Abstract
Bone quality is determined by a variety of compositional, micro- and ultrastructural properties of the mineralized tissue matrix. In contrast to X-ray-based methods, the interaction of acoustic waves with bone tissue carries information about elastic and structural properties of the tissue. Quantitative ultrasound (QUS) methods represent powerful alternatives to ionizing x-ray based assessment of fracture risk. New in vivo applicable methods permit measurements of fracture-relevant properties, [eg, cortical thickness and stiffness at fragile anatomic regions (eg, the distal radius and the proximal femur)]. Experimentally, resonance ultrasound spectroscopy and acoustic microscopy can be used to assess the mesoscale stiffness tensor and elastic maps of the tissue matrix at microscale resolution, respectively. QUS methods, thus, currently represent the most promising approach for noninvasive assessment of components of fragility beyond bone mass and bone microstructure providing prospects for improved assessment of fracture risk.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Today, the assessment of osteoporotic fracture risk and the therapeutic management of patients mostly rely on X-ray-based imaging modalities. However, these methods are far from being perfect because they do not provide all the information that is needed by clinicians, particularly the assessment of cortical bone properties. Consequently, the occurrence of osteoporotic bone fractures is still a largely unpredictable event, and the effects of treatment on fracture risk are difficult to assess. This can be explained by the multiplicity of bone quality factors that, in addition to bone quantity, determine bone strength and are currently poorly assessed by available X-ray based techniques. In the past 3 decades, researchers have turned to quantitative ultrasound (QUS) measurements to overcome these limitations. Mechanical waves such as ultrasound are inherently suited to probe mechanical properties. In addition to their affordable and nonionizing nature, they are probably in the best position among all the modalities to noninvasively provide the best estimate of bone fragility. This research field was stimulated by (1) experimental evidences from basic research showing the ability of ultrasonic waves to probe bone quality factors, eg, elasticity [1, 2, 3•], microstructure [3•, 4–10], bone matrix constituents (organic and mineral phases) [11–13, 14••], or microdamage accumulation [15–17], and (2) by the scalability of ultrasound for multi-scale assessment of the above mentioned features [3•, 18, 19].
Osteoporosis and other degenerative bone pathologies affect both cancellous and cortical bone compartments. Clinical bone assessment has long been focused on trabecular bone. It is only relatively recently that cortical bone got front stage attention with several reports showing that (1) most bone loss after age 65 occurs at peripheral sites and is cortical, not trabecular [20••] and (2) most fractures occur rather after than before an age of 65 years, are nonvertebral and occur predominantly at cortical sites [21, 22]. The disbalance between bone resorption and bone formation leads to a rarefaction of the trabecular network and accumulation of partially refilled basic multicellular units (BMUs) in cortical tissue. The latter result in cortical bone loss with cortical thinning, increased porosity, and consequently to a reduction of cortical bone stiffness and strength. Cortical bone loss and the resulting structural decay are poorly captured with currently clinically available techniques. Because of limits in spatial resolution and radiation exposure, dual energy X-ray absorptiometry (DXA), or quantitative computed tomography (QCT) provide only limited means to assess the age- or disease- related increase in porosity and the resulting increase in fragility.
Accounting for cortical bone morphology could improve the identification of individuals at high risk of fracture and therefore assist in pursuing patient specific treatment strategies [23]. The ability to measure decreases in cortical bone or tissue mineral density and cortical thickness, along with increases in cortical porosity are becoming accepted as surrogate markers for cortical bone fragility [20••, 24].
Established QUS methods are based on empirically observed relations between the measured sound velocities and attenuation values in trabecular and cortical tissue with BMD and fracture risk [25, 26]. The history and “golden age” of the clinical use of diagnostic ultrasound for the assessment of osteoporosis started with measurements of trabecular bone employing a QUS technology commonly referred to as heel transverse transmission [27]. Heel QUS technologies and implementations were introduced into clinical practice in the 1990s [28]. While strong clinical evidence was obtained in large scale prospective studies [29], showing equivalent fracture risk prediction capabilities compared with X-ray densitometry, an added-value of ultrasound technologies could not be established. In particular, our limited understanding of the interaction mechanisms between an ultrasound wave and the complex structure of cancellous bone did not allow a clear interpretation of the measured variables nor the identification of clear relationships between these variables and bone strength-related properties. As a direct continuation of these heel transverse transmission techniques studies, basic research aiming at elucidating interaction mechanisms, eg, wave scattering or propagation in poroelastic media, are actively continuing [30–35, 36••].
More recently, the research in the field has shifted toward measurements of physical cortical bone properties in order to answer the identified need to accurately quantify alterations of cortical bone and to fill the current technological gap. Several model-based approaches are currently being developed into effective clinical methods. In these approaches, strength related properties, eg, effective cortical stiffness, intra-cortical porosity, and cortical thickness are retrieved from the spectral analysis of guided and scattered waves by solving inverse problems.
Relations Between Bone Structure, Matrix Stiffness and Strength
The macroscopic mechanical properties of bones, particularly the resistance to fractures depend on both, the material properties of the bone tissue and on multi-scale structural features, eg, density and arrangement of the trabecular network, thickness and porosity of the cortical tissue, and the bone shape determining the moment of inertia. At its highest level of hierarchical organization, ie, the millimeter (mm)-scale, cortical bone can be considered as a 2-phase composite material: a heterogeneous mineralized extracellular tissue matrix (ECM) pervaded by hierarchical porous network. From a mechanical perspective, mm-scale elasticity that will be referred to as “effective elasticity” in what follows is determined by the properties of the two phases: (1) pore structure and relative volume and (2) matrix composition and microstructure.
Impact of the ECM Properties on Bone Stiffness and Strength
The extracellular tissue matrix (ECM) of bone consists of a network of hierarchically structured, heterogeneous, and anisotropic mineralized collagen fibrils. The major determinants of ECM properties are the degree of mineralization, the lamellar arrangement of mineralized collagen fibrils, the composition of the collagen cross-links, and the density of micro-cracks. Mechanical properties of mineralized collagen fibrils, determined primarily by the level of mineralization, are highly anisotropic [37, 38]. Therefore, fibril orientation [39, 40] is a major determinant of the elastic properties of bone at the coarser length scales [14••], enabling adaptation of the ECM to the governing type and direction of local mechanical stress and strain [41•]. Tissue aging leads to a fast primary and a slow secondary mineralization of the collagen fibrils [42], resulting in a tissue-age dependent variation of the elastic properties. The variation of the average ECM mineralization is small (around 2 %) in healthy subjects [43]. However, interstitial tissue, which is on average older compared with the more recently built secondary osteons, has been shown in human radius bone of elderly donors (age range between 68 and 90 years) to exhibit slightly higher (~10 %) mineralization values and much higher (~60 %) stiffness values compared with osteonal tissue regions [44, 45]. Moreover, the flexibility of the cross-linked collagen matrix decreases [46], suggesting that the tissue gets stiffer, but eventually also less tough [46]. This process contributes to the formation of micro-cracks, occurring already at physiological load magnitudes of the cyclic loading during everyday activities. The amount of micro-damage in bone tissue increases with age and although its direct relation to fragility is not clear, larger density of micro-cracks may increase the remodeling rate [47–49], leading to a more frequent occurrence of BMUs [50]. Moreover, the mechanical properties of collagen, providing the ductile component of the behavior of bone, have also been reported to degrade with age [51].
Orchestrated by osteocytes, the bone remodeling mechanisms of osteoclastic resorption and osteoblastic ECM synthesis not only ensure removal and replacement of micro-damaged tissue, but also provide a high capacity for adaptation to changes in mechanical conditions. Moreover, these mechanisms prevent tissue-ageing and, in combination with osteocytic osteolysis [52], participate in the maintenance of an almost constant serum calcium level throughout life-time. However, osteoporosis and other pathologies, eg, diabetes, kidney failure can disturb this equilibrium of bone resorption and formation and essentially reduce bone mass, potentially tissue quality and consequently bone strength.
Impact of Cortical Porosity on Bone Stiffness and Strength
Disbalanced intracortical remodeling leaves progressively more nonrefilled bone multicellular units (BMUs) in the cortex, which becomes thinner and contains particularly large coalescent basic multi-cellular units (BMUs) compared with the Haversian canals. Specifically in the endosteal sub-compartment, close clustering of BMUs enhances their chances to merge, leading to the so-called trabecularized cortex [20••, 53]. The relationship between porosity and effective elasticity has been investigated using both, experimental and theoretical approaches [54, 55]. The mm-scale elasticity was found to be highly correlated to the cortical porosity (adj.-R2 = 0.72–0.84), indicating that, for the elderly population, the effective elastic properties of the mineralized matrix do not undergo large variations among different donors, as reflected in the low coefficients of variation of matrix impedance (less than 6 %). The trend in the variation of mm-scale elasticity with porosity can be predicted by a 2-parameter micromechanical model [56, 57] consisting of an anisotropic matrix pervaded by cylindrical pores.
Decreased cortical thickness and increased porosity reduce bone strength [24], and are quantifiable ‘fingerprints’ of structural deterioration [23], which is likely to predict fracture risk and may be used as a marker of responsiveness to therapy. Patsch et al [58] have recently shown that women with type 2 diabetic related fragility fractures had a 4.7-fold greater relative cortical porosity than age-matched diabetic women without fractures. Furthermore, changes in cortical porosity were significantly related to deficits in macroscopic stiffness, failure load, and cortical load fraction at all investigated anatomic sites (ultra-distal and distal radius, distal tibia). It should be noted that relative variations of cortical porosity are amplified in changes in effective stiffness, For example, the aforementioned 2-parameter model [57] predicts that an increase of cortical porosity from 10 % to 20 % results in a decrease of effective elasticity of 25 %. Therefore, the separate estimations of cortical thickness and effective stiffness are anticipated to hold a high diagnostic value for the prediction of bone strength.
Ultrasound Based Assessment of Mechanical Properties at Various Length Scales
Nano and Microscale: Scanning Acoustic Microscopy
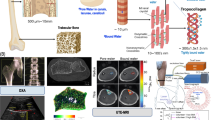
Scanning acoustic microscopy of bone specimens (SAM, Fig. 1) provides large scale (cm range) maps of structural and anisotropic micro-elastic properties at the tissue level with a spatial resolution down to the μm-range [59–61]. Compared with nanoindentation, which provides both elastic and post-yield properties but is destructive and has limited scanning capabilities, SAM is an attractive noncontact and nondestructive quantitative alternative to map linear elastic properties of flat sample surfaces. A face-to-face comparison between 200-MHz SAM and nanoindentation showed a strong correlation between estimates of the elastic moduli derived from both techniques on site-matched regions of human femoral cortical bone [62]. Recent systematic SAM-surveys have been conducted to study (1) the tissue level (25-μm length scale) properties, ie, tissue mineralization, matrix stiffness tensor, cortical porosity in cortical tissue within the human femoral shaft [41•], and (2) the age-dependent variation in the spatial distributions of microstructural and micro-elastic properties of the human femoral neck and shaft in 21 men (age range between 17 and 82 years) [63••]. Most importantly, these studies revealed (1) remarkable regional variations of stiffness with a coefficient of variation (CV) up to 45.5 % and porosity (CV = 47.5 %) in spite of a fairly invariable tissue mineralization (CV = 1.8 %) within the femoral shaft, and (2) an age-related increase of cortical porosity and stiffening of the cortical tissue matrix, as well as significant correlations between shaft and neck tissue stiffness values (R2 = .63). These findings support the hypothesis that no global relation between tissue mineralization and tissue elastic properties exists [41•]. In particular, elastic adaptation of the tissue matrix by local changes of the lamellar fibril orientation patterns [39] is not associated with a change of tissue mineralization, but can be studied by SAM [14••, 41•, 59, 61]. Further stiffening mechanisms not associated with an increase of tissue mineralization have been suggested to be linked to alterations of the collagen cross-link composition [46] and an agglomeration of mineral crystals [38, 64]. However, further site-matched studies of stiffness, toughness and chemical composition by SAM, nanoindentation, and optical spectroscopy are needed to unravel the associations between tissue mineralization, elastic and ultimate bone properties and their relations with respect to ageing, bone pathologies and drug treatments.
Differences in the tibia mid-shaft micro- and ultrastructure in patients of increasing age depicted by 50-MHz scanning acoustic microscopy (SAM). The progression of bone deterioration (from left to right) results in an accumulation of large BMUs, cortical thinning and changes in the tissue stiffness. The medial (upper) region can be assessed in vivo by ultrasound
Large scale micro-elastic maps of entire cross-sections obtained by 50-MHZ SAM have also been used as direct input for numerical homogenization models [3•, 38, 55], simulations of sound propagations through the femoral neck [65, 66], and in fracture healing studies [67]. High resolution SAM provides insight into the role of the organization of collagen micro-fibril and mineral nanocrystals on micro-elastic properties [60]. For example, the combination of 1-GHz SAM measurements of bone micro-elastic properties with site-matched synchrotron radiation micro-computed tomography (SR-μCT) and small angle X-ray scattering (SAXS) imaging of mineral content and nanostructure revealed that the periodic modulations of elasticity across osteonal bone [68] is essentially determined by the orientation of the mineral nanoparticles and to a lesser extent only by the particle size and density [14••].
The possibilities to assess both, structural and elastic material properties of the tissue across multiple length scales and to site-match this information with 3D micromorphology and tissue mineralization, eg, obtained by SR-μCT [38], opens new perspectives for the identification of elastic tissue alterations in response to ageing, pathologies and drug treatment, which may not be associated with remarkable alterations of the tissue mineralization.
Mesoscale (mm-Scale): Resonant Ultrasound Spectroscopy
The effective elastic stiffness tensor at the mesoscale (mm-scale) can be measured in parallelepiped samples (edge length typically larger than 5 mm) by time-of-flight measurements of compressional and shear waves in several directions [1]. Resonant ultrasound spectroscopy (RUS) is currently developed with the aim to become a routine technique for the accurate assessment of anisotropic elastic and viscoelastic properties of mineralized tissues. RUS, a method based on the comparison of measured and model-predicted resonant frequencies, allows estimating all the terms of the stiffness tensor of an anisotropic material from the measurement of the mechanical resonant behavior of a specimen. Although RUS was developed in the 1990s to measure metals [69, 70], the difficulty raised by the high level of mechanical damping of bone, which causes resonant peaks to overlap, has only been recently overcome [71]. Bernard et al [72••] have demonstrated the feasibility of measuring the stiffness tensor on small samples (edge length: 3–5 millimeters) with RUS with a good agreement with pulse-echo measurements. The method does not suffer from the drawbacks and limitations associated with the conventional time-of-flight approach, which has been used to measure bone elasticity by a number of authors. In particular, RUS is more precise and can measure smaller samples (eg, from femur or tibia cortex). It is, therefore, a keystone for future systematic routine measurements of the mesoscale stiffness tensor in cortical bone samples in larger cohorts.
Clinical Development
Although QUS measurements at sites containing predominantly trabecular bone have been most widely tested and validated clinically, this technology to date has not been shown to permit assessment of trabecular bone material properties. However, cortical bone is readily accessible for measurements at the radius and tibia and recently also measurements at the proximal femur, a site containing both cortical and trabecular bone have been reported both ex vivo and in vivo [26]. Because of the recognized importance of cortical bone efforts have been made to improve its measurement.
Axial Transmission with Guided Wave Analysis
The first reports on in-vivo measurements of cortical bone using ultrasound axial transmission trace back to more than half a century. A few clinical studies reported use of the velocity of ultrasound waves transmitted axially along the shaft of long bones (tibia) as a biomarker for monitoring bone fracture healing [73]. The technique was rapidly forgotten and it was not before the late 1990s that it was revived in the context of osteoporosis [74, 75]. The first version of the axial transmission approach essentially consisted in measuring the time-of-flight of the first arriving signal (FAS). FAS velocity discriminates patients with osteoporotic fractures from controls, although not better than X-ray densitometry [76]. FAS offers the advantage of a straightforward signal analysis, but it also has the disadvantage of being difficult to interpret physically. Empirically, the velocity of the FAS has been shown to depend on various bone properties, eg, cortical thickness, porosity, bone mineral density, and elasticity [19, 77], but until now no clear interpretation has been reached regarding the link between FAS velocity and these bone properties. Individual bone properties cannot be inferred from a single FAS measurement. Because the nature of the FAS changes with the frequency, a multiple frequency approach, in which FAS velocity is measured at different frequency has been described to enhance cortical bone status assessment [78–80].
A step forward toward the ultrasonic characterization of cortical bone has been made with reports showing that cortical bone behaves as a waveguide for ultrasound [81–83]. In the appropriate clinical frequency range (ie, roughly between 100 kHz and 2 MHz), cortical bone is a multi-modal waveguide (WG), which means that different modes coexist. The frequency-dependent propagation speed of each mode is determined by a specific combination of stiffness coefficients and thickness of the WG [84]. Thus, improving the characterization of individual bone properties can be sought by exploiting the waveguide character of cortical bone. Measurements of the dispersion relationships (or in other words, the frequency variation of the wave modes speed), together with appropriate waveguide modeling have, therefore, the potential for providing estimations of effective stiffness coefficients (which are largely determined by cortical porosity) and also of cortical thickness [81, 85–88]. Moilanen et al were the first to propose a low-frequency approach to excite and detect in-vivo a thickness-sensitive fundamental flexural guided wave and to infer from the measurement of its velocity dispersion characteristics estimates of cortical thickness [89, 90]. Another approach is to record the full response of the WG, enabling to measure the dispersion curves of multiple guided waves [91]. The ability of the method to recover parameters of interest such as the waveguide thickness and/or elastic coefficients has been validated on bone mimicking phantoms and on ex-vivo human radius specimens [88]. Using the empirical relations between effective stiffness and porosity [3•], the cortical porosity can be estimated from this measurement.
Recently, a sensor was developed, which, in addition to the measurement of axial transmission FAS velocity, could measure a perpendicular component, the tangential transmission FAS velocity. The feasibility of a direct estimation of elastic anisotropy at the tibial mid-shaft by simultaneously measuring both axial and tangential FAS components could be demonstrated at tibia shaft specimens [92].
Transverse Transmission at the Proximal Femur
An important limitation of QUS today is their limited access to peripheral skeletal sites only. QUS assessment at the hip is expected to provide better hip fracture risk prediction compared with QUS at peripheral sites. However, the complexity of the anatomy makes measurements at this site quite challenging. One of the most significant recent technological advances is the new QUS scanner developed by Barkmann et al [93] for direct assessment of skeletal properties at the proximal femur. In-vivo QUS-measurements have been performed at the proximal femur in a first clinical trial [94, 95]. These transverse-transmission measurements through the trochanter major and proximal shaft, consisting of predominantly trabecular and cortical tissues, respectively, could be used to discriminate between women with and without fractures as good as DXA [96•]. Both, direct waves through the trochanter major (trabecular tissue) and guided waves through the proximal shaft (cortical tissue) contributed to the estimation of fracture risk. New research, extending the concept of guided waves to the circumferential propagation in the cortex, led to the development of methods for specific measurements of the cortical shell at the proximal femur. Circumferential wave propagation could be tested ex-vivo on 9 femurs, and the time-of-flight of the FAS signal revealed a strong relationship with femur strength, as assessed by mechanical testing (R2 = .79) [97•]. Furthermore, simulations of ultrasound propagation through the femoral neck yielded associations between FAS propagation characteristics and bone properties, predominantly cortical porosity, indicating a possible added value of a QUS measurement at the femoral neck [66]. Former measurements of circumferential waves through human finger phalanges [98, 99] demonstrated the impact of cortical thickness, cortical porosity and apparent cortical density on the ultrasound parameters of FAS transmission. As the relative cortical thickness (cortical thickness divided by bone width) is similar in the long bones of finger phalanx and the femur shaft, comparable results can be expected for measurements at the femoral proximal shaft. However, US-transmission measurements at the proximal femur using a pair of single-element transducers limit the clinical applicability. Recently developed array systems increase the flexibility of such QUS measurements and may enable a better estimation of relevant bone properties by adjusting for the impact of bone geometry [100]. Developments of signal processing techniques inspired from time reversal are currently underway to extend the principles of measuring the dispersion curves to circumferential guided waves with the aim to assess the geometric and mechanical properties of the cortical shell of the femoral neck [101, 102].
Conclusions
Many advances have been achieved in recent years and a variety of different sophisticated ultrasound technologies capable of measuring elastic properties from the tissue level (ex-vivo) to the organ level (in- vivo) have been introduced and evaluated. Elastic properties of bone are nowadays widely used in fundamental studies, in conjunction with numerical models, to investigate the structure-function relationships and in clinical applications to predict fracture risk or to monitor fracture healing. Novel quantitative ultrasound technologies taking benefit of the scalability of ultrasound to noninvasively investigate elastic properties at multiple organization levels have emerged like, eg, scanning acoustic microscopy or resonant ultrasound spectroscopy. One important research goal is to provide guidance for the interpretation and the optimization of cortical bone QUS measurements in vivo. A secondary motivation is to contribute to fundamental knowledge of mechanical properties.
Basic research is continuing to gain better understanding of the interaction between ultrasound and bone structure or to investigate the nonlinear elastic properties in relation to bone microdamage.
Active research is carried out to develop new measurement modes as effective clinical tools, particularly to assess cortical bone at peripheral skeletal sites including the proximal femur as the location of the most severe osteoporotic fracture. Measuring guided waves in cortical bone has the potential to evaluate bone quality factors such as cortical thickness, elasticity, and with a-priori assumptions also porosity. Several GW-based approaches are currently in clinical testing.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Ashman RB, Cowin SC, Rho JY, Van Buskirk WC, Rice JC. A continuous wave technique for the measurement of the elastic properties of cortical bone. J Biomech. 1984;17:349–61.
Rho JY. An ultrasonic method for measuring the elastic properties of human tibial cortical and cancellous bone. Ultrasonics. 1996;34:777–83.
Granke M, Grimal Q, Saied A, Nauleau P, Peyrin F, Laugier P. Change in porosity is the major determinant of the variation of cortical bone elasticity at the millimeter scale in aged women. Bone. 2011;49:1020–6. A multi-scale assessment of cortical bone combining low frequency ultrasound and scanning acoustic microscopy that evidences that porosity explains most of the variations of effective elasticity (ie, mm-scale).
Baron C, Talmant M, Laugier P. Effect of porosity on effective diagonal stiffness coefficients (cii) and elastic anisotropy of cortical bone at 1 MHz: a finite-difference time domain study. J Acoust Soc Am. 2007;122:1810.
Wear KA. Ultrasonic scattering from cancellous bone: a review. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1432–41.
Hakulinen MA, Day JS, Toyras J, Weinans H, Jurvelin JS. Ultrasonic characterization of human trabecular bone microstructure. Phys Med Biol. 2006;51:1633–48.
Padilla F, Laugier P. Recent developments in trabecular bone characterization using ultrasound. Curr Osteoporos Rep. 2005;3:64–9.
Padilla F, Jenson F, Bousson V, Peyrin F, Laugier P. Relationships of trabecular bone structure with quantitative ultrasound parameters: in vitro study on human proximal femur using transmission and backscatter measurements. Bone. 2008;42:1193–202.
Padilla F, Jenson F, Laugier P. Estimation of trabecular thickness using ultrasonic backscatter. Ultrason Imaging. 2006;28:3–22.
Wear KA, Laib A. The dependence of ultrasonic backscatter on trabecular thickness in human calcaneus: theoretical and experimental results. IEEE Trans Ultrason Ferroelectr Freq Control. 2003;50:979–86.
Hoffmeister BK, Whitten SA, Kaste SC, Rho JY. Effect of collagen and mineral content on the high-frequency ultrasonic properties of human cancellous bone. Osteoporos Int. 2002;13:26–32.
Karjalainen JP, Toyras J, Riekkinen O, Hakulinen M, Jurvelin JS. Ultrasound backscatter imaging provides frequency-dependent information on structure, composition and mechanical properties of human trabecular bone. Ultrasound Med Biol. 2009;35:1376–84.
Riekkinen O, Hakulinen MA, Lammi MJ, Jurvelin JS, Kallioniemi A, Toyras J. Acoustic properties of trabecular bone—relationships to tissue composition. Ultrasound Med Biol. 2007;33:1438–44.
Granke M, Gourrier A, Rupin F, Raum K, Peyrin F, Burghammer M, et al. Microfibril orientation dominates the microelastic properties of human bone tissue at the lamellar length scale. PLoS One. 2013;8:e58043. A study showing that micro-fibril orientation is the main determinant of the observed undulations of micro-elastic properties measured by scanning acoustic microscopy in regions of constant mineralization in osteonal lamellar bone.
Muller M, Mitton D, Talmant M, Johnson P, Laugier P. Nonlinear ultrasound can detect accumulated damage in human bone. J Biomech. 2008;41:1062–8.
Haupert S, Guerard S, Peyrin F, Mitton D, Laugier P. Nondestructive characterization of cortical bone micro-damage by nonlinear resonant ultrasound spectroscopy. PLoS One. 2014;9:e83599.
Moreschi H, Calle S, Guerard S, Mitton D, Renaud G, Defontaine M. Monitoring trabecular bone microdamage using a dynamic acousto-elastic testing method. Proc Inst Mech Eng H. 2011;225:282–95.
Raum K, Reisshauer J, Brandt J. Frequency and resolution dependence of the anisotropic impedance estimation in cortical bone using time-resolved scanning acoustic microscopy. J Biomed Mater Res A. 2004;71A:430–8.
Raum K, Leguerney I, Chandelier F, Bossy E, Talmant M, Saied A, et al. Bone microstructure and elastic tissue properties are reflected in QUS axial transmission measurements. Ultrasound Med Biol. 2005;31:1225–35.
Zebaze RM, Ghasem-Zadeh A, Bohte A, Iuliano-Burns S, Mirams M, Price RI, et al. Intracortical remodeling and porosity in the distal radius and postmortem femurs of women: a cross-sectional study. Lancet. 2010;375:1729–36. A very important paper re-examining cortical bone as a source of bone loss in the appendicular skeleton. Following this paper, cortical bone got front stage attention from many researchers.
Kanis JA, Johnell O, Oden A, Dawson A, De LC, Jonsson B. Ten-year probabilities of osteoporotic fractures according to BMD and diagnostic thresholds. Osteoporos Int. 2001;12:989–95.
Riggs BL, Wahner HW, Dunn WL, Mazess RB, Offord KP, Melton III LJ. Differential changes in bone mineral density of the appendicular and axial skeleton with aging: relationship to spinal osteoporosis. J Clin Invest. 1981;67:328–35.
Nishiyama KK, Macdonald HM, Buie HR, Hanley DA, Boyd SK. Postmenopausal women with osteopenia have higher cortical porosity and thinner cortices at the distal radius and tibia than women with normal aBMD: an in vivo HR-pQCT study. J Bone Miner Res. 2010;25:882–90.
Burghardt AJ, Kazakia GJ, Ramachandran S, Link TM, Majumdar S. Age- and gender-related differences in the geometric properties and biomechanical significance of intracortical porosity in the distal radius and tibia. J Bone Miner Res. 2010;25:983–93.
Gluer CC. A new quality of bone ultrasound research. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1524–8.
Laugier P. Instrumentation for in vivo ultrasonic characterization of bone strength. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1179–96.
Langton CM, Palmer SB, Porter RW. The measurement of broadband ultrasonic attenuation in cancellous bone. Eng Med. 1984;13:89–91.
Gluer CC. Quantitative ultrasound techniques for the assessment of osteoporosis: expert agreement on current status. The International Quantitative Ultrasound Consensus Group. J Bone Miner Res. 1997;12:1280–8.
Marin F, Gonzalez-Macias J, Diez-Perez A, Palma S, Delgado-Rodriguez M. Relationship between bone quantitative ultrasound and fractures: a meta-analysis. J Bone Miner Res. 2006;21:1126–35.
Meziere F, Muller M, Dobigny B, Bossy E, Derode A. Simulations of ultrasound propagation in random arrangements of elliptic scatterers: occurrence of two longitudinal waves. J Acoust Soc Am. 2013;133:643–52.
Meziere F, Muller M, Bossy E, Derode A. Measurements of ultrasound velocity and attenuation in numerical anisotropic porous media compared with Biot's and multiple scattering models. Ultrasonics. 2013. doi:10.1016/j.ultras.2013.09.013.
Pakula M, Padilla F, Laugier P. Influence of the filling fluid on frequency-dependent velocity and attenuation in cancellous bones between 0.35 and 2.5 MHz. J Acoust Soc Am. 2009;126:3301–10.
Hoffman JJ, Nelson AM, Holland MR, Miller JG. Cancellous bone fast and slow waves obtained with Bayesian probability theory correlate with porosity from computed tomography. J Acoust Soc Am. 2012;132:1830–7.
Anderson CC, Bauer AQ, Holland MR, Pakula M, Laugier P, Bretthorst GL, et al. Inverse problems in cancellous bone: estimation of the ultrasonic properties of fast and slow waves using Bayesian probability theory. J Acoust Soc Am. 2010;128:2940–8.
Mizuno K, Somiya H, Kubo T, Matsukawa M, Otani T, Tsujimoto T. Influence of cancellous bone microstructure on two ultrasonic wave propagations in bovine femur: an in vitro study. J Acoust Soc Am. 2010;128:3181–9.
Laugier P, Haiat G, editors. Bone quantitative ultrasound. Dordrecht: Springer Science+Business Media B.V; 2011. The most recent and the most comprehensive view of the field of bone quantitative ultrasound.
Reisinger AG, Pahr DH, Zysset PK. Sensitivity analysis and parametric study of elastic properties of an unidirectional mineralized bone fibril-array using mean field methods. Biomech Model Mechanobiol. 2010;9:499–510.
Tiburtius S, Schrof S, Molnar F, Varga P, Peyrin F, Grimal Q, et al. On the elastic properties of mineralized turkey leg tendon tissue: multi-scale model and experiment. Biomech Model Mechanobiol. 2014. doi:10.1007/s10237-013-0550-8:.
Varga P, Pacureanu A, Langer M, Suhonen H, Hesse B, Grimal Q, et al. Investigation of the three-dimensional orientation of mineralized collagen fibrils in human lamellar bone using synchrotron X-ray phase nano-tomography. Acta Biomater. 2013;9:8118–27.
Reznikov N, Almany-Magal R, Shahar R, Weiner S. Three-dimensional imaging of collagen fibril organization in rat circumferential lamellar bone using a dual beam electron microscope reveals ordered and disordered sub-lamellar structures. Bone. 2013;52:676–83.
Rohrbach D, Lakshmanan S, Peyrin F, Langer M, Gerisch A, Grimal Q, et al. Spatial distribution of tissue level properties in a human femoral cortical bone. J Biomech. 2012;45:2264–70. The first systematic SAM-SRμCT study that measured the tissue scale stiffness tensor, degree of mineralization, and cortical porosity in the human femoral shaft.
Ruffoni D, Fratzl P, Roschger P, Klaushofer K, Weinkamer R. The bone mineralization density distribution as a fingerprint of the mineralization process. Bone. 2007;40:1308–19.
Roschger P, Paschalis EP, Fratzl P, Klaushofer K. Bone mineralization density distribution in health and disease. Bone. 2008;42:456–66.
Raum K, Cleveland RO, Peyrin F, Laugier P. Derivation of elastic stiffness from site-matched mineral density and acoustic impedance maps. Phys Med Biol. 2006;51:747–58.
Raum K, Leguerney I, Chandelier F, Talmant M, Saied A, Peyrin F, et al. Site-matched assessment of structural and tissue properties of cortical bone using scanning acoustic microscopy and synchrotron radiation muCT. Phys Med Biol. 2006;51:733–46.
Zimmermann EA, Schaible E, Bale H, Barth HD, Tang SY, Reichert P, et al. Age-related changes in the plasticity and toughness of human cortical bone at multiple length scales. Proc Natl Acad Sci U S A. 2011;108:14416–21.
Burr DB, Forwood MR, Fyhrie DP, Martin RB, Schaffler MB, Turner CH. Bone microdamage and skeletal fragility in osteoporotic and stress fractures. J Bone Miner Res. 1997;12:6–15.
Mori S, Burr DB. Increased intracortical remodeling following fatigue damage. Bone. 1993;14:103–9.
Sobelman OS, Gibeling JC, Stover SM, Hazelwood SJ, Yeh OC, Shelton DR, et al. Do micro-cracks decrease or increase fatigue resistance in cortical bone? J Biomech. 2004;37:1295–303.
Burr DB, Martin RB, Schaffler MB, Radin EL. Bone remodeling in response to in vivo fatigue microdamage. J Biomech. 1985;18:189–200.
Leng H, Reyes MJ, Dong XN, Wang X. Effect of age on mechanical properties of the collagen phase in different orientations of human cortical bone. Bone. 2013;55:288–91.
Wysolmerski JJ. Osteocytic osteolysis: time for a second look? Bonekey Rep. 2012;1:229.
Zebaze R, Ghasem-Zadeh A, Mbala A, Seeman E. A new method of segmentation of compact-appearing, transitional and trabecular compartments and quantification of cortical porosity from high resolution peripheral quantitative computed tomographic images. Bone. 2013;54:8–20.
Grimal Q, Raum K, Gerisch A, Laugier P. A determination of the minimum sizes of representative volume elements for the prediction of cortical bone elastic properties. Biomech Model Mechanobiol. 2011;10:925–37.
Grimal Q, Raum K, Gerisch A, Laugier P. Derivation of the mesoscopic elasticity tensor of cortical bone from quantitative impedance images at the micron scale. Comput Methods Biomech Biomed Engin. 2008;11:147–57.
Parnell WJ, Grimal Q. The influence of mesoscale porosity on cortical bone anisotropy. Investigations via asymptotic homogenization. J R Soc Interface. 2009;6:97–109.
Grimal Q, Rus G, Parnell WJ, Laugier P. A two-parameter model of the effective elastic tensor for cortical bone. J Biomech. 2011;44:1621–5.
Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, et al. Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res. 2013;28:313–24.
Lakshmanan S, Bodi A, Raum K. Assessment of anisotropic tissue elasticity of cortical bone from high-resolution, angular acoustic measurements. IEEE Trans Ultrason Ferroelectr Freq Control. 2007;54:1560–70.
Raum K. Microelastic imaging of bone. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1417–31.
Saied A, Raum K, Leguerney I, Laugier P. Spatial distribution of anisotropic acoustic impedance assessed by time-resolved 50-MHz scanning acoustic microscopy and its relation to porosity in human cortical bone. Bone. 2008;43:187–94.
Rupin F, Saied A, Dalmas D, Peyrin F, Haupert S, Raum K, et al. Assessment of microelastic properties of bone. using scanning acoustic microscopy: a face-to-face comparison with nanoindentation. Jpn J Appl Phys. 2009;48.
Malo MK, Rohrbach D, Isaksson H, Toyras J, Jurvelin JS, Tamminen IS, et al. Longitudinal elastic properties and porosity of cortical bone tissue vary with age in human proximal femur. Bone. 2013;53:451–8. An experimental SAM study showing the variability and associations of microscale tissue elastic properties and intracortical porosity in men with respect to age in proximal femur shaft and neck regions.
Chen PY, Toroian D, Price PA, McKittrick J. Minerals form a continuum phase in mature cancellous bone. Calcif Tissue Int. 2011;88:351–61.
Grimal Q, Rohrbach D, Grondin J, Barkmann R, Gluer CC, Raum K, et al. Modeling of femoral neck cortical bone. for the numerical simulation of ultrasound propagation. Ultrasound Med Biol. 2014. doi:10.1016/j.ultrasmedbio.2013.11.010.
Rohde K, Rohrbach D, Gluer CC, Laugier P, Grimal Q, Raum K, et al. Influence of porosity, pore size, and cortical thickness on the propagation of ultrasonic waves guided through the femoral neck cortex: a simulation study. IEEE Trans Ultrason Ferroelectr Freq Control. 2014;61:302–13.
Potsika VT, Grivas KN, Protopappas VC, Vavva MG, Raum K, Rohrbach D, et al. Application of an effective medium theory for modeling ultrasound wave propagation in healing long bones. Ultrasonics. 2013. doi:10.1016/j.ultras.2013.09.002.
Hofmann T, Heyroth F, Meinhard H, Franzel W, Raum K. Assessment of composition and anisotropic elastic properties of secondary osteon lamellae. J Biomech. 2006;39:2284–94.
Migliori A, Sarrao JL, Visscher WM, Bell TM, Lei M, Fisk Z, et al. Resonant Ultrasound spectroscopic techniques for measurement of the elastic-moduli of solids. Physica B. 1993;183:1–24.
Migliori A, Maynard JD. Implementation of a modern resonant ultrasound spectroscopy system for the measurement of the elastic moduli of small solid specimens. Rev Sci Instrum. 2005;76. doi:10.1063/1.2140494.
Bernard S, Grimal Q, Laugier P. Resonant ultrasound spectroscopy for viscoelastic characterization of anisotropic attenuative solid materials. J Acoust Soc Am. 2014;in press.
Bernard S, Grimal Q, Laugier P. Accurate measurement of cortical bone elasticity tensor with resonant ultrasound spectroscopy. J Mech Behav Biomed Mater. 2013;18:12–9. The first study showing that resonant ultrasound spectroscopy (RUS) can be applied to measure the full stiffness tensor of small bone specimens with high precision. This is an important milestone as RUS may be used in future systematic routine measurements of the mesoscale stiffness tensor in cortical bone samples for basic bone research, small animal phenotyping, etc.
Siegel IM, Anast GT, Fields T. The determination of fracture healing by measurement of sound velocity across the fracture site. Surg Gynecol Obstet. 1958;107:327–32.
Foldes AJ, Rimon A, Keinan DD, Popovtzer MM. Quantitative ultrasound of the tibia: a novel approach for assessment of bone status. Bone. 1995;17:363–7.
Moilanen P. Ultrasonic guided waves in bone. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1277–86.
Talmant M, Kolta S, Roux C, Haguenauer D, Vedel I, Cassou B, et al. In vivo performance evaluation of bi-directional ultrasonic axial transmission for cortical bone assessment. Ultrasound Med Biol. 2009;35:912–9.
Bossy E, Talmant M, Peyrin F, Akrout L, Cloetens P, Laugier P. An in vitro study of the ultrasonic axial transmission technique at the radius: 1-MHz velocity measurements are sensitive to both mineralization and intracortical porosity. J Bone Miner Res. 2004;19:1548–56.
Egorov V, Tatarinov A, Sarvazyan N, Wood R, Magidenko L, Amin S, et al. Osteoporosis detection in postmenopausal women using axial transmission multi-frequency bone ultrasonometer: clinical findings. Ultrasonics. 2013. doi:10.1016/j.ultras.2013.08.017.
Sarvazyan A, Tatarinov A, Egorov V, Airapetian S, Kurtenok V, Gatt Jr CJ. Application of the dual-frequency ultrasonometer for osteoporosis detection. Ultrasonics. 2009;49:331–7.
Tatarinov A, Egorov V, Sarvazyan N, Sarvazyan A. Multi-frequency axial transmission bone ultrasonometer. Ultrasonics. 2013. doi:10.1016/j.ultras.2013.09.025.
Lefebvre F, Deblock Y, Campistron P, Ahite D, Fabre JJ. Development of a new ultrasonic technique for bone and biomaterials in vitro characterization. J Biomed Mater Res. 2002;63:441–6.
Moilanen P, Nicholson PH, Karkkainen T, Wang Q, Timonen J, Cheng S. Assessment of the tibia using ultrasonic guided waves in pubertal girls. Osteoporos Int. 2003;14:1020–7.
Protopappas VC, Kourtis IC, Kourtis LC, Malizos KN, Massalas CV, Fotiadis DI. Three-dimensional finite element modeling of guided ultrasound wave propagation in intact and healing long bones. J Acoust Soc Am. 2007;121:3907–21.
Talmant M, Foiret J, Minonzio JG. Guided waves in cortical bone. In: Laugier P, Haiat G, editors. Bone quantitative ultrasound. Dordrecht: Springer Science+Business Media B.V; 2011. p. 147–80.
Moilanen P, Talmant M, Bousson V, Nicholson PH, Cheng S, Timonen J, et al. Ultrasonically determined thickness of long cortical bones: two-dimensional simulations of in vitro experiments. J Acoust Soc Am. 2007;122:1818.
Moilanen P, Talmant M, Nicholson PH, Cheng S, Timonen J, Laugier P. Ultrasonically determined thickness of long cortical bones: three-dimensional simulations of in vitro experiments. J Acoust Soc Am. 2007;122:2439–45.
Moilanen P, Nicholson PH, Kilappa V, Cheng S, Timonen J. Assessment of the cortical bone thickness using ultrasonic guided waves: modelling and in vitro study. Ultrasound Med Biol. 2007;33:254–62.
Foiret J, Minonzio JG, Talmant M, Laugier P. Cortical bone quality assessment using quantitative ultrasound on long bones. Conf Proc IEEE Eng Med Biol Soc. 2012;2012:1121–4.
Moilanen P, Maatta M, Kilappa V, Xu L, Nicholson PH, Alen M, et al. Discrimination of fractures by low-frequency axial transmission ultrasound in postmenopausal females. Osteoporos Int. 2013;24:723–30.
Kilappa V, Moilanen P, Xu L, Nicholson PH, Timonen J, Cheng S. Low-frequency axial ultrasound velocity correlates with bone mineral density and cortical thickness in the radius and tibia in pre- and postmenopausal women. Osteoporos Int. 2011;22:1103–13.
Minonzio JG, Talmant M, Laugier P. Guided wave phase velocity measurement using multi-emitter and multi-receiver arrays in the axial transmission configuration. J Acoust Soc Am. 2010;127:2913–9.
Daugschies M, Rohde K, Gluer CC, Barkmann R. The preliminary evaluation of a 1 MHz ultrasound probe for measuring the elastic anisotropy of human cortical bone. Ultrasonics. 2014;54:4–10.
Barkmann R, Laugier P, Moser U, Dencks S, Padilla F, Haiat G, et al. A method for the estimation of femoral bone mineral density from variables of ultrasound transmission through the human femur. Bone. 2007;40:37–44.
Barkmann R, Laugier P, Moser U, Dencks S, Klausner M, Padilla F, et al. In vivo measurements of ultrasound transmission through the human proximal femur. Ultrasound Med Biol. 2008;34:1186–90.
Barkmann R, Laugier P, Moser U, Dencks S, Klausner M, Padilla F, et al. A device for in vivo measurements of quantitative ultrasound variables at the human proximal femur. IEEE Trans Ultrason Ferroelectr Freq Control. 2008;55:1197–204.
Barkmann R, Dencks S, Laugier P, Padilla F, Brixen K, Ryg J, et al. Femur ultrasound (FemUS)–first clinical results on hip fracture discrimination and estimation of femoral BMD. Osteoporos Int. 2010;21:969–76. One of the most significant recent technological developments in bone QUS: the femur scanner to measure the hip.
Grimal Q, Grondin J, Guerard S, Barkmann R, Engelke K, Gluer CC, et al. Quantitative ultrasound of cortical bone in the femoral neck predicts femur strength: results of a pilot study. J Bone Miner Res. 2013;28:302–12. An experimental study showing the feasibility of measuring circumferential waves propating in the femoral neck. The results suggest that the measured waveform conveys important information of the neck biomechanical competence. This study and Barkman’s paper potentially opens new research routes for the future of clinical QUS.
Barkmann R, Lusse S, Stampa B, Sakata S, Heller M, Gluer CC. Assessment of the geometry of human finger phalanges using quantitative ultrasound in vivo. Osteoporos Int. 2000;11:745–55.
Sakata S, Barkmann R, Lochmuller EM, Heller M, Gluer CC. Assessing bone status beyond BMD: evaluation of bone geometry and porosity by quantitative ultrasound of human finger phalanges. J Bone Miner Res. 2004;19:924–30.
Rohde K, Rohrbach D, Glueer CC, Laugier P, Grimal Q, Raum K, et al. Influence of porosity, pore size and cortical thickness on the propagation of ultrasonic waves guided through the femoral neck cortex: a simulation study. IEEE Trans Ultrason Ferroelectr Freq Control. 2013;in press.
Nauleau P, Cochard E, Minonzio JG, Grimal Q, Laugier P, Prada C. Characterization of circumferential guided waves in a cylindrical cortical bone-mimicking phantom. J Acoust Soc Am. 2012;131:EL289–94.
Nauleau P, Grimal Q, Minonzio JG, Laugier P, Prada C. Circumferential guided wave measurements of a cylindrical fluid-filled femoral neck mimicking phantom. J Acoust Soc Am. 2014;in press.
Acknowledgements
K. Raum received funding from the Deutsche Forschungsgemeinschaft within the priority program SPP1420 "Biomimetic Materials Research: Functionality by Hierarchical Structuring of Materials" (grant RA 1380/7). Q. Grimal received grants from Agence Nationale de la Recherche (French minister for research) during the conduct of the study. P. Laugier is one of the co-founder of Azalee, a spin-off company to develop and commercialize a technology using ultrasound to measure cortical bone and assess its structural and mechanical properties. All authors are associated with the European Associated Laboratory “Ultrasound Based Assessment of Bone” (ULAB).
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
K. Raum, Q. Grimal, P. Varga, R. Barkmann, CC Glüer, and P. Laugier declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
All studies by the authors involving animal and/or human subjects were performed after approval by the appropriate institutional review boards. When required, written informed consent was obtained from all participants.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Raum, K., Grimal, Q., Varga, P. et al. Ultrasound to Assess Bone Quality. Curr Osteoporos Rep 12, 154–162 (2014). https://doi.org/10.1007/s11914-014-0205-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-014-0205-4