Abstract
Can osteoporosis disease management be cost effective? To answer that question, we conducted an extensive review of osteoporosis and fragility fracture prevention literature in peer-reviewed scientific journals and evidence-based guidelines from professional societies and government health organizations. We explored different strategies suggested by the literature to find how programs can be structured to be cost effective and to decrease fracture rates. We focused on ways to cost effectively identify, risk stratify, treat, and then track patients at risk for osteoporosis and fragility fractures. Studies have shown that osteoporosis management can decrease the hip fracture rate by 25% to 50% and be cost effective at the same time.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The problem of osteoporosis is now reaching epidemic proportions with the rapidly aging population [1]. A huge cost is associated with osteoporosis in terms of morbidity, mortality, and the financial impact on society. The most devastating complication of osteoporosis is a hip fracture. According to the most recent statistics published in the 2004 Report of the Surgeon General’s Workshop on Osteoporosis and Bone Health, of the 325,000 patients who sustain a hip fracture each year in the United States, 24% end up in nursing homes, 50% never reach their previous functional capacity, and 25% die within the first year after the fracture. The total cost of a hip fracture is estimated to be on average $40,000 for both acute and chronic care [2••].
Although hip fractures account for the majority of the total cost for fragility fractures, it is important to remember that each year over 2 million people in the United States experience a fragility fracture secondary to osteoporosis, resulting in an annual cost of $19 billion. The incidence of osteoporosis-related fractures and costs in the United States are projected to rapidly increase between now and the year 2025. A group commonly overlooked in osteoporosis guidelines and in cost analysis is men. Men account for more than 29% of fractures and 25% of the annual cost of fragility fractures [3].
We will follow the normal chain of logic in osteoporosis disease management. The first step is to identify the population at risk for fragility fractures that need to be screened with a dual-energy x-ray absorptiometry (DXA) scan or other test. The second step is to risk stratify the population with the results of the DXA and/or other clinical risk factors (CRFs) to determine proper treatments. The third step is to implement the treatment plan. The fourth step is to determine a strategy that will keep the at-risk patients on their treatments. The last step is to track the patients to assess the effectiveness of the program in keeping the patients on treatment and in decreasing the number of expected fragility fractures and the overall cost of osteoporosis disease management.
We systematically address every step in this logical sequence to find the most cost-effective ways to prevent osteoporosis and fragility fractures from occurring. We start the process by setting goals and understand what tools we have available to help make the diagnosis of osteoporosis and determine who is appropriate for treatment.
Goal
The goal is to decrease the rate of hip and other fragility fractures while being cost effective. Can this goal be achieved and if so how? Hip and other fragility fractures have been shown to decrease in programs that aggressively target osteoporosis. These programs have also shown that the savings from fracture prevention is greater than the cost of diagnosis and treatment of osteoporosis in these populations [4, 5].
Both these studies have shown that the greatest reduction in hip fractures and cost savings occur in patients over the age of 75 years old, but cost savings can even occur in the 65- to 75-year age groups. The two studies demonstrated that modification of the health care environment may be more effective than interventions targeting the busy provider. Both programs are in closed health systems that implemented osteoporosis disease management programs that provided clinical practice guidelines, physician and allied health care provider education, and used a systematic approach to order DXA testing and then get appropriate treatment for those patients who needed treatment. Over a 5-year period, the Geisinger program led to a decrease in the incidence of hip fractures among the clinic’s patients and an overall reduction of nearly $8 million in health care costs compared with the estimated costs if no intervention had been undertaken [5].
The populations at Geisinger and Kaiser are not the only groups that have demonstrated a decrease in hip fracture rate over the past 10 or more years. There is now good evidence that a 20% decrease in the US hip fracture rate occurred between 1993 and 2003 [6].
Similarly, in Canada, it was seen over the 21-year period of the study that age-adjusted hip fracture rates decreased by 31.8% in female (from 118.6 to 80.9 hip fractures per 100,000 person-years) and by 25.0% in male patients (from 68.2 to 51.1 hip fractures per 100,000 person-years) [7•]. The decrease in the hip fracture rate may be related to the increase in diagnosis and treatment of osteoporosis in regions such as Ontario, Canada [8]. European counties are also seeing a decline in hip fracture rates [9].
What steps can be taken to target and treat the at-risk patients to see a decrease in the hip fracture rate? The first step starts with correctly identifying the population at risk for hip and other fragility fractures.
Identify Your Population
The ability to identify the population at risk is seriously limited by our lack of an integrated health care delivery program in the United States. A review of quality measures that show how well we identify our population at risk for osteoporosis shows we are doing a poor job. They concluded there are large care gaps in patients at high osteoporosis risk and in the delivery of optimal osteoporosis management [10•]. A concerning fact may be that the elderly—the group that experiences the highest number of hip fractures—are also experiencing the largest care gap in osteoporosis disease management. This care gap in the elderly may reflect attitudes from patients and clinicians that osteoporosis management is of low priority in the elderly [11•]. A total of 27% of eligible women between 66 and 70 years of age received DXA testing compared with only 16% of women 81 to 85 years of age and 9.7% of women between 86 and 90 years of age [12].
When systematic case-finding strategies are implemented, the rate of DXA screening can more than triple and the rate of patients treated can be as many as 96% [13•]. Even when a fragility fracture has occurred and concrete local protocols are in place, we continue to see large care gaps in the management of osteoporosis [14•].
It is hard to be cost effective if you do not systematically identify your population at risk. Better systems are needed to prevent care gaps for osteoporosis management [15•]. Health care organizations that used administrative databases to identify all at-risk patients have an advantage over less organized programs that rely on case finding without this systematic approach [16].
Risk Stratify
Currently, multiple screening guidelines are available to risk stratify populations to determine who need DXA scans and treatment. Even though differences exist between these guidelines, most recommend routine DXA screening in women to begin around 65 years of age. For those guidelines that address osteoporosis screening in men, most start routine screening around 70 years of age. These recommendations reflect the increase in the prevalence of low bone mass with age in both sexes [17-19].
If the goal is to identify all patients at risk for fragility fractures, using only a DXA scan to identify your at-risk population is not sufficient. Only 44% of all nonvertebral fractures occurred in women with a T-score below ≤ 2.5; in men, this percentage was even lower (21%). There is a clear need for the development of more sensitive risk assessment tools, using not only DXA scans, but also other clinical predictors of fractures [20].
The World Health Organization (WHO) developed a fracture risk algorithm (FRAX), an algorithm to calculate a 10-year absolute hip and other fragility fracture score. This algorithm uses multiple risk factors including age, gender, race, body mass index (BMI), history of prior fractures, paternal history of hip fracture, steroid use, presence of secondary osteoporosis, presence of rheumatoid arthritis, current smoking, and drinking history. A risk score can be calculated with or without the T-score from DXA screening. The FRAX algorithm was combined with an updated economic analysis to evaluate existing National Osteoporosis Foundation (NOF) guidance for osteoporosis prevention and treatment [21••].
In the analysis by Dawson-Hughes et al. [21••], in Caucasian women of average risk the 10-year hip fracture probability is estimated at 2% at 65 years of age but exceeds the 3% cost-effectiveness threshold at older ages. Average-risk women of other races did not have 10-year hip fracture probabilities exceeding 3% until they were over 80 years of age, and comparable men not until 75 years of age. Treatment would appear cost effective in high-risk subsets of these populations. These findings are quite sensitive to different assumptions about drug costs, a major determinant of treatment cost effectiveness. The updated NOF economic analysis estimated drug costs at $600 per year in anticipation of generic bisphosphonate; however, if the drug cost were further reduced from $600 to $300, then the level of 10-year hip fracture risk that is cost effective to treat falls to 1.4% [21••]. Further reduction in the cost of treatment would lower the cost-effective thresholds to even lower levels.
The FRAX tool was designed for postmenopausal women and men between 40 and 90 years of age. FRAX is available on the Internet and in paper form. The current US FRAX tool has been updated, as it was found that the US FRAX tool was overestimating the hip fracture rates by 16%, with the greatest reductions observed among those below 65 years of age [22••]. How this change in the US FRAX system will affect the cost analysis of treatment remains to be determined.
FRAX can be used to classify patients into one of three categories: 1) Patients at very low risk who do not need a DXA or any further workup. 2) Patients who would benefit from being started on treatment without any need for a DXA scan as part of their workup. 3) Patients who would benefit from additional information from the DXA prior to determining a risk score to see if they require treatment. The screening and case-finding strategies using only DXA scans are specific (they identify high-risk patients) but lack sensitivity (they miss many who will experience fractures). Kanis et al. [23•] demonstrated that case finding can be enhanced by the FRAX CRFs, which provide information on fracture risk over and above that provided by results from a DXA scan.
Even without FRAX or a DXA scan, a fragility fracture in the elderly is accepted as evidence of osteoporosis. The National Institute of Clinical Excellence (NICE) recommends osteoporosis treatment in all patients with fragility fractures over 75 years of age without a DXA scan, and after a DXA scan in younger patients [24].
Tools to Calculate Treatment Thresholds Based on Absolute Fracture Risk
Tosteson et al. [25••] used a Markov-cohort model to determine the FRAX cost-effective threshold in the United States by using the following assumptions: 1) $600 per year drug cost for 5 years; 2) a 35% fracture reduction in the hip fracture rate; and 3) $60,000 per quality-adjusted life-year gained was observed for treatment relative to no intervention. They found the cost-effective thresholds for the 10-year absolute risk of hip fracture to be 3% and a 10-year absolute risk of major osteoporotic fracture to be 20% using FRAX [25••]. These thresholds were used in the new NOF Guideline that uses FRAX.
The osteoporosis guidelines from NICE and others mentioned earlier has raised some controversy. A major concern has been that despite a sixfold reduction in the price of alendronate, the estimates of cost effectiveness have barely changed. This has been achieved by alteration of some of the model assumptions, in the absence of new evidence, so that the cost effectiveness of alendronate has remained unchanged despite its fall in price. Furthermore, these changes to the model have had a negative impact on the cost effectiveness of the other treatments under consideration [26•].
There are now several cost-analysis papers that have made similar assumptions on the cost of treatment. These include recent studies using Markov modeling [27, 28] and the Geisinger cost-analysis study that placed the cost of treatment at $500 (US 2003) per year and the cost of a DXA scan at $100 (US 2003). In the Geisinger study, the majority of the savings came from the decrease in hip fracture incidence. Significant separation in the cost curves began in the third year and became quite apparent by the fifth year. Net savings were achieved in the 65- to 75-year age group (US $3.1 million) and 75+ year (US $7.2 million) age group over the 5- year study period. However, the 55- to 65-year age group experienced a net loss of US $2.4 million above expected expenses, not unexpected given the low hip fracture rate in this age group [5].
There is no right or wrong methodologic approach, and the approach used is dependent on the socioeconomic setting. In the United States, for example, screening of men and women (at the ages of 70 and 65 years, respectively) with a DXA scan is recommended. In the United Kingdom, DXA tests are reserved for individuals with CRFs for fracture. In addition, intervention costs are much lower in the United Kingdom than in the United States. An intervention threshold based on cost utility alone in the United Kingdom would permit intervention in many individuals in whom this was considered clinically inappropriate, and many more than would be deemed eligible in the United States. Because intervention thresholds are in part based on cost effectiveness, and in part on clinical considerations, the examples above are not necessarily applicable to other countries, in which the willingness to pay and/or the costs of osteoporosis or intervention may differ.
Like the NOF Osteoporosis Guideline, the UK National Osteoporosis Guideline also recommends using FRAX with assessment and treatment thresholds in the absence of a DXA test and with DXA tests to compute fracture probability for men and women. Both guidelines recommend generic alendronate as the first-line treatment in the majority of cases [29•].
Since 2005, Canada has used a simplified risk assessment tool based on sex, age, DXA, and two CRFs, prior fracture, and systemic corticosteroid. Basal 10-year fracture risk from age and minimum T-score (lumbar spine, femur neck, trochanter, total hip) was categorized as low (< 10%), moderate (10% to 20%), or high (> 20%). This simplified system for estimating 10-year absolute fracture risk does not require any detailed calculations or access to a computer. All data required to use the system can be summarized on a pocket-sized laminated card. They were unable to compare their simplified fracture risk assessment and the FRAX tool. Whether there is incremental benefit with the additional CRFs of the FRAX system is currently unclear and warrants future study [30•].
Hopefully, a comparison analysis with the Canadian and FRAX tools will happen soon because the ease of use and interpretation of the Canadian risk calculator has several advantages over the more complex FRAX risk calculator. For risk assessment to be cost effective we need simple tools that will be consistently used. The NOF and others have chosen the FRAX tool, but that tool will continue to undergo improvements as it becomes more widely accepted into clinical practice and eventually incorporated directly into DXA reports.
Treatment
It is estimated that at least 72% of US white women ≥ 65 years of age and 93% of those ≥ 75 years of age would be recommended for drug treatment. Application of the new NOF Guidelines would result in recommending a very large proportion of white women in the United States for pharmacologic treatment of osteoporosis [31•]. For men, DXA screening followed by bisphosphonate therapy for those with osteoporosis may be cost effective for men 65 years of age or older with a self-reported prior clinical fracture [32].
For nonwhite patients, future fracture risk is lower in than white women and men who present with a prior fracture. However, if they have at least low bone mass (T-score, -2.0) and one or more risk factors (eg, smoking and alcohol use), their absolute hip fracture probability is elevated beyond the treatment threshold, although still less than that of postmenopausal white women [21••].
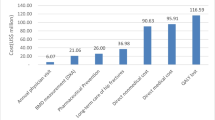
The population at the highest risk for hip fractures is the elderly patients who are over 75 years of age (Fig. 1, Tables 1 and 2). Treatment has been found to work well in the older age population. Although all patients should be carefully evaluated and screened prior to treatment for vitamin D levels, renal function, and calcium level, this should be done especially in the elderly [33].
Bisphosphonate therapy has also been shown to be considerably more cost effective in patients with low BMI. This was related to the inverse relationship between BMI and risk of fracture and to the higher prevalence of other CRFs in patients with low BMI [34].
Cost-effective analysis of osteoporosis treatment options may provide an estimate of the appropriate intervention thresholds for populations, but the choice of thresholds for individual patients is also dependent on their own risk/benefit balance. It should be noted that guidelines relying only on cost-effective models do not reflect many of the individual-level factors and are sometimes in direct contradiction to the clinical demands of society. Guidelines for prevention of osteoporotic fractures need to consider population-specific and individual-specific factors. The goal is to achieve “individualized intervention thresholds” that will better direct clinicians in using individual care plans to bring maximum benefit to the patients and the society [35•].
The switch to generic alendronate may have a huge impact on the cost model for many organizations and many countries. Even prior to generic alendronate, the cost effectiveness of alendronate in osteoporosis management was discussed [5, 26•, 36]. The cost savings are now considerably higher after the significant cost drop in generic alendronate. This allows a change in the cost model that can support additional patients to receive treatment who were previously above the threshold of cost [2••]. The cost savings then can drive a shift in case finding. The thresholds for screening now can also shift to a lower age level based on the decrease in cost of treatment. A business case could even be made for giving generic alendronate to all individuals who have no medical contraindications above a certain cutoff age in which the cost of treatment is lower than the total cost of managing hip and other fragility fractures in that subpopulation. Thus far, no organization or country has yet to make the recommendation to routinely treat all of its patients above a certain age threshold. However, in the United Kingdom, a recommendation is in place to treat with generic alendronate without the need for further workup if a woman is 75 years of age or older and has a prior fragility fracture, and if the responsible clinician considers a DXA scan to be clinically inappropriate or unfeasible [24].
Track
Increased risks of fragility fractures were found in patients with low medication compliance. Use of bisphosphonates was associated with fracture risk reductions after 6 to 12 months of treatment, but only 58% of the patients were treated for at least 1 year. Improvement in long-term persistence of bisphosphonate treatment may be important to reduce the impact of osteoporosis-related fractures [37].
The potentially important drivers of cost effectiveness include reduced drug effectiveness due to poor compliance, offset time, fracture risk, anti-fracture drug effect, and drug price. Optimal adherence was associated with fewer osteoporotic fractures, and the impact was more evident among those with prior fractures [38].
Improve
An example of a systems approach to osteoporosis disease management is the use of frequent reports to identify which patients require treatment and getting that list of patients to primary care providers or care managers. The ideal solution would be a real-time program that prevents care gaps from occurring. An example of a common care gap is the lack of coordination after a fracture has occurred. In a randomized controlled trial conducted in hip fracture survivors, it was demonstrated that the use of a hospital-based osteoporosis case manager could lead to a 51% rate of bisphosphonate treatment within 6 months of fracture (vs 22% for controls; P < 0.001) and result in 67% of patients receiving guideline-concordant appropriate care (vs 26% for controls; P < 0.001) [39]. Creative ideas like this may assist in decreasing care gaps.
Conclusions
It has been shown that osteoporosis disease management can be cost effective and decrease the hip fracture rate at the same time. Effective tools and treatments are now available to assist the clinician to help identify and risk stratify patients at risk for a fragility fracture. We now have FRAX that drastically improves the ability to risk assess patients to assist us in determining who needs screening and who needs treatment. The NOF and others have recommended the routine use of the FRAX tool in their guidelines. It is important to develop a systematic approach to identify the population at risk that needs screening to help eliminate care gaps in osteoporosis disease management. Better systems must be put in place so that no patient falls through the cracks by not having their osteoporosis diagnosed and treated.
References
Papers of particular interest, published recently, have been highlighted as follows: • Of importance, •• Of major importance
Office of the Surgeon General, US Department of Health and Human Services: Bone Health and Osteoporosis: A Report of the Surgeon General. Washington, DC: US Department of Health and Human Services; 2004.
•• Tosteson AN, Burge RT, Marshall DA, Lindsay R: Therapies for treatment of osteoporosis in US women: cost-effectiveness and budget impact considerations. Am J Manag Care 2008, 14:605–615. This paper evaluates the cost effectiveness of osteoporosis treatments for women at high fracture risk and estimates the population-level impact of providing bisphosphonate therapy to all eligible high-risk US women. Osteoporosis treatment of high-risk women is cost-effective, with bisphosphonates providing the most benefit at lowest cost. For highest-risk women, costs are offset by savings from fracture prevention.
Burge R, Dawson-Hughes B, Solomon DH, et al.: Incidence and economic burden of osteoporosis-related fractures in the United States, 2005-2025. J Bone Miner Res 2007, 22:465–475.
Dell R, Greene D, Schelkun SR, Williams K: Osteoporosis disease management: the role of the orthopaedic surgeon. J Bone Joint Surg Am 2008, 90(Suppl 4):188–194.
Newman ED, Ayoub WT, Starkey RH, et al.: Osteoporosis disease management in a rural health care population: hip fracture reduction and reduced costs in postmenopausal women after 5 years. Osteoporos Int 2003, 14:146–151.
Gehlbach SH, Avrunin JS, Puleo E: Trends in hospital care for hip fractures. Osteoporos Int 2007, 18:585–591.
• Leslie WD, O'Donnell S, Jean S, et al.: Trends in hip fracture rates in Canada. Osteoporosis Surveillance Expert Working Group. JAMA 2009, 302:883–889. Age-standardized rates of hip fracture have steadily declined in Canada since 1985 and more rapidly during the later study period. The factors primarily responsible for the earlier reduction in hip fractures are unknown.
Jaglal SB, Weller I, Mamdani M, et al.: Population trends in BMD testing, treatment, and hip and wrist fracture rates: are the hip fracture projections wrong? J Bone Miner Res 2005, 20:898–905.
Kannus P, Niemi S, Parkkari J, et al.: Nationwide decline in incidence of hip fracture. J Bone Miner Res 2006, 21:1836–1838.
• Curtis JR, Adachi JD, Saag KG: Bridging the osteoporosis quality chasm. J Bone Miner Res 2009, 24:3–7. There are a growing number of well-studied therapeutic options and emerging international consensus on what constitutes quality in osteoporosis and who needs to be treated. As a stark distinction from this evidence base, the care gap between adults at high osteoporosis risk and the delivery of optimal osteoporosis management is large. The osteoporosis care gap needs to be narrowed to reduce health care disparities and the burden of fractures. Evidence of implementation strategies that directly target providers, patients, and health care systems offer partial solutions.
• Vondracek SF, Linnebur SA: Diagnosis and management of osteoporosis in the older senior. Clin Interv Aging 2009, 4:121–136. It is important for health care providers to be fully aware of the potential risks and benefits of diagnosing and treating osteoporosis in the older senior population. Data indicate that bone mineral density (BMD) testing is underutilized and drug therapy is often not initiated when indicated in this population. BMD testing with central DXA is essential and cost effective in this population.
Neuner JM, Binkley N, Sparapani RA, et al.: Bone density testing in older women and its association with patient age. J Am Geriatr Soc 2006, 54:485–489.
• Geusens P, Dumitrescu B, van Geel T, et al.: Impact of systematic implementation of a clinical case-finding strategy on diagnosis and therapy of postmenopausal osteoporosis. J Bone Miner Res 2008, 23:812–818. By using the Osteoporosis Self-Assessment Index, based only on age and weight and fracture history, a case-finding strategy was developed for patients who needed DXA screening. This strategy nearly tripled referrals for DXA, and 96% of patients found to have osteoporosis had treatment. This indicates the need for better case-finding strategies with fewer barriers for referral for DXA and with higher accuracy for predicting osteoporosis.
• Kurup HV, Andrew JG: Secondary prevention of osteoporosis after Colles fracture: current practice. Joint Bone Spine 2008, 75:50–52. The National Institute of Clinical Excellence in the United Kingdom recommends osteoporosis treatment in all fragility fractures in patients over 75 years of age without a DXA scan, and after a DXA scan in younger patients.
• Ayoub WT, Newman ED, Blosky MA, et al.: Improving detection and treatment of osteoporosis: redesigning care using the electronic medical record and shared medical appointments. Osteoporos Int 2009, 20:37–42. The redesigned process was highly effective in improving BMD testing for women 65 years of age. The shared medical appointment was shown to be a more effective method to make calcium and vitamin D recommendations, to evaluate secondary causes of low bone density, and to prescribe prescription medications, compared with the usual care with the primary care physician.
Harrington JT, Barash HL, Day S, Lease J: Redesigning the care of fragility fracture patients to improve osteoporosis management: a health care improvement project. Arthritis Rheum 2005, 53:198–204.
National Osteoporosis Foundation: NOF’s Clinician’s Guide to Prevention and Treatment of Osteoporosis. Available at http://www.nof.org/Professionals/Clinicians_Guide.htm. Accessed August 22, 2009.
The International Society for Clinical Densitometry (ISCD): Official Positions. Available at http://iscd.org/Visitors/positions/OfficialPositionsText.cfm. Accessed August 22, 2009.
U.S. Department of Human and Health Services: U.S. Preventive Services Task Force Osteoporosis Screening. Available at http://www.ahrq.gov/clinic/3rduspstf/Osteoporosis/. Accessed August 22, 2009.
Schuit SC, van der Klift M, Weel AE, et al.: Fracture incidence and association with bone mineral density in elderly men and women: the Rotterdam Study. Bone 2004, 34:195–202.
•• Dawson-Hughes B, Tosteson AN, Melton LJ 3rd, et al.: Implications of absolute fracture risk assessment for osteoporosis practice guidelines in the USA. National Osteoporosis Foundation Guide Committee. Osteoporos Int 2008, 19:449–458. The new WHO fracture prediction algorithm was combined with an updated economic analysis to evaluate existing NOF guidance for osteoporosis prevention and treatment. It is cost effective to treat patients with a fragility fracture and those with osteoporosis by WHO criteria, as well as older individuals at average risk and osteopenic patients with additional risk factors. However, the estimated 10-year fracture probability was lower in men and nonwhite women compared with postmenopausal white women.
•• Ettinger B, Black DM, Dawson-Hughes B, et al.: Updated fracture incidence rates for the US version of FRAX. Osteoporos Int 2010, 21:25–33. Compared with rates used in the current FRAX tool, 2006 hip fracture rates are about 16% lower, with the greatest reductions observed among those below 65 years of age; major osteoporotic fracture rates are about one quarter lower, with similar reductions across all ages.
• Kanis JA, McCloskey EV, Johansson H, Oden A: Approaches to the targeting of treatment for osteoporosis. Nat Rev Rheumatol 2009, 5:425–431. The article uses FRAX in a case-finding strategy, based on the assessment of fracture probability using CRFs and, where appropriate, additional testing such as BMD, is recommended. These case-finding strategies have been validated from a health-economic perspective.
National Institute for Health and Excellence: Alendronate, etidronate, risedronate, raloxifene, strontium ranelate and teriparatide for the secondary prevention of osteoporotic fragility fractures in postmenopausal women. Available at http://www.nice.org.uk/nicemedia/pdf/TA161guidanceword.pdf. Accessed September 6, 2009.
•• Tosteson AN, Melton LJ 3rd, Dawson-Hughes B, et al.: Cost-effective osteoporosis treatment thresholds: the United States perspective. National Osteoporosis Foundation Guide Committee. Osteoporos Int 2008, 19:437–447. Osteoporosis treatment was cost effective when the 10-year hip fracture probability reached approximately 3%.
• Kanis JA, Compston JE.: NICE continues to muddy the waters of osteoporosis. National Osteoporosis Guideline Group of the UK. Osteoporos Int 2008, 19:1105–1107. The osteoporosis guidelines from NICE and others mentioned earlier has raised some controversy. A major concern has been that despite a sixfold reduction in the price of alendronate, the estimates of cost effectiveness have barely changed. This has been achieved by alteration of some of the model assumptions, in the absence of new evidence, so that the cost effectiveness of alendronate has remained unchanged despite its fall in price. Furthermore, these changes to the model have had a negative impact on the cost effectiveness of the other treatments under consideration.
Schousboe JT, Ensrud KE, Nyman JA, et al.: Universal bone densitometry screening combined with alendronate therapy for those diagnosed with osteoporosis is highly cost-effective for elderly women. J Am Geriatr Soc 2005, 53:1697–1704.
Black DM, Palermo L, Grima DT: Developing better economic models of osteoporosis: considerations for the calculation of the relative risk of fracture. Value Health 2006, 9:54–58.
• Compston J, Cooper A, Cooper C, et al.: Guidelines for the diagnosis and management of osteoporosis in postmenopausal women and men from the age of 50 years in the UK. National Osteoporosis Guideline Group (NOGG). Maturitas 2009, 62:105–108. Patients are identified opportunistically using a case-finding strategy on the finding of a previous fragility fracture or the presence of significant CRFs. Some of these risk factors act independently of BMD to increase fracture risk, whereas others increase fracture risk through their association with low BMD (eg, some of the secondary causes of osteoporosis).
• Leslie WD, Tsang JF, Lix LM: Simplified system for absolute fracture risk assessment: clinical validation in Canadian women. Manitoba Bone Density Program. J Bone Miner Res 2009, 24:353–360. A simplified risk assessment system from sex, age, DXA, and two CRFs—prior fracture and systemic corticosteroid use—has been used in Canada since 2005. This simplified fracture risk assessment system provides an assessment of fracture risk that is consistent with observed fracture rates.
• Donaldson MG, Cawthon PM, Lui LY, et al.: Estimates of the proportion of older white women who would be recommended for pharmacologic treatment by the new U.S. National Osteoporosis Foundation Guidelines. J Bone Miner Res 2009, 24:675–680. Application of NOF Guidelines to the Study of Osteoporotic Fractures data estimated that at least 72% of US white women ≥ 65 years of age and 93% of those ≥ 75 years of age would be recommended for drug treatment. Application of the new NOF Guidelines would result in recommending a very large proportion of white women in the United States for pharmacologic treatment of osteoporosis.
Schousboe JT, Taylor BC, Fink HA, et al.: Cost-effectiveness of bone densitometry followed by treatment of osteoporosis in older men. JAMA 2007, 298:629–637.
Hochberg MC, Thompson DE, Black DM, et al.: Effect of alendronate on the age-specific incidence of symptomatic osteoporotic fractures. FIT Research Group. J Bone Miner Res 2005, 20:971–976.
van Staa TP, Kanis JA, Geusens P, et al.: The cost-effectiveness of bisphosphonates in postmenopausal women based on individual long-term fracture risks. Value Health 2007, 10:348–357.
• Moayyeri A: Identification of factors influencing the intervention thresholds for treatment of osteoporosis based on 10-year absolute fracture risks. J Clin Densitom 2009, 12:1–4. Current guidelines are mainly based on offering cost-effective treatments to patients with certain characteristics ignoring their individual risk of fracture (which can be easily estimated by FRAX) and likeliness to benefit from treatment (determined by some other individual-level factors, such as compliance, preference, comorbidities).
Kanis JA, Adams J, Borgström F, et al.: The cost-effectiveness of alendronate in the management of osteoporosis. Bone 2008, 42:4–15.
Gallagher AM, Rietbrock S, Olson M, van Staa TP: Fracture outcomes related to persistence and compliance with oral bisphosphonates. J Bone Miner Res 2008, 23:1569–1575.
Ström O, Borgström F, Kanis JA, Jönsson B: Incorporating adherence into health economic modelling of osteoporosis. Osteoporos Int 2009, 20:23–34.
Majumdar SR, Lier DA, Beaupre LA, et al.: Osteoporosis case manager for patients with hip fractures: results of a cost-effectiveness analysis conducted alongside a randomized trial. Arch Intern Med 2009, 169:25–31.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dell, R., Greene, D. Is Osteoporosis Disease Management Cost Effective?. Curr Osteoporos Rep 8, 49–55 (2010). https://doi.org/10.1007/s11914-010-0009-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-010-0009-0