Abstract
Being a connective tissue, bone can increase or decrease its mass through the process of remodeling. Using a discovery in the mid-1980s—that tumor necrosis factor (TNF) could dramatically increase formation of osteoclasts (the cells that break down bone)—researchers at Amgen (Thousand Oaks, CA) discovered a TNF-like molecule that regulated bone resorption. Elevations in the expression of this molecule, receptor activator of nuclear factor-κB ligand (RANKL), can cause excessive bone destruction. A blocking antibody to RANKL named denosumab inhibits osteoclast formation and bone degradation. In a large multicenter clinical trial, known as the FREEDOM trial (Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months), the effects of denosumab were tested in 60- to 90-year-old women over 3 years. Statistically significant reductions in fracture risk at the vertebral column, hip, and nonvertebral sites were associated with increases in bone mineral density (BMD) and reciprocal decreases in markers of bone resorption. However, the FREEDOM trial did not test the most beneficial use of a resorption blocking drug—to target the rapid bone loss that occurs in late perimenopause and early postmenopause. One adverse effect from denosumab is cellulitis, and research in animals suggests that RANKL/RANK interaction is needed for Langerhans cell (LC) survival in the skin. Further mechanistic and clinical studies on the role of RANKL in the skin are needed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
The Discovery of RANKL
Bone is a connective tissue with the ability to increase its mass when stimulated and rapidly destroy itself when not needed. To resist stresses, bone constantly undergoes remodeling. The remodeling process involves resorption of bone by osteoclasts followed by the synthesis of bone by osteoblasts. Over the past two decades remarkable progress has been made in understanding each of the individual remodeling steps. One notable breakthrough was that the cytokine RANKL is necessary and sufficient for the formation and function of osteoclasts.
In the mid-1980s, researchers from the late Greg Mundy’s laboratory discovered that TNF could dramatically increase osteoclast formation [1]. Using this discovery, a decade later researchers at the Amgen pharmaceutical company screened an expression sequence tag database for potential bone-altering TNF-related proteins and identified a novel soluble TNF receptor [2]. Remarkably, when expressed in the livers of transgenic mice, this secreted receptor increased bone density by decreasing bone resorption, prompting the authors to name it osteoprotegerin (commonly abbreviated as OPG) [2].
The discovery of OPG prompted the Amgen group to search for the TNF-like molecule that this receptor was neutralizing to suppress bone resorption. They reasoned that osteoclasts, which are derived from monocytic hematopoietic precursors, might differentiate when exposed to this TNF-like molecule. Through expression cloning they identified a ligand for OPG that existed in both transmembrane and soluble forms [3]. OPG ligand, now called RANKL, when applied to hematopoietic precursors could induce osteoclast formation with only the co-addition of macrophage colony-stimulating factor [3]; prior to that discovery, osteoclasts were only formed when grown in co-cultures with stromal cells. These findings defined a major paradigm in osteoclast biology: RANKL and OPG act as the key positive and negative regulators of osteoclastogenesis, respectively.
Mechanisms of Osteoporosis
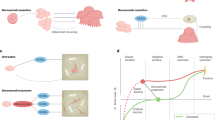
Osteoporosis, which results when bone destruction is greater than bone formation, is an umbrella term for distinct disease entities. In “high-turnover osteoporosis” there is excessive osteoclastic bone destruction, with osteoblasts unable to adequately keep up their rate of bone formation. A second form of osteoporosis, called “low-turnover osteoporosis,” results from a normal-paced remodeling cycle (Fig. 1) but feeble osteoblastic bone deposition. Either form can ultimately predispose to fragility fractures.
The effects of denosumab and bisphosphonates on bone remodeling cycle. a Normal remodeling cycle. During bone formation, osteoblasts secrete regulators, such as transforming growth factor-β (TGF-β) bound to an inhibitory latent protein (LP), which become deposited within the matrix. When osteoclasts resorb bone, their acidic vacuole frees these molecules. One of these molecules, TGF-β, serves as an attractant to recruit bone marrow stem cells (BMSCs) toward resorption sites [19, 20]. BMSCs subsequently differentiate into osteoblasts to fill in the cavity. b Remodeling on bisphosphonates. With bisphosphonates, resorption is prematurely halted because bisphosphonates inhibit signals necessary to keep the osteoclasts active. This results in significantly reduced resorption; moreover, reduced resorption decreases osteoblastic activity, as less cavities need filling. c Remodeling on denosumab. With denosumab, osteoclast formation is prevented. This blocks bone resorption. Because no resorption occurs, there is no osteoblastic activity as there are no cavities to fill
What would cause the osteoclasts to degrade bone at a higher rate than the body’s ability to rebuild bone? If one were to examine a plot of BMD versus age in both genders, one would be drawn to a precipitous drop in bone mass for women. This rapid loss of bone occurs during late perimenopause [4, 5]. During this phase of rapid, late perimenopausal bone loss, estrogen levels are surprisingly normal. In 2006 we suggested that elevations in the circulating levels of the pituitary-derived follicle-stimulating hormone (FSH) likely account for the enhanced bone loss occurring during late perimenopause; specifically, elevated FSH levels can increase RANKL-mediated osteoclast formation and function [6, 7]. Estrogen prevents bone loss by inhibiting RANKL-mediated osteoclastogenesis.
Denosumab FREEDOM Clinical Trial
All lines of evidence thus point to excessive activation of the osteoclast as a crucial event causing bone loss. Could the discovery that RANKL is the crucial mediator of osteoclastogenesis be targeted to therapeutic advantage in osteoporosis? Amgen has pursued the RANKL/OPG interplay by several mechanisms, such as gene therapy leading to OPG overexpression, or the creation of a neutralizing antibody to RANKL. This latter approach—the creation of a monoclonal anti-RANKL antibody known as denosumab—recently underwent a large multicenter clinical trial to assess its efficacy in preventing fractures.
This trial, known as the FREEDOM trial, consisted of giving 60- to 90-year-old women subcutaneous injections of 60 mg of denosumab or placebo every 6 months for 3 years [8]. The results from the approximately 7,800 women who completed the trial demonstrated remarkable increases in lumbar spine (∼8%) and hip (∼4%) BMD [8]. These increased BMDs seen with denosumab treatment resulted in statistically significant reductions in vertebral, nonvertebral, and hip fracture incidences [8]. The relative risk reduction was profound: 68% at the vertebral column, 40% at the hip, and 20% at nonvertebral sites.
Not surprisingly, the increases in BMD were reciprocally mirrored by decreases in serum markers of osteoclast bone resorption. Serum C-telopeptide of type I collagen (CTX) remained greater than 60% suppressed by denosumab throughout the 3-year trial [8]. A marker of bone formation, serum procollagen type I N-terminal propeptide (PINP), was also decreased expectedly in the denosumab-treated group compared with placebo controls. Overall, these results together suggest that denosumab is able to suppress bone turnover and thereby cause increases in bone mass and decreases in fracture incidence.
On closer inspection, the FREEDOM trial has several interesting peculiarities. One is that the placebo group’s lumbar spine and hip BMD did not significantly decrease over the 3-year duration of the study [8]. This suggests that bone resorption is not excessively activated in these individuals—this is supported by the lack of any increases in serum markers of bone resorption, such as CTX [8]. Recent studies, such as one examining quantitative computed tomography (QCT) by Genant et al. [9], have shown that denosumab increases BMD; they have, however, documented simultaneous decreases in BMD in the placebo-treated group [9].
The participants’ age and, more specifically, proximity to the hormonal changes associated with menopause, likely explain the aforementioned discrepancies. The mean age of women in the FREEDOM trial was 72 years, whereas the age of participants in the QCT trial was 59 to 60 years [8, 9]. As stated in the preceding sections, the most dramatic drop in BMD occurs in late perimenopause and early postmenopause. It is likely that the best benefit of inhibiting osteoclast resorption with either denosumab or bisphosphonates occurs during times of excessive bone destruction (ie, giving osteoclast inhibitors during the late perimenopause or early menopause will likely have the greatest benefit on BMD) [10]. Toward this end, although the QCT trial noted similar increases in BMD from baseline in the denosumab-treated group, the relative change to placebo controls was greater because those in the placebo group were actively losing bone [9]. Future studies are needed to assess the effect osteoclast inhibitors, including denosumab, during the menopausal transition.
Adverse Effects of Denosumab
Although every drug has adverse effects, the initial studies with denosumab are remarkable for the relative paucity of significant side effects. The one statistically significant adverse effect noted in the FREEDOM trial was cellulitis. There was greater incidence of cellulitis in the denosumab-treated group versus placebo [8] although these events were collected as adverse events, not predetermined end points. In addition to cellulitis, several clinicians have voiced their concerns about possible immunologic side effects of the drug. Is there a connection between cellulitis and possible immunologic changes?
When RANKL and its receptor RANK were first characterized, in addition to osteoclasts, this cytokine/receptor pair regulated lymph node formation and mammary gland development, but was also expressed on dendritic cells (DCs) [11, 12]. It is now realized that T lymphocytes express RANKL, and that interaction with RANK expressed on DCs functions to stimulate naive T-cell proliferation and enhance DC survival [13].
Knocking out either RANK or RANKL does not affect the development or distribution of DCs, suggesting initially that inhibiting this interaction would be of little consequence [14]. However, in the skin, RANKL promotes LC (a type of DC) activation and survival [15]. In mice deficient in RANKL, the number of epidermal LCs is reduced, suggesting that this interaction may be important in humans as well [15].
How is the presence of LCs beneficial at preventing cellulitis? No one knows for sure, but clues to a possible mechanism come from experiments with ultraviolet (UV) radiation. Skin keratinocytes upon exposure to UV radiation upregulate RANKL expression, and subsequently stimulate LC cytokine secretion [14]. The types of cytokines secreted by LCs cause regulatory T cells to act to suppress local and systemic inflammatory reactions [14]. Thus, it is tempting to speculate that the increased incidence of cellulitis seen with denosumab is related to alterations in RANKL/RANK signaling in skin, possibly through altering regulatory T cells; more research is needed in this area.
What about other possible adverse effects from denosumab? One area that bone turnover plays a vital role in is situations known to produce hypocalcemia. Bisphosphonates can cause symptomatic hypocalcemia, particularly in vitamin D–deficient states causing secondary hyperparathyroidism. However, it is interesting to note that hypocalcemia was absent in the adverse-effect profile of the FREEDOM trial. It is possible the few remaining osteoclasts present in bone are sufficiently responsive that hypocalcemia is not observed.
Another burgeoning area is the role that osteoclastic resorption plays in glucose metabolism. New data indicate that osteoclasts regulate insulin secretion from the pancreas through resorption-mediated release of decarboxylated osteocalcin [16]. Currently, we are not aware of any studies on the effects of bisphosphonate or denosumab evaluating their effects on insulin production. However, it is interesting to note that treatment with bisphosphonates can decrease decarboxylated osteocalcin, while not affecting carboxylated osteocalcin (which is thought to be directly released from osteoblasts independently of osteoclastic resorption) [17].
Conclusions
Overall, the future is bright for denosumab. Its effects on suppressing osteoclastogenesis are unmatched, and the drug has relatively few adverse effects of consequence. Denosumab’s role in skin LC function needs to be further explored, and clinical caution is advised. With more research, there is significant potential for using agents that modulate RANKL/RANK. Just recently, it was discovered that this pair interacting in the central nervous system controls the generation of fever [18].
References
Bertolini DR, Nedwin GE, Bringman TS, et al.: Stimulation of bone resorption and inhibition of bone formation in vitro by human tumour necrosis factors. Nature 1986, 319:516–518.
Simonet WS, Lacey DL, Dunstan CR, et al.: Osteoprotegerin: a novel secreted protein involved in the regulation of bone density. Cell 1997, 89:309–319.
Lacey DL, Timms E, Tan HL, et al.: Osteoprotegerin ligand is a cytokine that regulates osteoclast differentiation and activation. Cell 1998, 93:165–176.
Randolph JF Jr, Sowers M, Bondarenko IV, et al.: Change in estradiol and follicle-stimulating hormone across the early menopausal transition: effects of ethnicity and age. J Clin Endocrinol Metab 2004, 89:1555–1561.
Sowers MR, Jannausch M, McConnell D, et al.: Hormone predictors of bone mineral density changes during the menopausal transition. J Clin Endocrinol Metab 2006, 91:1261–1267.
Iqbal J, Sun L, Kumar TR, et al.: Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A 2006, 103:14925–14930.
Sun L, Peng Y, Sharrow AC, et al.: FSH directly regulates bone mass. Cell 2006, 125:247–260.
Cummings SR, San Martin J, McClung MR, et al.: Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009, 361:756–765.
Genant HK, Engelke K, Hanley DA, et al.: Denosumab improves density and strength parameters as measured by QCT of the radius in postmenopausal women with low bone mineral density. Bone 2010, 47:131–139.
Iqbal J, Zaidi M: Understanding estrogen action during menopause. Endocrinology 2009, 150:3443–3445.
Fata JE, Kong YY, Li J, et al.: The osteoclast differentiation factor osteoprotegerin-ligand is essential for mammary gland development. Cell 2000, 103:41–50.
Theill LE, Boyle WJ, Penninger JM: RANK-L and RANK: T cells, bone loss, and mammalian evolution. Annu Rev Immunol 2002, 20:795–823.
Leibbrandt A, Penninger JM: Novel functions of RANK(L) signaling in the immune system. Adv Exp Med Biol 2010, 658:77–94.
Leibbrandt A, Penninger JM: RANK/RANKL: regulators of immune responses and bone physiology. Ann N Y Acad Sci 2008, 1143:123–150.
Barbaroux JB, Beleut M, Brisken C, et al.: Epidermal receptor activator of NF-kappaB ligand controls Langerhans cells numbers and proliferation. J Immunol 2008, 181:1103–1108.
Ferron M, Wei J, Yoshizawa T, et al.: Insulin signaling in osteoblasts integrates bone remodeling and energy metabolism. Cell 2010, 142:296–308.
Hirao M, Hashimoto J, Ando W, et al.: Response of serum carboxylated and undercarboxylated osteocalcin to alendronate monotherapy and combined therapy with vitamin K2 in postmenopausal women. J Bone Miner Metab 2008, 26:260–264.
Hanada R, Leibbrandt A, Hanada T, et al.: Central control of fever and female body temperature by RANKL/RANK. Nature 2009, 462:505–509.
Iqbal J, Sun L, Zaidi M: Coupling bone degradation to formation. Nat Med 2009, 15:729–731.
Tang Y, Wu X, Lei W, et al.: TGF-beta1-induced migration of bone mesenchymal stem cells couples bone resorption with formation. Nat Med 2009, 15:757–765.
Acknowledgments
Drs. Mone Zaidi and Li Sun are supported by grants from the US National Institutes of Health. Dr. Jameel Iqbal acknowledges the support of the American Federation for Aging Research.
Disclosures
Dr. Zaidi consults for Genentech, Amgen, and Warner Chilcott. Dr. Zaidi is also a named inventor of a pending patent application related to osteoclastic bone resorption filed by the Mount Sinai School of Medicine (MSSM). In the event the pending or issued patent is licensed, he would be entitled to a share of any proceeds MSSM receives from the licensee. No other potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Iqbal, J., Sun, L. & Zaidi, M. Denosumab for the Treatment of Osteoporosis. Curr Osteoporos Rep 8, 163–167 (2010). https://doi.org/10.1007/s11914-010-0034-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-010-0034-z