. J Transl Med. 2011;9(1):86. https://doi.org/10.1186/1479-5876-9-86.
Lässer C, Seyed Alikhani V, Ekström K, Eldh M, Torregrosa Paredes P, Bossios A, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. J Transl Med. 2011;9(1):9. https://doi.org/10.1186/1479-5876-9-9.
Edgar JR. Q&A: what are exosomes, exactly? BMC Biol. 2016;14(1):46. https://doi.org/10.1186/s12915-016-0268-z.
Avcı E, Edibe Avcı BS, Banu Balcı-Peynircioğlu P. An overview of exosomes: from biology to emerging roles in immune response. Acta Med Austriaca. 2015;47:2–10.
Vlassov AV, Magdaleno S, Setterquist R, Conrad R. Exosomes: current knowledge of their composition, biological functions, and diagnostic and therapeutic potentials. Biochim Biophys Acta BBA - Gen Subj. 2012;1820(7):940–8. https://doi.org/10.1016/j.bbagen.2012.03.017.
Qin Y, Sun R, Wu C, Wang L, Zhang C. Exosome: a novel approach to stimulate bone regeneration through regulation of osteogenesis and angiogenesis. Int J Mol Sci. 2016;17(5):712. https://doi.org/10.3390/ijms17050712.
Xie Y, Chen Y, Zhang L, Ge W, Tang P. The roles of bone-derived exosomes and exosomal microRNAs in regulating bone remodelling. J Cell Mol Med. 2017;21(5):1033–41. https://doi.org/10.1111/jcmm.13039.
Fleming A, Sampey G, Chung MC, Bailey C, Hoek V, Kashanchi MLF, et al. The carrying pigeons of the cell: exosomes and their role in infectious diseases caused by human pathogens. Pathog Dis. 2014;71:109–20. https://doi.org/10.1111/2049-632X.12135.
Gibbons D Stem cell stories that caught our eye: our earliest days, cell therapy without the cells and unproven therapies. Stem Cellar. 2015. https://blog.cirm.ca.gov/2015/09/04/stem-cell-stories-that-caught-our-eye-our-earliest-days-cell-therapy-without-the-cells-and-unproven-therapies/. Accessed 20 Sept 2017.
Narayanan R, Huang C-C, Ravindran S. Hijacking the cellular mail: exosome mediated differentiation of mesenchymal stem cells. Stem Cells Int. 2016;2016:1–11. https://doi.org/10.1155/2016/3808674.
Li P, Kaslan M, Lee SH, Yao J, Gao Z. Progress in exosome isolation techniques. Theranostics. 2017;7(3):789–804. https://doi.org/10.7150/thno.18133.
Zeringer E, Barta T, Li M, Vlassov AV. Strategies for isolation of exosomes. Cold Spring Harb Protoc 2015;2015(4) pdb.top074476. https://doi.org/10.1101/pdb.top074476.
Baranyai T, Herczeg K, Onódi Z, Voszka I, Módos K, Marton N, et al. Isolation of exosomes from blood plasma: qualitative and quantitative comparison of ultracentrifugation and size exclusion chromatography methods. PLoS One. 2015;10(12):e0145686. https://doi.org/10.1371/journal.pone.0145686.
Momen-Heravi F, Balaj L, Alian S, Mantel P-Y, Halleck AE, Trachtenberg AJ, et al. Current methods for the isolation of extracellular vesicles. Biol Chem. 2013;394(10):1253–62. https://doi.org/10.1515/hsz-2013-0141.
Nakano M, Nagaishi K, Konari N, Saito Y, Chikenji T, Mizue Y, et al. Bone marrow-derived mesenchymal stem cells improve diabetes-induced cognitive impairment by exosome transfer into damaged neurons and astrocytes. Sci Rep. 2016;6(24805):1–14. https://doi.org/10.1038/srep24805.
Li D, Liu J, Guo B, Liang C, Dang L, Lu C, et al. Osteoclast-derived exosomal miR-214-3p inhibits osteoblastic bone formation. Nat Commun. 2016;7:10872. https://doi.org/10.1038/ncomms10872.
Rager TM, Olson JK, Zhou Y, Wang Y, Besner GE. Exosomes secreted from bone marrow-derived mesenchymal stem cells protect the intestines from experimental necrotizing enterocolitis. J Pediatr Surg. 2016;51(6):942–7. https://doi.org/10.1016/j.jpedsurg.2016.02.061.
Xu J-F, Yang G, Pan X-H, Zhang S-J, Zhao C, Qiu B-S, et al. Altered microRNA expression profile in exosomes during osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. PLoS One. 2014;9(12):e114627. https://doi.org/10.1371/journal.pone.0114627.
Ge M, Wu Y, Ke R, Cai T, Yang J, Mu X. Value of osteoblast-derived exosomes in bone diseases. J Craniofac Surg. 2017; 28(4): 866–70. https://doi.org/10.1097/SCS.0000000000003463.
Marton N, Kovács OT, Baricza E, Kittel Á, Győri D, Mócsai A, Meier FMP, Goodyear CS, McInnes IB, Buzás EI, Nagy G. Extracellular vesicles regulate the human osteoclastogenesis: divergent roles in discrete inflammatory arthropathies. Cell Mol Life Sci. 2017;74(19):3599–611. https://doi.org/10.1007/s00018-017-2535-8.
• Lu Z, Chen Y, Dunstan C, Roohani-Esfahani S, Zreiqat H. Priming adipose stem cells with tumor necrosis factor-alpha preconditioning potentiates their exosome efficacy for bone regeneration. Tissue Eng Part A. 2017; https://doi.org/10.1089/ten.tea.2016.0548. This paper presents new information on the use of exosomes in bone regeneration.
Sun W, Zhao C, Li Y, Wang L, Nie G, Peng J, et al. Osteoclast-derived microRNA-containing exosomes selectively inhibit osteoblast activity. Cell Discov. 2016;2:201615. https://doi.org/10.1038/celldisc.2016.15.
Zhang J, Liu X, Li H, Chen C, Hu B, Niu X, et al. Exosomes/tricalcium phosphate combination scaffolds can enhance bone regeneration by activating the PI3K/Akt signaling pathway. Stem Cell Res Ther. 2016;7(1):136. https://doi.org/10.1186/s13287-016-0391-3.
Zhu W, Huang L, Li Y, Zhang X, Gu J, Yan Y, et al. Exosomes derived from human bone marrow mesenchymal stem cells promote tumor growth in vivo. Cancer Lett. 2012;315(1):28–37. https://doi.org/10.1016/j.canlet.2011.10.002.
Xu S, Wang Z. Bone marrow mesenchymal stem cell-derived exosomes enhance osteoclastogenesis during alveolar bone deterioration in rats. RSC Adv. 2017;7(34):21153–63. https://doi.org/10.1039/C6RA27931G.
Wang J, Hendrix A, Hernot S, Lemaire M, Bruyne ED, Valckenborgh EV, et al. Bone marrow stromal cell–derived exosomes as communicators in drug resistance in multiple myeloma cells. Blood. 2014;124(4):555–66. https://doi.org/10.1182/blood-2014-03-562439.
• Cui Y, Luan J, Li H, Zhou X, Han J. Exosomes derived from mineralizing osteoblasts promote ST2 cell osteogenic differentiation by alteration of microRNA expression. FEBS Lett. 2016;590(1):185–92. https://doi.org/10.1002/1873-3468.12024. This study identifies the presence of several novel miRNAs in the exosomes and their role in osteoblast differentiation.
Huang C-C, Narayanan R, Alapati S, Ravindran S. Exosomes as biomimetic tools for stem cell differentiation: applications in dental pulp tissue regeneration. Biomaterials. 2016;111:103–15. https://doi.org/10.1016/j.biomaterials.2016.09.029.
Ge M, Ke R, Cai T, Yang J, Mu X. Identification and proteomic analysis of osteoblast-derived exosomes. Biochem Biophys Res Commun. 2015;467(1):27–32. https://doi.org/10.1016/j.bbrc.2015.09.135.
Zhang Y, Song Y, Ravindran S, Gao Q, Huang CC, Ramachandran A, et al. DSPP contains an IRES element responsible for the translation of dentin phosphophoryn. J Dent Res. 2014;93(2):155–61. https://doi.org/10.1177/0022034513516631.
Kolhe R, Hunter M, Liu S, Jadeja RN, Pundkar C, Mondal AK, et al. Gender-specific differential expression of exosomal miRNA in synovial fluid of patients with osteoarthritis. Sci Rep. 2017;7(1):2029. https://doi.org/10.1038/s41598-017-01905-y.
Haraszti RA, Didiot M-C, Sapp E, Leszyk J, Shaffer SA, Rockwell HE, et al. High-resolution proteomic and lipidomic analysis of exosomes and microvesicles from different cell sources. J Extracell Vesicles. 2016;5(1):32570. https://doi.org/10.3402/jev.v5.32570.
Colombo M, Raposo G, Théry C. Biogenesis, secretion, and intercellular interactions of exosomes and other extracellular vesicles. Annu Rev Cell Dev Biol. 2014;30(1):255–89. https://doi.org/10.1146/annurev-cellbio-101512-122326.
Théry C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nat Rev Immunol. 2002;2(8):569–79. https://doi.org/10.1038/nri855.
Ravindran S, Narayanan K, Eapen AS, Hao J, Ramachandran A, Blond S, et al. Endoplasmic reticulum chaperone protein GRP-78 mediates endocytosis of dentin matrix protein 1. J Biol Chem. 2008;283(44):29658–70. https://doi.org/10.1074/jbc.M800786200.
Ramachandran A, Ravindran S, Huang C-C, George A. TGF beta receptor II interacting protein-1, an intracellular protein has an extracellular role as a modulator of matrix mineralization. Sci Rep. 2016;6(37885):1–16. https://doi.org/10.1038/srep37885.
Ekström K, Omar O, Granéli C, Wang X, Vazirisani F, Thomsen P. Monocyte exosomes stimulate the osteogenic gene expression of mesenchymal stem cells. PLoS One. 2013;8(9):e75227. https://doi.org/10.1371/journal.pone.0075227.
Bruno S, Grange C, Deregibus MC, Calogero RA, Saviozzi S, Collino F, et al. Mesenchymal stem cell-derived microvesicles protect against acute tubular injury. J Am Soc Nephrol. 2009;20(5):1053–67. https://doi.org/10.1681/ASN.2008070798.
Herrera MB, Fonsato V, Gatti S, Deregibus MC, Sordi A, Cantarella D, et al. Human liver stem cell-derived microvesicles accelerate hepatic regeneration in hepatectomized rats. J Cell Mol Med. 2010;14(6B):1605–18. https://doi.org/10.1111/j.1582-4934.2009.00860.x.
Li J, Liu K, Liu Y, Xu Y, Zhang F, Yang H, et al. Exosomes mediate the cell-to-cell transmission of IFN-α-induced antiviral activity. Nat Immunol. 2013;14(8):793–803. https://doi.org/10.1038/ni.2647.
Gimona M, Pachler K, Laner-Plamberger S, Schallmoser K, Rohde E. Manufacturing of human extracellular vesicle-based therapeutics for clinical use. Int J Mol Sci. 2017;18(6):1190. https://doi.org/10.3390/ijms18061190.
Burke J, Kolhe R, Hunter M, Isales C, Hamrick M, Fulzele S. Stem cell-derived exosomes: a potential alternative therapeutic agent in orthopaedics. Stem Cells Int. 2016;2016:1–6. https://doi.org/10.1155/2016/5802529.
Furuta T, Miyaki S, Ishitobi H, Ogura T, Kato Y, Kamei N, et al. Mesenchymal stem cell-derived exosomes promote fracture healing in a mouse model. Stem Cells Transl Med. 2016;5(12):1620–30. https://doi.org/10.5966/sctm.2015-0285.
Wei J, Li H, Wang S, Li T, Fan J, Liang X, et al. Let-7 enhances osteogenesis and bone formation while repressing adipogenesis of human stromal/mesenchymal stem cells by regulating HMGA2. Stem Cells Dev. 2014;23(13):1452–63. https://doi.org/10.1089/scd.2013.0600.
Zhang Y, Xie R-L, Croce CM, Stein JL, Lian JB, van Wijnen AJ, et al. A program of microRNAs controls osteogenic lineage progression by targeting transcription factor Runx2. Proc Natl Acad Sci U S A. 2011;108(24):9863–8. https://doi.org/10.1073/pnas.1018493108.
Qi X, Zhang J, Yuan H, Xu Z, Li Q, Niu X, et al. Exosomes secreted by human-induced pluripotent stem cell-derived mesenchymal stem cells repair critical-sized bone defects through enhanced angiogenesis and osteogenesis in osteoporotic rats. Int J Biol Sci. 2016;12(7):836–49. https://doi.org/10.7150/ijbs.14809.
Zhang S, Chu WC, Lai RC, Lim SK, Hui JHP, Toh WS. Exosomes derived from human embryonic mesenchymal stem cells promote osteochondral regeneration. Osteoarthr Cartil. 2016;24(12):2135–40. https://doi.org/10.1016/j.joca.2016.06.022.
Acknowledgements
A. P greatly acknowledges the scholarship from the Rosztoczy Foundation.
Funding
This work was supported by the Brodie Endowment Fund and the National Institutes of Health grant DE11657.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Adrienn Pethő, Yinghua Chen, and Anne George declare no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Skeletal Development
Rights and permissions
About this article
Cite this article
Pethő, A., Chen, Y. & George, A. Exosomes in Extracellular Matrix Bone Biology. Curr Osteoporos Rep 16, 58–64 (2018). https://doi.org/10.1007/s11914-018-0419-y
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-018-0419-y