Abstract
Background
Sarcopenic obesity (SO) is a condition combining two important public health issues commonly seen amongst older individuals, obesity and sarcopenia. Depressive symptoms are common among older people, whose population is increasing worldwide. Obesity and sarcopenia alone, are clearly associated with depression while the coexistence of these two conditions (SO) upon depressive disorders is currently unclear. We aimed to systematically review the association between primary SO and depressive disorders.
Methods
Searches were run on MEDLINE, EMBASE, PsycINFO, and CINAHL (inception to June 2019). One reviewer screened titles, abstracts, and full-texts, with 10% checked independently by a second reviewer. Cohort and cross-sectional studies were included. Two reviewers independently assessed risk of bias using the Mixed Methods Appraisal Tool. Results were narratively synthesised.
Results
Out of the 7 studies eligible for inclusion, evidence of sarcopenic obesity as a predictor of depressive symptoms was found in two studies. The main observed trend was that diagnosing sarcopenia using muscle strength led to significant associations between sarcopenic obesity and depressive symptoms. Two cross-sectional studies found a significant association between SO and depressive symptoms, whilst three others found no statistically significant associations. All possessed some methodological limitations.
Discussion
This is the first review to systematically examine a potential relationship between sarcopenic obesity and depressive disorders. Currently, the results are heterogeneous due to the large variability in assessment methods and outcome measurements. Future longitudinal studies would achieve greater confidence in the provisional conclusion that sarcopenic obesity, when measured using muscle strength, is associated with depressive symptoms.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2015, it was estimated that 39% of the world’s population were overweight or obese (1) and this is progressively increasing. Obesity is associated with a range of adverse outcomes, including a greater risk of diabetes, cardiovascular disease and mortality (2). Among obese populations, depression’s prevalence is elevated and a bidirectional effect is usually implied in studies. It has been observed that in extreme weight measurements (underweight and obese), the prevalence of depression is higher, by 23% among obese participants (3). Concurrently, depressed individuals are significantly more likely to develop obesity than those who are not depressed (4). Additionally, obesity increases the risk of depression (5). A meta-analysis including cross-sectional studies revealed a strong positive association between obesity and depression in the general population, confirming the previous findings (6).
Approximately 20% of people aged over 60 experience a mental disorder while amongst them 7% are diagnosed with depression (7). Depression is associated with greater functional decline, poorer quality of life and increased use of healthcare services. Common characteristics include feelings of sadness, emptiness, irritability and mood dysregulations along with cognitive and physical impairments which disrupt the person’s functionality (8). It has also been argued that additional changes in physiological function that should be measured such as sleep disturbances, loss of appetite, fatigue, hopelessness and cognitive impairments should be taken into account when diagnosing depression in older people (9).
There is a global increase in the older population and by the year 2050, the proportion of the world’s population aged over 65 years old will reach 22%, compared to the 12% documented in 2015 (7). Ageing is accompanied by losses in muscle mass and muscle strength leading to ‘primary sarcopenia’ (10). There are different working groups on sarcopenia (European Working Group on Sarcopenia in Older People (EWGSOP), the Asian Working Group (AWGS), International Working Group on Sarcopenia) which all exhibit differences in standards and cutoff points for defining sarcopenia. The Asian Working Group (AWGS) considers muscle mass as a primary indicator but uses different cut-off values due to morphological differences between the Caucasians and Asians (11). The International Working Group on Sarcopenia based the diagnosis on low fat-free mass combined with inadequate physical functioning (12). A recent meta-analysis concluded that sarcopenia is positively associated with depression rates among the elderly (13). Whilst sarcopenia is recognised as a disease primarily affecting older people, it may also be a comorbidity of a health condition, which is defined as secondary sarcopenia (14). Chronic inflammation from a health condition is paired with an increased circulation of pro-inflammatory cytokines which shift the balance of protein turnover promoting muscles’ catabolism and thus, secondary sarcopenia (15).
Obesity can exacerbate sarcopenia (10) through further loss of muscle mass due to its infiltration by the adipose tissue (16). Sarcopenic obesity (SO) is therefore a combination of excessive adipose tissue and decreased skeletal muscle mass and strength (17). Factors involved in the pathogenesis of sarcopenic obesity include ageing, sedentary lifestyle, increased energy intake, insulin resistance, inflammation, and oxidative stress (18).
A plethora of complications are attributed to sarcopenic obesity. In a meta-analysis of prospective studies, there was a 24% increase in the mortality rate attributed to all-cause mortality in sarcopenic obese adults compared to the non-sarcopenic obese adults (19). The disability burden is exacerbated; weakness due to sarcopenia is combined with the need to support added weight from obesity (20). Reduced muscle strength increases the risk of falling which in combination with age-related declines in bone density, poses a major risk of fractures (21). There is a negative correlation between body mass index (BMI) and quality of life, which increases in the presence of sarcopenia (22). There are various ways in which SO appears to influence the quality of life, including reducing exercise ability, daily activity and self- care (23).
In a longitudinal study, low grip strength, used as the measurement for the diagnosis of sarcopenia, was associated with depressive symptoms and interestingly, the relationship was present only between those participants classified as obese (24), posing an intriguing scenario of a potential synergistic effect of increased fat mass and sarcopenia on the onset of depression. There is some evidence stemming from individual studies suggesting that increased fat mass and decreased muscle mass, alone, are associated with a worse psychological condition (21).
Although previous reviews have assessed the relationship between sarcopenia and depression (13), no previous review has assessed the relationship between sarcopenic obesity and depression. Therefore, the main aim of this review is to investigate the association between sarcopenic obesity and depression. In parallel, it may trigger future research on a possible ability of sarcopenic obesity to predict the development of depressive disorders. The review focuses on primary sarcopenic obesity as secondary sarcopenic obesity may have other confounding factors in relation to the health condition itself and depression.
Methods
We carried out a systematic review of observational studies, assessing the association between sarcopenic obesity (SO) and depression.
Data sources and search strategy
We searched MEDLINE, EMBASE, PsycINFO, and CINAHL (inception to June 2019), using a combination of subject heading and free text terms relating to obesity (including “high fat mass”, “adiposity”, “increased BMI”), sarcopenia (including “reduced muscle mass”, “reduced muscle strength”, “muscle atrophy”), and depressive disorders (including “depressive symptoms”, “low mood”, “dysthymia”). All relevant papers written in any language were included to avoid language bias. We did not search for or include grey literature as this was felt to be an unlikely source of cohort and cross-sectional studies.
Study selection
Titles and abstracts were screened by one reviewer (IP) for eligibility, with 10% checked by a second reviewer (RF). Agreement was 97.46%. Full texts were screened by one reviewer (IP), and two additional reviewers (RF and AS) checked those assessed as eligible for inclusion and those considered unclear.
Inclusion criteria: cohort and cross-sectional studies; adults (>18 years of age, as although typically age-related declines in muscle strength and function are only seen after age 50, there are individual variations affecting this (25)), with primary (age-related) sarcopenia and co-existing obesity, located either in the community or in a specific care setting; assessing depression as an outcome using a questionnaire or self-reported or clinician diagnosis, including major depression or depressive symptomatology. As the European Working Group definition is relatively recent and is not used worldwide, in order to find the full range of relevant studies, primary sarcopenia in this review could be measured as muscle mass, muscle strength or both (although currently, low handgrip strength is the main determinant for the diagnosis of the condition). As the relationship between factors can be bi-directional, it was also regarded as appropriate to include and discuss studies assessing obesity status and depressive symptoms as predictors for a measured decline in muscle strength (sarcopenia), to investigate the direction of relationships between these variables.
Exclusion criteria: Studies only assessing outcomes such as quality of life, wellbeing or other psychiatric conditions were excluded. Studies focusing on patients with a particular health condition (e.g. cancer, diabetes) were excluded, as features of individual diseases may independently influence the risk of depression.
Quality assessment
The risk of bias within eligible studies was assessed with section 3 of the Mixed Methods Appraisal Tool (MMAT) (26). A second reviewer (RF) independently rated each study as well, with disagreements resolved through email discussion.
Data extraction and data synthesis
A data extraction form was designed to extract all relevant and necessary data including each study’s design, the number and age of participants, the diagnostic tools used to assess sarcopenic obesity and outcome measured (major depression, depressive symptomatology, onset of depressive symptoms, etc.) and tool(s) used to assess it.
Meta-analysis was intended to be undertaken where possible. However, in many cases, this was precluded due to heterogeneity in methods and in measures used between studies. In two studies which were eligible for meta-analysis, there was substantial statistical heterogeneity (I2 = 86%) due to large variation in sample sizes, and so we have not reported this in our results. The remaining studies were tabulated and narratively synthesised. Due to the wide diversity in definitions and measures used for both predictors and outcomes, studies are critically compared and contrasted, with considerations for quality and methodological approaches.
Results
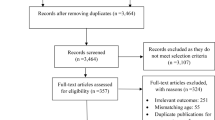
Out of the 757 initial citations identified, of which 556 remained after removing duplicate data, the final screening identified seven studies fulfilling the inclusion criteria (Figure 1.). Out of the nineteen studies which were screened full- text, twelve were excluded. A common justification for exclusion was that some of the studies were not assessing the predictor of interest, which was the co-existence of sarcopenia and obesity defined as sarcopenic obesity (n=6), or they did not measure for the outcome of interest, which was depressive symptoms (n=2), or both (n=2). Two studies were excluded due to their design (they were reviews).
Of the seven included studies, four were cross- sectional, two used a longitudinal design while one of them (27) incorporated both design methods. Five assessed the relationship between sarcopenic obesity and depression, and two assessed whether obesity and depressive symptoms predicted declines on handgrip strength. Two were carried out in the USA, one in Japan, one in England, two in Korea and one in the Netherlands. Participants’ mean ages varied from 43.3 years to 77.1 years, and sample sizes ranged from 506 to 11521.
Sarcopenic obesity was assessed with a combination of measuring sarcopenia and obesity. Obesity was measured either using BMI, (24, 28, 29, 30), waist circumference (23, 27) or body fat percentage (31); whilst sarcopenia was assessed using handgrip strength in five studies (24, 27, 28, 30, 31) and appendicular skeletal mass in two studies (23, 29).
Depressive symptoms were measured using: a positive response to the question ‘In the past year, have you felt sadness or despair continuously for two or more weeks that was severe enough to interfere with daily life?’ alone (23) or also asking for self-reported diagnosis (29), the Centre of Epidemiological Studies Depression Scale (24, 32) or a shorter adaptation (28, 33); 15- item self-reported Geriatric Depression Scale (GDS), (31) validated for the Japanese population (34); the Patient Health Questionnaire (PHQ-9) (30), which is 9-item depression screener (35); or the Composite International Diagnostic Interview (CIDI version 2.1) (36) to assess the presence of depression or dysthymia according to the (DSM-IV-TR) (27).
Risk of bias within included studies
Table 1, summarises the quality assessment process.
Overall, the majority of studies met most quality criteria. The purpose of this study was to detect adults with age-related sarcopenia so the population included in each study was judged as appropriately selected. Since there is no consensus respecting the definition of sarcopenic obesity no strict judgment could be made regarding the appropriateness of the measurement of the predictor, so this was divided into appropriateness of sarcopenia measure and appropriateness of obesity measure. Moreover, given the small amount of literature available, it was thought as most appropriate to be more lenient in quality assessment regarding variability between the studies’ definitions.
This fact, along with the differences in population characteristics across the studies (different ethnicities, races, ages included) increases the heterogeneity. All studies adjusted their models for covariates (demographic, socioeconomic or clinical factors), but different kinds and numbers were used across them.
Longitudinal associations between sarcopenic obesity and depression
Within the two longitudinal studies (Table 2.), there was high quality evidence that sarcopenic obesity was associated with higher risk of depressive symptoms at 6 years follow up, which weakened slightly but was still significant after adjusting for covariates (adjusted OR 1.79 (1.10, 2.89), reference group non-obese, non-sarcopenic adults) (24). In a smaller depressed Dutch cohort, there was a significant interaction between sarcopenia and obesity (when measured continuously as low handgrip strength and waist circumference) in predicting non-remission of depressive symptoms after 2 years; in the sarcopenic group an increase in waist circumference was associated with an adjusted OR 1.06 [1.01 – 1.11] (27). When assessed dichotomously, this association was not found (OR=1.54 [95% CI: 0.75 – 3.16], p=.241).
Cross-sectional associations between sarcopenic obesity and depression
Evidence for cross-sectional associations was conflicting (Table 3). Associations were mostly observed in studies of better quality. Sarcopenic obesity was significantly associated with an increased risk of depressive symptoms in one high quality large Japanese study in older adults (adjusted OR 2.79 (1.43–5.43), p=0.003, compared to the reference group of non-obese, non-sarcopenic adults) (31). In the adjusted model, there was also a significant interaction between sarcopenia and obesity as two independent variables. However, the increased risk of depressive symptoms was seen most strongly in those aged 65–74 years old (adjusted OR 6.05 (1.89–19.38), p=0.003) and became non-significant in those aged >75years old (adjusted OR (1.77 (0.75–4.18), p=0.2) (31).
Within the Korean National Health Study, sarcopenic obesity was examined in relation to the umbrella term of “psychological health”, separately in two age groups; >=60 years old and those of a younger age. Focusing on subgroup analysis, associations with depressive symptoms, suicidal ideation and perceived stress for those aged >=60 were non- significant (adjusted OR 0.95 (CI 0.66–1.38)) compared to the younger age group who exhibited significant associations with both perceived stress and suicidal ideation, but not depressive symptoms. Interestingly, in the <60 age group associations of SO with perceived stress were stronger than in the general obesity group, implying an important additional effect of sarcopenia on obesity (23).
Two other studies did not find an association. In one large Korean study, a self-reported depression diagnosis and depressive symptoms had a higher prevalence within sarcopenic obese participants, but this was not statistically significant in an unadjusted Chi-squared test across any age group (29). Similarly, the NESDO found no cross-sectional association between sarcopenic obesity and depression (adjusted OR 1.09 [0.54 – 2.23]), whether measured continuously or dichotomously (27). However, NESDO recruited participants on the basis of having depression, in addition to a smaller number of healthier controls, and the representativeness of this cohort is questionable.
Studies of obesity and depression as a predictor of sarcopenia
In a moderate quality longitudinal study (28), older adults with the coexistence of an increased BMI and depressive mood did not exhibit significant declines in handgrip strength compared to the reference group of those with increased BMI and no depressive symptoms (adjusted OR not reported, no difference when adjusted for antidepressant use). However, within this study, an overweight BMI cutoff (>25kg/m2) rather than an obese cutoff, was used and participant’s age range may be considered high (71–92 years). In another high-quality cross-sectional study, among obese adults aged >60, both men (adjusted OR −3.72 (−7.00 to −0.43)) and women (adjusted OR −1.83 (−2.87 to −0.78)) with moderate to severe depressive symptoms had lower handgrip strength compared to those with no depressive symptomatology (30). Associations were not significant for those with mild depressive symptoms (Table 4.).
Discussion
The original motive of the present review was the examination of the effect of sarcopenic obesity on depressive disorders. The included studies found limited evidence that sarcopenic obesity increases the risk of depressive symptoms and non-remission of depression. Cross-sectional studies were heterogenous, but suggested associations may exist in studies where handgrip strength is used as the primary definition for sarcopenia, with stronger associations in younger populations. These heterogeneous results may be explained in a number of ways.
Firstly, studies using muscle strength to define sarcopenia were more likely to report significant associations with depressive symptoms. The two studies using muscle mass to define sarcopenia without considering muscle strength did not find an association. The revised guidelines for sarcopenia (10), suggest that handgrip strength is a better indicator of muscle function and is preferred over measures of muscle mass alone, although this derives from a European Working Group, and other groups (e.g. the Asian Working Group) may use measures more relevant to their populations. Although low muscle mass explains 13% of the variance in muscle strength (38), other parameters such as age and fat mass are equally recognised as crucial determinants (39). As obesity can incorporate an increase in muscle as well as fat mass, in obese individuals quantity of muscle mass is commonly found to be normal while its quality is insufficient (40). While in normal-weight individuals mood is not correlated with muscle mass, studies using hand-grip strength as a useful measurement of general muscle function, report a significant association between low hand- grip strength and depressive symptomatology (41, 42). In obese individuals, anxiety and depression levels are influenced by lean mass reductions paired with its infiltration by the adipose tissue (43). Other studies have also concluded that it is only in the presence of obesity when sarcopenia is related to some health complications (44, 45), nominating muscle tissue’s condition as a reason for variations in health between obese and non-obese individuals. Another study suggested a synergistic effect of low grip strength and depression on all-cause mortality in older adults (46).
Another explanation may be the obesity definitions used. In an earlier study examining the relationship between body composition and depression amongst older people no association between central obesity and depression can be found (47). At the same time, what is already known is that abdominal fat is more pathogenic than subcutaneous. In a large studied sample from NHANES, participants classified as overweight using BMI, with an increased waist circumference, had a greater prevalence of depression (48). On the current review, studies using waist circumference (23, 27), were less likely to find a cross sectional association than those using BMI as a diagnostic criterion.
Similarly, use of lower obesity cutoffs for BMI (25 rather than 30kg/m2), may also impact upon outcomes. The cut off point for obesity diagnosis in Caucasians is 30 kg/m2, while for Asian populations BMI should be 27.5 kg/m2 or higher (49, 50). The earliest study included found no association between elevated BMI (>25kg/m2) and depression based on baseline measurements (28). This is in contrast with current knowledge since the relationship between depression and obesity is confirmed by a plethora of evidence (4, 5). A meta-analysis including cross-sectional studies revealed a strong positive association between obesity and depression in the general population confirming the previous findings (6). In those papers finding a significant association, these were much stronger in younger than older populations, which may be related to better mortality outcomes in obese and overweight older adults (51). One paper included in the current review found a significant association between depressive symptoms and SO in adults <65years old (23). This could be an insight suggesting that it is equally important for younger obese adults to be screened for primary sarcopenia since deteriorations on muscle mass may vary depending on general health and physical condition.
Measurement of depressive symptoms may also influence results. Self-reported mental health is a methodologically vulnerable tool since the way people perceive their health is controversial and could be easily influenced by many factors or even vary from day to day and rely on experiences during the past days (52). Studies using a validated questionnaire were more likely to report associations with sarcopenic obesity than those using a non-validated single question (23, 29). This may relate to the broadness of the population captured — one study (30) found that an association was only present in those with more severe symptoms, although one study using self-reported depression diagnoses found no association (28). No studies measured the association between sarcopenic obesity and clinician-diagnosed Major Depressive Disorder alone.
Overall completeness, applicability, and quality of evidence
There was a limited number of papers eligible for inclusion in this review, suggesting conclusions may be open to change in the future. Most of these were conducted recently, reflecting that sarcopenic obesity is a condition which is gaining attention and being more explicitly defined and understood. The results of included studies are supported by the existing knowledge of biological and pathophysiological mechanisms and previous work on the field, but currently are very heterogeneous in the measures used. However, findings do stem from different countries all over the world.
Strengths and limitations
To the best of our knowledge, up to date, this is the first systematic review investigating the association between sarcopenic obesity and depressive disorders. Two reviewers assessed the quality of included studies.
However, limitations exist in the heterogeneity of measurement methods used. Although currently sarcopenic obesity is identified as a condition on its own, its assessment is still based on individual measurements of obesity and sarcopenia, with no universal definition for combining the two. Some of the papers adjusted the results for some comorbidities but possibly, results cannot be generalised to populations with greater comorbidity. Only a few studies adjusted for anti-depressant use, which may be a strong predictor of depressive symptoms. Another limitation is the observational design of the studies which restricts the ability to draw conclusions regarding causal relationships. Likewise, many of the included studies were cross-sectional, limiting conclusions that can be drawn regarding the direction of the relationship.
Implications for practice and policy
The evidence suggests that there may be a link between sarcopenic obesity and depressive symptoms, but that this is likely to be stronger in younger populations and when sarcopenia is defined using handgrip strength. There is some evidence for an interaction between sarcopenia and obesity, and that the link between sarcopenic obesity and depression may be bi-directional, but this requires further investigation. Health professionals should be aware that for those who are obese, low muscle strength may further increase the risk of depressive symptoms in addition to other health issues. In that case, they should be screened regularly for depressive symptoms so that the effects of sarcopenic obesity may be combated with appropriate treatment strategies. Current evidence suggests that the most effective strategies are those incorporating hypocaloric diet (no more than 200–700 Kcals deficit to minimize muscle mass loss) along with aerobic training for fat loss, resistance training and increased protein intake to preserve the quality and quantity of muscles (53). The type of protein is important which should be high quality, evenly distributed through a day aiming to achieve an intake every 3 to 4 hours (53).
Implications for future research
Further longitudinal studies need to be undertaken to provide definitive evidence for a relationship between sarcopenic obesity and depressive symptoms. There needs to be an international consensus regarding the tools, measurements and cut-off points used in the diagnosis of sarcopenic obesity. The absence of a clear, universally established definition introduces a big variation in the rates of sarcopenic obesity depending on the used criteria (54), however this may be challenging given the necessary differences in cut-offs between different populations. More attention should be drawn towards the importance of the in-depth study of the condition and recognition of potentially modifiable risk factors could trigger prevention strategies on a population basis. In a society in which 39% of the population are overweight, sarcopenic obesity and its resulting effects is likely to become a larger issue in future.
Furthermore, so far there is not an identified, accepted mechanism by which sarcopenic obesity manifests the disturbances in depressive disorders. Gaining an understanding of the underlying pathophysiological mechanisms would help design appropriate tackle strategies.
Author’s conclusions
This review found limited evidence that sarcopenic obesity increases the risk of depressive symptoms, with some conflicting findings. Reductions in obesity and sarcopenia levels may have the potential to reduce the risk of depressive symptoms in addition to improving functioning and reducing the risk of comorbidities such as disability, stress and anxiety disorders, metabolic impairments such as insulin resistance and quality of life in general.
References
Chooi, Y. C., Ding, C., Magkos, F. The epidemiology of obesity. Metabolism, 2018;92, pp. 6–10. https://doi.org/10.1016/j.metabol.2018.09.005
Djalalinia, S., Qorbani, M., Peykari, N., & Kelishadi, R. Health impacts of Obesity. Pakistan journal of medical sciences, 2015;31(1), pp. 239–242. https://doi.org/10.12669/pjms.311.7033
Carey, M., Small, H., Yoong, S. L., Boyes, A., Bisquera, A., & Sanson-Fisher, R. Prevalence of comorbid depression and obesity in general practice: a cross-sectional survey. The British journal of general practice: the journal of the Royal College of General Practitioners, 2014;64(620), e122–e127. https://doi.org/10.3399/bjgp14X677482
Blaine, B. Does Depression Cause Obesity? A Meta-analysis of Longitudinal Studies of Depression and Weight Control. Journal of Health Psychology, 2008;13(8), pp. 1190–1197. https://doi.org/10.1177/1359105308095977.
Luppino, F.S., de Wit, L.M., Bouvy, P.F., Stijnen, T., Cuijpers, P., Penninx, B.W., Zitman, F.G. Overweight, Obesity, and Depression: A Systematic Review and Metaanalysis of Longitudinal Studies. Arch Gen Psychiatry, 2010;67(3), pp. 220–229. https://doi.org/10.1001/archgenpsychiatry.2010.2
Wit, L., Luppino, F., Straten, A., Penninx, B.W., Zitman, F., Cuijpers, P. Depression and Obesity: A Meta-Analysis of Community-Based Studies. Psychiatry Res, 2010;178(2), pp. 230–5. https://doi.org/10.1016/j.psychres.2009.04.015
World Health Organization, 2017. Mental health of older adults [Fact sheet]. Available at: https://www.who.int/news-room/fact-sheets/detail/mental-health-of-older-adults
American Psychiatric Association, 2013. Diagnostic and statistical manual of mental disorders (5th ed.). https://doi.org/10.1176/appi.books.9780890425596
Luppa, M., Sikorski, C., Luck, T., Ehreke, L., Konnopka, A., Wiese, B., Weyerer, S., König, H.H., Riedel-Heller, S.G. Age- and gender-specific prevalence of depression in latest-life - Systematic review and meta-analysis. Journal of Affective Disorders, 2012;136(3), 212–221. https://doi.org/10.1016/j.jad.2010.11.033
Cruz-Jentoft, A. J., Bahat, G., Bauer, J., Boirie, Y., Bruyère, O., Cederholm, T., Cooper, C., Landi, F., Rolland, Y., Aihie Sayer, A., Schneider, S., C Sieber, C., Topinková, E., Vandewoude, M., Visser, M., Zamboni, M. Sarcopenia: revised European consensus on definition and diagnosis. Age and Ageing. 2019;48(1), pp.16–31. https://doi.org/10.1093/ageing/afy169
Chen, L.-K., Lee, W.-J., Peng, L.-N., Liu, L.-K., Arai, H., & Akishita, M. Recent Advances in Sarcopenia Research in Asia: 2016 Update From the Asian Working Group for Sarcopenia. Journal of the American Medical Directors Association, 2016;17(8), 767.e1–767.e7. https://doi.org/10.1016/j.jamda.2016.05.016
Fielding, R., Vellas, B., Evans, W., et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, aetiology, and consequences. J Am Med Dir Assoc., 2011;12(4), pp.249–56. https://doi.org/10.1016/j.jamda.2011.01.003
Chang, K.V., Hsu, T.H., Wu, W.Y., Huang, K.C., Han, DS. Is sarcopenia associated with depression? A systematic review and meta-analysis of observational studies. Age and Ageing, 2017;46(5), pp. 738–746. https://doi.org/10.1093/ageing/afx094
Fan, J., Kou, X., Yang, Y., Chen, N. MicroRNA-Regulated Proinflammatory Cytokines in Sarcopenia. Mediators of inflammation, 2016, 1438686. https://doi.org/10.1155/2016/1438686
Pérez-Baos, S., Prieto-Potin, I., Román-Blas, J. A., Sánchez-Pernaute, O., Largo, R., Herrero-Beaumont, G. Mediators and Patterns of Muscle Loss in Chronic Systemic Inflammation. Frontiers in physiology; 2018;9, pp. 409. https://doi.org/10.3389/fphys.2018.00409
Kalinkovich, A., Livshits, G. Sarcopenic obesity or obese sarcopenia: A cross talk between age-associated adipose tissue and skeletal muscle inflammation as a main mechanism of the pathogenesis. Ageing Res Rev, 2016;35, pp. 200–21. https://doi.org/10.1016/j.arr.2016.09.008
Yu, S., Umapathysivam, K., Visvanathan, R. Sarcopenia in older people. International Journal of Evidence-Based Healthcare, 2014;12(4), 227–243. https://doi.org/10.1097/xeb.0000000000000018
Polyzos, S. A., Margioris, A. N. Sarcopenic obesity. Hormones, 2018;17(3), pp. 321–331. https://doi.org/10.1007/s42000-018-0049-x
Tian, S., Xu, Y. Association of sarcopenic obesity with the risk of all-cause mortality: A meta-analysis of prospective cohort studies. Geriatrics & Gerontology International, 2015;16(2), 155–166. https://doi.org/10.1111/ggi.12579
Roubenoff, R. Sarcopenic Obesity: The Confluence of Two Epidemics. Obesity Research, 2004;12(6), pp. 887–8. https://doi.org/10.1038/oby.2004.107
Batsis, J. A., Villareal, D. T. Sarcopenic obesity in older adults: aetiology, epidemiology and treatment strategies. Nature reviews. Endocrinology, 2018;14(9), pp. 513–537. https://doi.org/10.1038/s41574-018-0062-9
Ul-Haq Z., Mackay D.F., Fenwick E. & Pell J.P. Meta-analysis of the association between body mass index and health-related quality of life among adults, assessed by the SF-36. Obesity (Silver Spring), 2013;21(3), E322–E327. https://doi.org/10.1002/oby.20107
Cho, Y., Shin, S.Y, Shin, M.J. Sarcopenic obesity is associated with lower indicators of psychological health and quality of life in Koreans. Nutr. Res., 2015;35(5), pp. 384–92. https://doi.org/10.1016/j.nutres.2015.04.002
Hamer, M., Batty, G. D., & Kivimaki, M. Sarcopenic obesity and risk of new onset depressive symptoms in older adults: English Longitudinal Study of Ageing. International journal of obesity (2005), 2015;39(12), pp. 1717–1720. https://doi.org/10.1038/ijo.2015.124
Keller, K., Engelhardt, M. Strength and muscle mass loss with aging process. Age and strength loss. Muscles, ligaments and tendons journal, 2014;3(4), pp. 346–350. PMID: 24596700; PMCID: PMC3940510.
Hong, Q. N., FÀBregues, S., Bartlett, G., Boardman, F., Cargo, M., Dagenais, P., Gagnong, M., Griffithsd, F., Nicolauh, B., O’Cathaini, A., Rousseauj, M., Vedela, I., Pluye, P. The Mixed Methods Appraisal Tool (MMAT) version 2018 for information professionals and researchers. Education for Information, 2018;1–7. https://doi.org/10.3233/efi-180221
Kokkeler, K. J., van den Berg, K.S., Comijs, H.C., Oude Voshaar, R.C., Marijnissen, R.M. Sarcopenic obesity predicts nonremission of late-life depression. International Journal of Geriatric Psychiatry, 2019;34(8), pp. 1226–1234. https://doi.org/10.1002/gps.5121
Rantanen, T., Penninx, B.W., Masaki, K., Lintunen, T., Foley, D., Guralnik, J.M. Depressed mood and body mass index as predictors of muscle strength decline in old men. Journal of the American Geriatrics Society, 2000;48(6), pp. 613–617. https://doi.org/10.1111/j.1532-5415.2000.tb04717.x
Byeon, C. H., Kang, K.Y., Kang, S.H. Kim, H. K., Bae, E. J. Sarcopenia Is Not Associated with Depression in Korean Adults: Results from the 2010–2011 Korean National Health and Nutrition Examination Survey. Korean Journal of Family Medicine, 2016;37(1), pp. 37–43. https://doi.org/10.4082/kjfm.2016.37.1.37
Smith, L., White, S., Stubbs, B., Hu., L., Veronese, N., Vancampfort, D., Hamer, M., Gardner, B., Yang, L. Depressive symptoms, handgrip strength, and weight status in US older adults. Journal of Affective Disorders, 2018;238, pp. 305–310. https://doi.org/10.1016/j.jad.2018.06.016
Ishii S, Chang C, Tanaka T, Kuroda A, Tsuji T, Akishita M, Iijima, K. The Association between Sarcopenic Obesity and Depressive Symptoms in Older Japanese Adults. PLoS ONE, 2016;11(9): e0162898. https://doi.org/10.1371/journal.pone.0162898
Irwin, M., Artin, K.H., Oxman, M.N. Screening for depression in the older adult: criterion validity of the 10-item Center for Epidemiological Studies Depression Scale (CES-D). Arch Intern Med, 1999;159(15), pp. 1701–1704. https://doi.org/10.1001/archinte.159.15.1701
Radloff, L.S. The CES-D scale. A self-report depression scale for research in the general population. Appl Psycho1 Meas, 1977;1, pp. 385–401. https://doi.org/10.1177/014662167700100306
Schreiner, A.S., Hayakawa, H., Morimoto, T., Kakuma, T. Screening for late life depression: cut-off scores for the Geriatric Depression Scale and the Cornell Scale for Depression in Dementia among Japanese subjects. Int J Geriatr Psychiatry, 2003;18(6), pp. 498–505. https://doi.org/10.1002/gps.880
Kroenke, K., Spitzer, R.L., Williams, J.B. The PHQ-9: validity of a brief depression severity measure. J. Gen. Intern. Med., 2001;16(9), pp. 606–613. https://doi.org/10.1046/j.1525-1497.2001.016009606.x
Wittchen, H.U., Robins, L.N., Cottler, L.B., Sartorius, N., Burke, J.D., Regier, D. Cross-cultural feasibility, reliability and sources of variance of the Composite International Diagnostic Interview (CIDI). The Multicenter WHO/ADAMHA Field Trials. Br J Psychiatry, 1991;159, pp. 645–653. https://doi.org/10.1192/bjp.159.5.645
Liberati, A., Altman, D.G., Tetzlaff, J., Mulrow, C., Gøtzsche, P.C., Ioannidis, J., Clarke, M., Devereaux, P. J., Kleijnen, J., Moher, D. The PRISMA statement for reporting systematic and meta-analyses of studies that evaluate interventions: explanation and elaboration. PLoS Medicine, 2009;6(7):e1000100. https://doi.org/10.1371/journal.pmed.1000100
Chen, L., Nelson, D. R., Zhao, Y., Cui, Z., & Johnston, J. A. Relationship between muscle mass and muscle strength, and the impact of comorbidities: a population-based, cross-sectional study of older adults in the United States. BMC Geriatrics, 2013;13(1). https://doi.org/10.1186/1471-2318-13-74
Newman, A., Haggerty, C.L., Goodpaster, B., Harris, T., Kritchevsky, S., Nevitt, M., Miles, T.P., Visser, M.; Health Aging And Body Composition Research Group. Strength and muscle quality in a cohort of well-functioning older adults: The Health Aging and Body Composition Study. J Am Geriatr Soc., 2003;51(3), pp. 323–330. https://doi.org/10.1046/j.1532-5415.2003.51105.x
De Simone, G., Pasanisi, F., Ferrara, A. L., Roman, M. J., Lee, E. T., Contaldo, F., Howard, B., Devereux, R. B. Relative fat-free mass deficiency and left ventricular adaptation to obesity: The Strong Heart Study. International Journal of Cardiology, 2013;168(2), 729–733. https://doi.org/10.1016/j.ijcard.2012.09.055
Fukumori, N., Yamamoto, Y., Takegami, M., Yamazaki, S., Onishi, Y., Sekiguchi, M., Otani, K., Konno S., Kikuchi S., Fukuhara, S. Association between hand-grip strength and depressive symptoms: Locomotive Syndrome and Health Outcomes in Aizu Cohort Study (LOHAS). Age and Ageing, 2015;44(4), 592–598. https://doi.org/10.1093/ageing/afv013
Ashdown-Franks, G., Stubbs, B., Koyanagi, A., Schuch, F., Firth, J., Veronese, N., & Vancampfort, D. Handgrip strength and depression among 34,129 adults aged 50 years and older in six low- and middle-income countries. Journal of Affective Disorders, 2018. https://doi.org/10.1016/j.jad.2018.09.036
Cugini, P., Cilli, M., Salandri, A., Ceccotti, P., Di Marzo, A., Rodio, A., Fontana, S., Pellegrino, A.M., De Francesco, G.P., Coda, S., De Vito, F., Colosi, L., Petrangeli, C.M., Giovannini, C. Anxiety, depression, hunger and body composition: III. Their relationships in obese patients. Obesity, 1999;4(3), pp. 115–120. https://doi.org/10.1007/bf03339726
Rolland, Y., Lauwers-Cances, V., Cristini, C., Abellan van Kan, G., Janssen, I., Morley, J., Vellas, B. Difficulties with physical function associated with obesity, sarcopenia, and sarcopenic-obesity in community-dwelling elderly women: the EPIDOS (EPIDemiologie de l’OSteoporose) Study, The American Journal of Clinical Nutrition, 2009;89(6); pp. 1895–1900. https://doi.org/10.3945/ajcn.2008.26950
Tolea, M.I., Chrisphonte, S., Galvin, J.E. Sarcopenic obesity and cognitive performance. Clin Interv Aging; 2018;13, pp. 1111–1119. https://doi.org/10.2147/CIA.S164113
Park, S., Cho, J., Kim, D. et al. Handgrip strength, depression, and all-cause mortality in Korean older adults. BMC Geriatr 2019;19, 127. https://doi.org/10.1186/s12877-019-1140-0
Kim, N.H., Kim, H.S., Eun, C.R., Seo, J.A., Cho, H.J., Kim, S.G., Choi, K.M., Baik, S.H., Choi, D.S., Park, M.H., Han, C., Kim, N.H. Depression is associated with sarcopenia, not central obesity, in elderly Korean men. J Am Geriatr Soc, 2011;59(11), pp. 2062–8. https://doi.org/10.1111/j.1532-5415.2011.03664.x
Zhao, G., Ford, E. S., Li, C., Tsai, J., Dhingra, S., & Balluz, L. S. Waist circumference, abdominal obesity, and depression among overweight and obese U.S. adults: national health and nutrition examination survey 2005–2006. BMC Psychiatry, 2011;11(1). https://doi.org/10.1186/1471-244x-11-130
Choo, V. WHO reassesses appropriate body-mass index for Asian populations. The Lancet, 2002;360(9328), 235. https://doi.org/10.1016/s0140-6736(02)09512-0
Stegenga, H., Haines, A., Jones, K., Wilding, J. Guideline Development Group. Identification, assessment, and management of overweight and obesity: summary of updated NICE guidance. BMJ, 2014;27;349:g6608. https://doi.org/10.1136/bmj.g6608
McGee, D. L. Body mass index and mortality: a meta-analysis based on person-level data from twenty-six observational studies. Annals of Epidemiology; 2005;15(2), pp. 87–97. https://doi.org/10.1016/j.annepidem.2004.05.012
Northrup, D.A. The Problem of the Self- Report in Survey Research. Institute for Social Research. 1996;11(3).
Trouwborst, I., Verreijen, A., Memelink, R., Massanet, P., Boirie, Y., Weijs, P., & Tieland, M. Exercise and Nutrition Strategies to Counteract Sarcopenic Obesity. Nutrients; 2018;10(5), pp. 605. https://doi.org/10.3390/nu10050605
De Rosa, E., Santarpia, L., Marra, M., Sammarco, R., Amato, V., Onufrio, M., De Simone, G., Pasanisi, F. Preliminary evaluation of the prevalence of sarcopenia in obese patients from Southern Italy. Nutrition, 2015;31(1), 79–83. https://doi.org/10.1016/j.nut.2014.04.025
Funding
The authors received no financial support for the authorship of this article.
Author information
Authors and Affiliations
Corresponding author
Additional information
How to cite this article: I. Pilati, A. Slee, R. Frost. Sarcopenic Obesity and Depression: A Systematic Review. J Frailty Aging 2022;11(1)51-58; https://doi.org/10.14283/jfa.2021.39
Conflict of Interest
The authors declare that they have no conflict of interest.
Electronic Supplementary Material
Rights and permissions
About this article
Cite this article
Pilati, I., Slee, A. & Frost, R. Sarcopenic Obesity and Depression: A Systematic Review. J Frailty Aging 11, 51–58 (2022). https://doi.org/10.14283/jfa.2021.39
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.14283/jfa.2021.39