Abstract
While it is understood that body composition impacts on physical conditions, such as diabetes and cardiovascular disease, it is only now apparent that body composition might play a role in the genesis of common mental disorders, depression and anxiety. Sarcopenia occurs in ageing and comprises a progressive decline in muscle mass, strength and function, leading to frailty, decreased independence and poorer quality of life. This review presents an emerging body of evidence to support the hypothesis that shared pathophysiological pathways for sarcopenia and the common mental disorders constitute links between skeletal muscle and brain function. Contracting skeletal muscle secretes neurotrophic factors that are known to play a role in mood and anxiety, and have the dual role of nourishing neuronal growth and differentiation, while protecting the size and number of motor units in skeletal muscle. Furthermore, skeletal muscle activity has important immune and redox effects that impact behaviour and reduce muscle catabolism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
While it is known that body composition impacts on medical disorders, such as diabetes and cardiovascular disease, it is now apparent that body composition is associated with the genesis of the common mental disorders, depression and anxiety. It is clear that obesity is linked to the risk for depression in a bi-directional manner [1]. While there is some evidence linking obesity with anxiety disorders, this association remains unclear because of the cross-sectional nature of most of the studies to-date and the heterogeneity of anxiety disorders [2]. However, the possible role of muscle, which is the component of body composition important for vitality and well-being, remains largely unexplored.
Sarcopenia, which means ‘poverty of flesh’, occurs in ageing and comprises a progressive deterioration in the mass, strength and function of muscle tissue [3]. Such deterioration can have serious health consequences, and sarcopenia is the prime driver of the decreased independence and quality of life associated with ageing [4]. Peak muscle mass and strength are attained in late adolescence/early adulthood followed by an age-related decline; decreases in both total and appendicular skeletal muscle mass can become particularly marked in the elderly [5•]. Achievement of optimal peak muscle mass and performance, and a slowing or reversal of age-related muscle deterioration [6, 7], should be a focus of health recommendations across the lifecourse, aimed at reducing the burden of disability and frailty in later life.
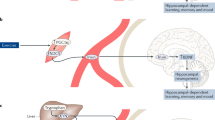
The purpose of this review is to address the hypothesis that sarcopenia is linked to the common mental disorders. The face validity for this tenet derives from the evidence of common pathophysiological pathways for sarcopenia, anxiety and depression, that involve neurotrophins, oxidative stress and inflammation, and modulated by lifestyle behaviours (Fig. 1). For example, contracting skeletal muscle is a major source of neurotrophic factors which appear to play a critical role in psychiatric illness. Furthermore, skeletal muscle activity has important immune and redox effects that both reduce muscle catabolism [8] and enhance mood [9].
Associations Between Sarcopenia and Common Mental Disorders
Using observational cross-sectional data from community-dwelling people aged 60 years and older in Ireland, the odds ratio (OR) for physical frailty was associated with a higher likelihood of anxiety and depressive symptoms, independent of age, sex and past mood and anxiety (OR 4.3, 95 % CI 1.5–11.9) [10••]. In this study, physical frailty was measured using the Fried biological syndrome model that incorporated slow walking speed, poor grip strength, physical inactivity, poor endurance and weight loss [11]. In a cross-sectional Korean study, also involving men and women aged 60 years and older, individuals with self-reported depression or those taking antidepressants had lower appendicular skeletal muscle mass than those free of depression or antidepressant use (4.2 % lower in men and 3.7 % lower in women) [12]. Multivariable logistic analyses revealed that the odds for depression in men were 51 % lower for each standard deviation increase in appendicular skeletal muscle mass; however, the association was not significant in the adjusted model for women. In another study, this time involving hospitalised patients, those identified with sarcopenia were more likely to suffer from depression, have a longer length of hospital stay, were at greater risk of non-elective readmission and had a higher mortality rate [13].
There are some published data from the USA on sarcopenia as a therapeutic target in depression. In a clinical trial involving a home-based telemedicine exercise intervention for middle-aged and older individuals, management of sarcopenia using resistance training was associated with a reduction in depressive symptoms [14]. Similarly, in an Australian secondary prevention study of individuals at risk of type 2 diabetes, a 10-week programme of resistance training at low-moderate and moderate-high intensities involving major muscle groups, resulted in improved muscle strength and reduced depressed mood, such that the percentage change in relative muscle strength correlated with the change in scores for the Cardiac Depression Scale (r = -0.46, p = 0.008) [15].
Potential Mechanisms Linking Sarcopenia and Common Mental Disorders
Neurotrophins
Neurotrophins promote neuronal survival, differentiation and synaptic potentiation. Brain-derived neurotrophic factor (BDNF) acts on neurons in both the central and peripheral nervous systems and drives neurogenesis in the hippocampus [16]. The hippocampus is a key region of the brain, implicated in psychiatric illness; it has been shown that hippocampal volume is reduced in adults with depression [17], while antidepressants increase neurogenesis [18]. Despite its name, BDNF is produced in a variety of tissues, including skeletal muscle. BDNF is expressed in myoblasts in culture and in myogenic progenitors, known as satellite cells, which are responsible for growth and repair of skeletal muscle [19]; however, the role of satellite cells in the regeneration of old muscle remains unclear [20]. Skeletal muscle-derived BDNF functions as a retrograde survival factor for regenerating motor neurons [19], with the capacity to reduce the denervation-induced atrophy of skeletal muscle seen in sarcopenia. Similarly, neurotrophin-3, a key brain neurotrophin, is expressed by muscle [21]. BDNF is one of the most explored biomarkers in depression [22], and increases in BDNF parallel improvements in mood [23]. Neurotrophin-3 is similarly implicated in depression, albeit with a much smaller evidence base [24].
Inflammation and Oxidative Stress
Many studies report a role of chronic inflammation and oxidative stress in the pathophysiology of many common chronic diseases, especially in association with ageing [25, 26, 27•]. In an observational study of more than 2000 participants in the Health, Aging and Body Composition Study, tumour necrosis factor alpha (TNFα) was consistently associated with declining muscle mass and strength [28]. Obesity is an inflammatory state, as adipocytes synthesise and secrete pro-inflammatory cytokines [29]. Serum markers, such as C-reactive protein (CRP) and interleukin (IL)-6, increase with increasing fat mass—and are negatively associated with appendicular lean mass [30]. The occurrence of sarcopenia in the context of obesity, particularly abdominal obesity, which is also seen with ageing [31], constitutes the condition known as sarcopenic obesity [32, 33]. Sarcopenic obesity is similarly an inflammatory state, and this condition contributes to catabolic effects on muscle [34].
Studies in rats show that a reduction of low-grade inflammation by ibuprofen slows loss of muscle and limits the development of sarcopenia [35]. Nuclear factor–kappaB (NF-κB) is thought to upregulate IL-6 and TNFα [36]. Furthermore, reactive oxygen species (ROS) serve as second messengers for TNFα in skeletal muscle, activating NF-κB both directly and indirectly [37]. Increased TNFα expression in serum and muscle promotes apoptosis in the mitochondria, causing loss of muscle fibres [38]; indeed, TNFα is recognised as one of the primary signals inducing apoptosis in muscle—and brain. The glutathione system dampens ROS in response to inactivity-mediated skeletal muscle atrophy [39].
Low levels inflammation and oxidative stress also play a role in depression [40•], and administration of exogenous cytokines such as IL-1, interferon alpha (IFNα) and TNFα evokes a depressed mood state [41]. Over a decade ago, elevated levels of complement component (C)4, IL-6 and CRP, but not C3, were found to be associated with depression [42]. Oxidative stress induced by administration of l-buthionine-(S,R)-sulfoximine (BSO) [43] and xanthine plus xanthine oxidase (X + XO) [44] promotes NF-κB-dependent inflammation [45] and causes anxiety-like behaviour in rats.
Antidepressant treatment normalises markers of oxidative stress and inflammation [46] and a positive correlation is seen between oxidative stress index values and depression severity [47], suggesting an immunomodulatory role for antidepressant medication. Similarly, etanercept, a TNFα inhibitor used to treat psoriasis, decreases anxiety and depression in a rodent model [48], and improves fatigue and reduces depression in psoriasis patients with high baseline CRP [49]. More recently, elevated translocator protein density, which is a central marker of neuroinflammation, is found in the brain during episodes of major depression [50].
In an Australian study that examined the association between serum levels of high-sensitivity (hs) CRP and risk of de novo major depressive disorder (MDD) over a decade among randomly selected women with no prior history of depression, MDD risk increased by 44 % for each standard deviation increase in log-transformed hsCRP (adjusted HR 1.44, 95 %CI 1.04–1.99) [51]. Serum hsCRP was thus identified as an independent predictor of de novo MDD risk. Complementing these data, exposure to statins and aspirin, which have anti-inflammatory properties, have been shown to reduce the risk for depression in both clinical [52, 53] and community [54] settings, implicating both inflammation and the glutathione antioxidant system in the pathophysiology of mood disorders. Clinical trials also show that treatment with N-acetyl cysteine (NAC), a precursor of cysteine and glutathione that has anti-inflammatory effects, improves depressive symptoms in unipolar and bipolar depression [55–57]. Taken in aggregate, this series of reports supports a role of inflammation and oxidative stress in the pathophysiology of both sarcopenia and the common mental disorders.
Sarcopenia, Mood and Anxiety: What Common Role Does Lifestyle Play?
Exercise
In separate studies in Australia, ambulatory activity was reported to be associated with the maintenance of leg strength and muscle quality in older women [58], and habitual physical activity was found to be associated with a reduced likelihood of new depressive and anxiety disorders in older men and women [59]; both of these associations were driven by leisure-time activities. While resistance training is a recognised strategy for slowing or preventing the age-related loss of skeletal muscle mass and function [60], exercise and physical activity are recognised as effective strategies for treating depression and anxiety [61, 62, 63•] and reducing the risk for new onset of these disorders [59, 64•]. Contracting skeletal muscle produces BDNF [65], and exercise has been shown to enhance circulating BDNF in a range of diverse settings such as during moderate exercise in patients with multiple sclerosis [66], endurance training in healthy young men [67] and in sprint athletes [68]. Insulin-like growth factor-1 (IGF-1) is one of the factors involved in the expression of BDNF; it is increased after exercise and has neurological effects [69] as well as modulating skeletal muscle growth and atrophy [70]. Exercise also increases a number of myokines such as IL-6 [71] that impact on lipid and glucose metabolism. Recovery after the exercise-induced IL-6 spike dampens the inflammatory response and oxidative burst activity [72]; the anti-inflammatory effects of BDNF [73] may play a role in this recovery. Chronic exercise is therefore known to down-regulate systemic inflammation and is the most evidence-based management strategy for insulin resistance [74]. Given the role of inflammation in depression [75], it similarly suggests that exercise-induced downregulation of IL-6 and the anti-inflammatory effects of BDNF may be relevant pathways.
Nutrition
Adequate nutrition is essential for maintaining skeletal muscle and mental well-being. Prospective data describe a protective effect of dietary protein for sarcopenia [76] and positive associations of protein intakes with whole body muscle mass, appendicular muscle mass and upper arm muscle area [77]. Furthermore, muscle mass losses are considerably reduced for individuals with high protein intakes [78], and there are some data showing that consuming the recommended intake of red meat may be protective against depression [79]. Circulating levels of IGF-1 are positively related to protein intakes [80].
There is some evidence to suggest that antioxidants might protect skeletal muscle against oxidative stress. Higher dietary intakes of antioxidants, particularly vitamin C, were associated with better knee extension strength in a study of Italians aged 65 years and older [81]. Data from the Fourth Korean National Health and Nutrition Examination Survey showed that frequent consumption of fruit was associated with a lower likelihood of sarcopenia in elderly men and women, while a similar negative association was observed for frequent vegetable consumption, albeit in men only [82]. There are other data that support a role for poor diet as a risk factor for common mental disorders. Individuals with diets characterised by higher intakes of unhealthy ‘Western-type’ foods are more likely to have either MDD or dysthymia, while those with better quality diets, rich in vegetables, lean meats, fish and wholegrains, are less likely to have either mood or anxiety disorders [83–85]. These findings accord with the description of a diet high in fat and refined carbohydrate being regarded as an inflammatory dietary pattern that increases inflammation [86] and might increase the risk for physical and mental disorders initiated and exacerbated by chronic inflammation.
Low levels of circulating vitamin D can cause myopathy, which is linked to muscle weakness; serum levels of 25-hydroxyvitamin D below 50 nmol/L are associated with increased body sway, while levels below 30 nmol/L compromise muscle strength [87]. There is some evidence that low vitamin D levels also increase the risk for depression [88]. Hypovitaminosis D as a shared risk factor between muscle weakness and mood disorders might be explained, at least in part, by vitamin D’s immunomodulatory effects [89].
Smoking
Sarcopenia, anxiety and depression are among the many diseases attributed to, or exacerbated by, smoking cigarettes/tobacco [90–94]. A recent meta-analysis suggested that smoking was a risk factor for sarcopenia; however, interpretation of the results from multiple studies was hampered by the heterogeneity in methods used for identifying sarcopenia [95]. In a cohort study of children and adolescents in Norway, smoking in adolescence increased the risk for early adulthood anxiety [93]. Using prospective Australian data, smoking was identified as a risk marker for the onset of de novo MDD and, compared with non-smokers, the risk for MDD more than doubled for heavy smokers (> 20 cigarettes/day) [94]. It is noteworthy that muscle dysfunction is common for patients with chronic obstructive pulmonary disease [96], and this is a population also vulnerable to depression [99]. Cigarette smoke generates free radicals, causing lipid peroxidation, oxidation of proteins and other tissue damage in smokers [97]. There is some evidence that cigarette smoke activates NF-κB and increases TNFα release and production [98]. The milieu of increased inflammation and oxidative stress increases muscle catabolism [99, 100] and also increases the risk profiles for anxiety and depression [9, 101].
Conclusions
Establishing a link between sarcopenia and common mental disorders may have major translational, clinical and public health implications, as sarcopenia is a condition open to modification. Currently, there is no coherent public health strategy for preventing common mood disorders, at least in part due to lack of knowledge of potentially reversible risk factors that might include modifiable health behaviours. While physical exercise is recognised as a valuable therapeutic modality for both depression and anxiety, the role of sarcopenia in vulnerability for psychiatric illness is largely unexplored.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Luppino FS, de Wit LM, Bouvy PF, Stijnen T, Cuijpers P, Penninx BW, et al. Overweight, obesity, and depression: a systematic review and meta-analysis of longitudinal studies. Arch Gen Psychiatry. 2010;67:220–9.
Lykouras L, Michopoulos J. Anxiety disorders and obesity. Psychiatriki. 2011;22:307–13.
Rosenberg IH. Sarcopenia: origins and clinical relevance. J Nutr. 1997;127(Suppl):990S–1.
Janssen I, Heymsfield SB, Ross R. Low relative skeletal muscle mass (sarcopenia) in older persons is associated with functional impairment and physical disability. J Am Geriatr Soc. 2002;50:889–96.
Gould H, Brennan SL, Kotowicz MA, Nicholson GC, Pasco JA. Total and appendicular lean mass reference ranges for Australian men and women: the Geelong Osteoporosis Study. Calcif Tissue Int. 2014;94(4):363–72. Provides normative data for low lean mass.
Roubenoff R. Sarcopenia: a major modifiable cause of frailty in the elderly. J Nutr Health Aging. 2000;4:140–2.
Sakuma K, Yamaguchi A. Molecular mechanisms in aging and current strategies to counteract sarcopenia. Curr Aging Sci. 2011;3:90–101.
Koltun DO, Marquart TA, Shenk KD, Elzein E, Li Y, Nguyen M, et al. New fatty acid oxidation inhibitors with increased potency lacking adverse metabolic and electrophysiological properties. Bioorg Med Chem Lett. 2004;14:549–52.
Maes M, Ruckoanich P, Chang YS, Mahanonda N, Berk M. Multiple aberrations in shared inflammatory and oxidative & nitrosative stress (IO&NS) pathways explain the co-association of depression and cardiovascular disorder (CVD), and the increased risk for CVD and due mortality in depressed patients. Prog Neuropsychopharmacol Biol Psychiatry. 2011;35:769–83.
Ni Mhaolain AM, Fan CW, Romero-Ortuno R, Cogan L, Cunningham C, Kenny RA, et al. Frailty, depression, and anxiety in later life. Int Psychogeriatr. 2012;24:1265–74. Primary data that supports the contention that there is a link between frailty and the common mental disorders.
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56:M146–56.
Kim NH, Kim HS, Eun CR, Seo JA, Cho HJ, Kim SG, et al. Depression is associated with sarcopenia, not central obesity, in elderly Korean men. J Am Geriatr Soc. 2011;59:2062–8.
Gariballa S, Alessa A. Sarcopenia: prevalence and prognostic significance in hospitalized patients. Clin Nutr. 2013;32:772–6.
Sparrow D, Gottlieb DJ, Demolles D, Fielding RA. Increases in muscle strength and balance using a resistance training program administered via a telecommunications system in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:1251–7.
Levinger I, Selig S, Goodman C, Jerums G, Stewart A, Hare DL. Resistance training improves depressive symptoms in individuals at high risk for type 2 diabetes. J Strength Cond Res. 2011;25:2328–33.
Binder DK, Scharfman HE. Brain-derived neurotrophic factor. Growth Factors. 2004;22:123–31.
Campbell S, Marriott M, Nahmias C, MacQueen GM. Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry. 2004;161:598–607.
Malberg JE, Eisch AJ, Nestler EJ, Duman RS. Chronic antidepressant treatment increases neurogenesis in adult rat hippocampus. J Neurosci. 2000;20:9104–10.
Mousavi K, Jasmin BJ. BDNF is expressed in skeletal muscle satellite cells and inhibits myogenic differentiation. J Neurosci. 2006;26:5739–49.
Grounds MD. Therapies for sarcopenia and regeneration of old skeletal muscles: more a case of old tissue architecture than old stem cells. Bioarchitecture. 2014;4:81–7.
Ren JC, Fan XL, Song XA, Shi L. Decreased neurotrophin-3 expression of intrafusal muscle fibers in rat soleus muscles under simulated weightlessness. Sheng Li Xue Bao. 2011;63:75–80.
Molendijk ML, Spinhoven P, Polak M, Bus BA, Penninx BW, Elzinga BM. Serum BDNF concentrations as peripheral manifestations of depression: evidence from a systematic review and meta-analyses on 179 associations (N = 9484). Mol Psychiatry. 2014;19:791–800.
Polyakova M, Stuke K, Schuemberg K, Mueller K, Schoenknecht P, Schroeter ML. BDNF as a biomarker for successful treatment of mood disorders: a systematic & quantitative meta-analysis. J Affect Disord. 2015;174:432–40.
Pae CU, Marks DM, Han C, Patkar AA, Steffens D. Does neurotropin-3 have a therapeutic implication in major depression? Int J Neurosci. 2008;118:1515–22.
Khansari N, Shakiba Y, Mahmoudi M. Chronic inflammation and oxidative stress as a major cause of age-related diseases and cancer. Recent Patents Inflamm Allergy Drug Discov. 2009;3:73–80.
Goldberg EL, Dixit VD. Drivers of age-related inflammation and strategies for healthspan extension. Immunol Rev. 2015;265:63–74.
Moylan S, Berk M, Dean OM, Samuni Y, Williams LJ, O’Neil A, et al. Oxidative & nitrosative stress in depression: why so much stress? Neurosci Biobehav Rev. 2014;45:46–62. Presents mechanistic pathways for oxidative and nitrosative stress in depression.
Schaap LA, Pluijm SM, Deeg DJ, Harris TB, Kritchevsky SB, Newman AB, et al. Higher inflammatory marker levels in older persons: associations with 5-year change in muscle mass and muscle strength. J Gerontol A Biol Sci Med Sci. 2009;64:1183–9.
Coppack SW. Pro-inflammatory cytokines and adipose tissue. Proc Nutr Soc. 2001;60:349–56.
Cesari M, Kritchevsky SB, Baumgartner RN, Atkinson HH, Penninx BW, Lenchik L, et al. Sarcopenia, obesity, and inflammation—results from the trial of angiotensin converting enzyme inhibition and novel cardiovascular risk factors study. Am J Clin Nutr. 2005;82:428–34.
Pasco JA, Nicholson GC, Brennan SL, Kotowicz MA. Prevalence of obesity and the relationship between the body mass index and body fat: cross-sectional, population-based data. PLoS One. 2012;7:e29580.
Baumgartner RN. Body composition in healthy aging. Ann N Y Acad Sci. 2000;904:437–48.
Stenholm S, Harris TB, Rantanen T, Visser M, Kritchevsky SB, Ferrucci L. Sarcopenic obesity: definition, cause and consequences. Curr Opin Clin Nutr Metab Care. 2008;11:693–700.
Pasini E, Aquilani R, Dioguardi FS, D’Antona G, Gheorghiade M, Taegtmeyer H. Hypercatabolic syndrome: molecular basis and effects of nutritional supplements with amino acids. Am J Cardiol. 2008;101:11E–5.
Rieu I, Magne H, Savary-Auzeloux I, Averous J, Bos C, Peyron MA, et al. Reduction of low grade inflammation restores blunting of postprandial muscle anabolism and limits sarcopenia in old rats. J Physiol. 2009;587:5483–92.
Chung HY, Cesari M, Anton S, Marzetti E, Giovannini S, Seo AY, et al. Molecular inflammation: underpinnings of aging and age-related diseases. Ageing Res Rev. 2009;8:18–30.
Reid MB, Li YP. Tumor necrosis factor-alpha and muscle wasting: a cellular perspective. Respir Res. 2001;2:269–72.
Phillips T, Leeuwenburgh C. Muscle fiber specific apoptosis and TNF-alpha signaling in sarcopenia are attenuated by life-long calorie restriction. FASEB J. 2005;19:668–70.
Agostini F, Dalla Libera L, Rittweger J, Mazzucco S, Jurdana M, Mekjavic IB, et al. Effects of inactivity on human muscle glutathione synthesis by a double-tracer and single-biopsy approach. J Physiol. 2010;588:5089–104.
Berk M, Williams LJ, Jacka FN, O’Neil A, Pasco JA, Moylan S, et al. So depression is an inflammatory disease, but where does the inflammation come from? BMC Med. 2013;11:200. Presents mechanistic pathways for neuroinflammation.
Connor TJ, Leonard BE. Depression, stress and immunological activation: the role of cytokines in depressive disorders. Life Sci. 1998;62:583–606.
Berk M, Wadee AA, Kuschke RH, O’Neill-Kerr A. Acute phase proteins in major depression. J Psychosom Res. 1997;43:529–34.
Salim S, Sarraj N, Taneja M, Saha K, Tejada-Simon MV, Chugh G. Moderate treadmill exercise prevents oxidative stress-induced anxiety-like behavior in rats. Behav Brain Res. 2010;208:545–52.
Salim S, Asghar M, Chugh G, Taneja M, Xia Z, Saha K. Oxidative stress: a potential recipe for anxiety, hypertension and insulin resistance. Brain Res. 2010;1359:178–85.
Salim S, Asghar M, Taneja M, Hovatta I, Chugh G, Vollert C, et al. Potential contribution of oxidative stress and inflammation to anxiety and hypertension. Brain Res. 2011;1404:63–71.
Herken H, Gurel A, Selek S, Armutcu F, Ozen ME, Bulut M, et al. Adenosine deaminase, nitric oxide, superoxide dismutase, and xanthine oxidase in patients with major depression: impact of antidepressant treatment. Arch Med Res. 2007;38:247–52.
Yanik M, Erel O, Katoi M. The relationship between potency of oxidative stress and severity of depression. Acta Neuropsychiatr. 2004;16:200–3.
Bayramgurler D, Karson A, Ozer C, Utkan T. Effects of long-term etanercept treatment on anxiety- and depression-like neurobehaviors in rats. Physiol Behav. 2013;119:145–8.
Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, et al. Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet. 2006;367:29–35.
Setiawan E, Wilson AA, Mizrahi R, Rusjan PM, Miler L, Rajkowska G, et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry. 2015;72:268–75.
Pasco JA, Nicholson GC, Williams LJ, Jacka FN, Henry MJ, Kotowicz MA, et al. Association of high-sensitivity C-reactive protein with de novo major depression. Br J Psychiatry. 2010;197:372–7.
Stafford L, Berk M. The use of statins after a cardiac intervention is associated with reduced risk of subsequent depression: proof of concept for the inflammatory and oxidative hypotheses of depression? J Clin Psychiatry. 2012;72:1229–35.
Gougol A, Zareh-Mohammadi N, Raheb S, Farokhnia M, Salimi S, Iranpour N, et al. Simvastatin as an adjuvant therapy to fluoxetine in patients with moderate to severe major depression: a double-blind placebo-controlled trial. J Psychopharmacol. 2015;29:575–81.
Pasco JA, Jacka FN, Williams LJ, Henry MJ, Nicholson GC, Kotowicz MA, et al. Clinical implications of the cytokine hypothesis of depression: the association between use of statins and aspirin and the risk of major depression. Psychother Psychosom. 2010;79:323–5.
Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I, et al. N-acetyl cysteine for depressive symptoms in bipolar disorder—a double-blind randomized placebo-controlled trial. Biol Psychiatry. 2008;64:468–75.
Berk M, Ng F, Dean O, Dodd S, Bush AI. Glutathione: a novel treatment target in psychiatry. Trends Pharmacol Sci. 2008;29:346–51.
Dodd S, Maes M, Anderson G, Dean OM, Moylan S, Berk M. Putative neuroprotective agents in neuropsychiatric disorders. Prog Neuropsychopharmacol Biol Psychiatry. 2013;42:135–45.
Scott D, Blizzard L, Fell J, Jones G. Ambulatory activity, body composition, and lower-limb muscle strength in older adults. Med Sci Sports Exerc. 2009;41:383–9.
Pasco JA, Williams LJ, Jacka FN, Henry MJ, Coulson CE, Brennan SL, et al. Habitual physical activity and the risk for depressive and anxiety disorders among older men and women. Int Psychogeriatr. 2011;23:292–8.
Chodzko-Zajko WJ, Proctor DN, Fiatarone Singh MA, Minson CT, Nigg CR, Salem GJ, et al. American college of sports medicine position stand. Exercise and physical activity for older adults. Med Sci Sports Exerc. 2009;41:1510–30.
Thomson D, Turner A, Lauder S, Gigler ME, Berk L, Singh AB, et al. A brief review of exercise, bipolar disorder, and mechanistic pathways. Front Psychol. 2015;6:147.
Ensari I, Greenlee TA, Motl RW, Petruzzello SJ. Meta-analysis of acute exercise effects on state anxiety: an update of randomized controlled trials over the past 25 years. Depress Anxiety. 2015. doi:10.1002/da.22370.
Rebar AL, Stanton R, Geard D, Short C, Duncan MJ, Vandelanotte C. A meta-meta-analysis of the effect of physical activity on depression and anxiety in non-clinical adult populations. Health Psychol Rev. 2015;5:1–78. Provides evidence of the therapeutic benefits of physical activity on the common mental disorders.
Mammen G, Faulkner G. Physical activity and the prevention of depression: a systematic review of prospective studies. Am J Prev Med. 2013;45:649–57. Provides evidence of the preventive effects of physical activity on the onset of depression.
Matthews VB, Astrom MB, Chan MH, Bruce CR, Krabbe KS, Prelovsek O, et al. Brain-derived neurotrophic factor is produced by skeletal muscle cells in response to contraction and enhances fat oxidation via activation of AMP-activated protein kinase. Diabetologia. 2009;52:1409–18.
Gold SM, Schulz KH, Hartmann S, Mladek M, Lang UE, Hellweg R, et al. Basal serum levels and reactivity of nerve growth factor and brain-derived neurotrophic factor to standardized acute exercise in multiple sclerosis and controls. J Neuroimmunol. 2003;138:99–105.
Zoladz JA, Pilc A, Majerczak J, Grandys M, Zapart-Bukowska J, Duda K. Endurance training increases plasma brain-derived neurotrophic factor concentration in young healthy men. J Physiol Pharmacol. 2008;59 Suppl 7:119–32.
Correia PR, Scorza FA, Gomes da Silva S, Pansani A, Toscano-Silva M, de Almeida AC, et al. Increased basal plasma brain-derived neurotrophic factor levels in sprint runners. Neurosci Bull. 2011;27:325–9.
Carro E, Trejo JL, Busiguina S, Torres-Aleman I. Circulating insulin-like growth factor I mediates the protective effects of physical exercise against brain insults of different etiology and anatomy. J Neurosci. 2001;21:5678–84.
Tomori K, Kobayash R, Koseki T, Ohta Y. Effect of neuromuscular electrical stimulation of denervated muscle on the mRNA expression of IGFs in rat skeletal muscle and sciatic nerve. J Phys Ther Sci. 2009;21:269–73.
Febbraio MA, Pedersen BK. Muscle-derived interleukin-6: mechanisms for activation and possible biological roles. FASEB J. 2002;16:1335–47.
Walsh NP, Gleeson M, Shephard RJ, Woods JA, Bishop NC, Fleshner M, et al. Position statement. Part one: Immune function and exercise. Exerc Immunol Rev. 2011;17:6–63.
Makar TK, Trisler D, Sura KT, Sultana S, Patel N, Bever CT. Brain derived neurotrophic factor treatment reduces inflammation and apoptosis in experimental allergic encephalomyelitis. J Neurol Sci. 2008;270:70–6.
Stuart MJ, Baune BT. Depression and type 2 diabetes: inflammatory mechanisms of a psychoneuroendocrine co-morbidity. Neurosci Biobehav Rev. 2012;36:658–76.
Maes M, Anderson G, Kubera M, Berk M. Targeting classical IL-6 signalling or IL-6 trans-signalling in depression? Expert Opin Ther Targets. 2014;18:495–512.
Stookey JD, Adair L, Stevens J, Popkin BM. Patterns of long-term change in body composition are associated with diet, activity, income and urban residence among older adults in China. J Nutr. 2001;131:2433S–40.
Meng X, Zhu K, Devine A, Kerr DA, Binns CW, Prince RL. A 5-year cohort study of the effects of high protein intake on lean mass and BMC in elderly postmenopausal women. J Bone Miner Res. 2009;24:1827–34.
Houston DK, Nicklas BJ, Ding J, Harris TB, Tylavsky FA, Newman AB, et al. Dietary protein intake is associated with lean mass change in older, community-dwelling adults: the Health, Aging, and Body Composition (Health ABC) Study. Am J Clin Nutr. 2008;87:150–5.
Jacka FN, Pasco JA, Williams LJ, Mann N, Hodge A, Brazionis L, et al. Red meat consumption and mood and anxiety disorders. Psychother Psychosom. 2012;81:196–8.
Norat T, Dossus L, Rinaldi S, Overvad K, Gronbaek H, Tjonneland A, et al. Diet, serum insulin-like growth factor-I and IGF-binding protein-3 in European women. Eur J Clin Nutr. 2007;61:91–8.
Cesari M, Pahor M, Bartali B, Cherubini A, Penninx BW, Williams GR, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr. 2004;79:289–94.
Kim J, Lee Y, Kye S, Chung YS, Kim KM. Association of vegetables and fruits consumption with sarcopenia in older adults: the Fourth Korea National Health and Nutrition Examination Survey. Age Ageing. 2015;44:96–102.
Jacka FN, Pasco JA, Mykletun A, Williams LJ, Hodge AM, O’Reilly SL, et al. Association of Western and traditional diets with depression and anxiety in women. Am J Psychiatry. 2010;167:305–11.
Jacka FN, Kremer PJ, Berk M, de Silva-Sanigorski AM, Moodie M, Leslie ER, et al. A prospective study of diet quality and mental health in adolescents. PLoS One. 2011;6:e24805.
Jacka FN, Pasco JA, Mykletun A, Williams LJ, Nicholson GC, Kotowicz MA, et al. Diet quality in bipolar disorder in a population-based sample of women. J Affect Disord. 2011;129:332–7.
Tabung FK, Steck SE, Zhang J, Ma Y, Liese AD, Agalliu I, et al. Construct validation of the dietary inflammatory index among postmenopausal women. Ann Epidemiol. 2015;25:398–405.
Pfeifer M, Begerow B, Minne HW. Vitamin D and muscle function. Osteoporos Int. 2002;13:187–94.
Anglin RE, Samaan Z, Walter SD, McDonald SD. Vitamin D deficiency and depression in adults: systematic review and meta-analysis. Br J Psychiatry. 2013;202:100–7.
Correale J, Ysrraelit MC, Gaitan MI. Immunomodulatory effects of Vitamin D in multiple sclerosis. Brain. 2009;132:1146–60.
Castillo EM, Goodman-Gruen D, Kritz-Silverstein D, Morton DJ, Wingard DL, Barrett-Connor E. Sarcopenia in elderly men and women: the Rancho Bernardo study. Am J Prev Med. 2003;25:226–31.
Lee JS, Auyeung TW, Kwok T, Lau EM, Leung PC, Woo J. Associated factors and health impact of sarcopenia in older Chinese men and women: a cross-sectional study. Gerontology. 2007;53:404–10.
Landi F, Liperoti R, Fusco D, Mastropaolo S, Quattrociocchi D, Proia A, et al. Prevalence and risk factors of sarcopenia among nursing home older residents. J Gerontol A Biol Sci Med Sci. 2012;67:48–55.
Moylan S, Gustavson K, Karevold E, Overland S, Jacka FN, Pasco JA, et al. The impact of smoking in adolescence on early adult anxiety symptoms and the relationship between infant vulnerability factors for anxiety and early adult anxiety symptoms: the TOPP Study. PLoS One. 2013;8:e63252.
Pasco JA, Williams LJ, Jacka FN, Ng F, Henry MJ, Nicholson GC, et al. Tobacco smoking as a risk factor for major depressive disorder: population-based study. Br J Psychiatry. 2008;193:322–6.
Steffl M, Bohannon RW, Petr M, Kohlikova E, Holmerova I. Relation between cigarette smoking and sarcopenia - meta analysis. Physiol Res. 2014.
Degens H, Gayan-Ramirez G, van Hees HW. Smoking-induced skeletal muscle dysfunction: from evidence to mechanisms. Am J Respir Crit Care Med. 2015;191:620–5.
Ozguner F, Koyu A, Cesur G. Active smoking causes oxidative stress and decreases blood melatonin levels. Toxicol Ind Health. 2005;21:21–6.
Preciado D, Kuo E, Ashktorab S, Manes P, Rose M. Cigarette smoke activates NFkappaB-mediated Tnf-alpha release from mouse middle ear cells. Laryngoscope. 2010;120:2508–15.
Rom O, Kaisari S, Aizenbud D, Reznick AZ. Sarcopenia and smoking: a possible cellular model of cigarette smoke effects on muscle protein breakdown. Ann N Y Acad Sci. 2012;1259:47–53.
Rom O, Kaisari S, Aizenbud D, Reznick AZ. The effects of acetaldehyde and acrolein on muscle catabolism in C2 myotubes. Free Radic Biol Med. 2013;65:190–200.
Moylan S, Jacka FN, Pasco JA, Berk M. How cigarette smoking may increase the risk of anxiety symptoms and anxiety disorders: a critical review of biological pathways. Brain Behav. 2013;3:302–26.
Acknowledgments
LJW is supported by a NHMRC Career Development Fellowship (1064272); FNJ is supported by a post-graduate scholarship from the Australian Rotary Health Fund and a Postdoctoral Training Fellowship from the NHMRC; SLB-O is supported by an Alfred Deakin Postdoctoral Research Fellowship; MB is supported by a NHMRC Senior Principal Research Fellowship. These funding organisations played no role in the preparation, review and approval of the manuscript.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
JAP has received grants from the National Health and Medical Research Council (NHMRC), the BUPA Foundation and the Western Alliance; LJW has received grants from Eli Lilly, Pfizer, The University of Melbourne, Deakin University and the NHMRC; FNJ has received grants from the Brain and Behaviour Research Institute, the NHMRC, Australian Rotary Health, the Geelong Medical Research Foundation, the Ian Potter Foundation, Eli Lilly, the Meat and Livestock Board and The University of Melbourne and has received speakers honoraria from Sanofi-Synthelabo, Janssen Cilag, Servier, Pfizer, Health Ed, Network Nutrition, Angelini Farmaceutica, and Eli Lilly; NS has received grants from the NHMRC, Financial Markets Foundation for Children and Deakin University; SLB-O has received grants from the Victorian Orthopaedic Research Trust, Arthritis Australia (Zimmer Australia), The University of Melbourne, Arthritis Victoria and the Australian Association of Gerontology, Deakin University and the City of Greater Geelong; MB has received grants from the NIH, Simons Autism Foundation, Stanley Medical Research Foundation, NHMRC and CRC for Mental Health, he has been a paid consultant for Astra Zeneca, Eli Lilly, Glaxo SmithKline, Janssen Cilag, Lundbeck and Pfizer and a paid speaker for Astra Zeneca, Eli Lilly, Glaxo SmithKline, Lundbeck and Merck; FNJ has received grants from the Brain and Behaviour Research Institute, NHMRC, Australian Rotary Health, the Meat and Livestock Board and has been a paid speaker for Sanofi-Synthelabo, Janssen Cilag, Servier and Health Ed.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Nutrition, Exercise, and Lifestyle in Osteoporosis
Rights and permissions
About this article
Cite this article
Pasco, J.A., Williams, L.J., Jacka, F.N. et al. Sarcopenia and the Common Mental Disorders: a Potential Regulatory Role of Skeletal Muscle on Brain Function?. Curr Osteoporos Rep 13, 351–357 (2015). https://doi.org/10.1007/s11914-015-0279-7
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-015-0279-7