Abstract
Purpose of review
Triple negative breast cancer (TNBC) accounts for approximately 10–15% of all breast cancers and it is associated with a poor prognosis. However, recent new effective treatment strategies have improved its outcomes. The aim of this review is to provide an overview on the emerging therapeutics for TNBC, describing both previously approved therapies that are currently being repurposed, as well as new target therapies that may improve patient outcomes.
Recent findings
Emerging therapies are forthcoming in TNBC’s treatment landscape, including new post-neoadjuvant chemotherapy strategies, PARP inhibitors, immune checkpoint inhibitors, and antibody-drug conjugates. Combination of different therapies such as AKT/PI3K/mTOR-inhibitors, other immunotherapeutic agents, CDK-inhibitors, antiandrogens, antiangiogenics, and histone deacetylase inhibitors is under clinical investigation.
Summary
The treatment landscape for TNBC is gradually evolving towards a more personalized approach with promising expectations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Triple-negative breast cancer (TNBC) accounts for about 10–15% of newly diagnosed breast cancers (BC) and is associated with worse overall survival (OS) compared to other BC subtypes (5-year OS of 76.5% versus 94% for luminal BC) [1]. More than 30% of patients with TNBC eventually develop metastatic disease and relapses often occur during the first 2–3 years from diagnosis [2, 3].
This prognosis reflects an intrinsic aggressive behavior since TNBC is often associated with high histological grade and high proliferation index (ki67) [4] as well as the lack of actionable oncogenic targets, namely hormone receptors and human epidermal growth factor receptor-2 (HER2) [5].
For many years, chemotherapy has been the only available systemic treatment option for TNBC, but, recently, a deeper understanding of genomic and molecular characteristics of TNBC has led to the introduction of new target therapies. TNBC is no longer considered a single entity since different subtypes have been identified, depending on different protein expressions, genomic alterations, and/or mRNA signatures [6].
Lehmann et al. evaluated gene expression profiles of TNBC and identified six subtypes: two basal-like, immunomodulatory, mesenchymal, mesenchymal stem–like, and luminal androgen receptor (LAR) subtypes [7]. Another classification, proposed by Burnstein et al. distinguishes four different subtypes with its own characteristics and prognosis: LAR, mesenchymal, basal-like immunosuppressed, and basal-like immune-activated [8]. Each molecular subtype showed different degrees of sensitivity to targeted therapies [7]. Thus far, these classifications have no direct implications in clinical practice. However, molecular analyses of TNBC are leading towards a more tailored approach in clinical trials.
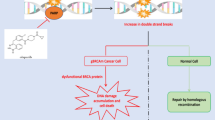
The aim of this review is to provide an overview on the emerging therapeutics for TNBC treatment, describing both previously approved therapies which are currently being evaluated in different scenarios (i.e., therapies approved in the metastatic setting, under evaluation in the early setting), and new therapies that may improve patient outcomes (Fig. 1).
Current Treatment Strategy for TNBC
Early Setting
The standard of care is represented by dose-dense anthracycline-based chemotherapy followed by a taxane [9]. While clinical data suggest that TNBC is particularly sensitive to platinum salts and support the use of platinum-based chemotherapy in the advanced setting [10, 11], the use of carboplatin in the neoadjuvant setting is still a matter of debate [12,13,14]. Platinum-based neoadjuvant regimens are associated with higher pathological complete response (pCR) rates [12]. However, there is no conclusive data on long-term outcome benefit, although some adjuvant data recently became available for anthracycline-free platinum-containing regimens [15,16,17].
Advanced Setting
In current clinical practice, a proposed treatment algorithm for advanced TNBC relies on the BRCA mutational status and PD-L1 expression [3]. In the presence of a germline BRCA mutation, platinum-based chemotherapy or PARP inhibitors (PARPi) represent first-line treatment options. In case of PD-L1 expression (defined as PD-L1≥1% on immune cells with the SP142 assay, Ventana), first-line treatment with atezolizumab and nab-paclitaxel should be considered. For BRCA-wild-type TNBC without PD-L1 expression, chemotherapy is the first-line treatment option [3]. Sequential single-agent chemotherapy represents the optimal approach, while combinations should be reserved for patients with high disease burden, rapid clinical progression or visceral crisis. Anthracyclines or taxanes are recommended first-line options, provided patients did not progress on these regimens in the early setting. Other treatment options exist and their choice depends on patients preferences, comorbidities and safety profile [10]. Inclusion in clinical trials should be considered at any disease stage, where available.
Repurposing Previously Approved Therapies into New Settings
Chemotherapy in the Post-neoadjuvant Setting
The CREATE-X study showed that the use of adjuvant capecitabine in HER2-negative BC without pCR after neoadjuvant chemotherapy (NAC) provided a statistically significant disease-free survival (DFS) and OS benefit, which was more prominent in the TNBC subgroup [18••]. In a large meta-analysis, (neo)adjuvant capecitabine was able to decrease the risk of a DFS event by 21% in TNBC [19]. Based on these data, adjuvant capecitabine is nowadays considered a standard option for patients with residual disease after NAC, according to European Society for Medical Oncology (ESMO) and American Society of Clinical Oncology (ASCO) guidelines [9, 20].
Additionally, data from two randomized phase III trials of adjuvant capecitabine in TNBC were recently presented [21, 22]. In these trials, patients were not selected for residual disease as in CREATE-X. Both studies concluded that adjuvant capecitabine improved DFS rates without significant OS benefit [21, 22].
Besides capecitabine, other post-neoadjuvant strategies are being evaluated for TNBC with residual disease after NAC. Examples are platinum-based chemotherapy vs. capecitabine in patients with ≥1cm residual TNBC after NAC (NCT02445391) or cisplatin plus gemcitabine as post-neoadjuvant treatment for non-pCR TNBC after standard NAC (NCT04297267).
PARP Inhibitors in BRCA-Mutated TNBC and Beyond
Approximately 11% of patients affected by TNBC are carriers of a germline pathogenic variant in BRCA1 or BRCA2 (gBRCA) [23]. BRCA genes code for proteins involved in the repair of double-strand DNA breaks through homologous recombination repair (HRR) [24]. Therefore, BRCA-mutated cells are unable to use HRR pathway and rely on complementary DNA repair processes, which involve poly ADP-ribose polymerase (PARP) proteins. As a consequence, the use of PARP inhibitors (PARPi) induces cell death because of accumulation of unrepaired DNA damages, a concept known as “synthetic lethality” [25,26,27]. Two PARPi (olaparib and talazoparib) have been approved in monotherapy as treatment options for advanced gBRCA-mutated HER2-negative BC [28••, 29••], based on data from OlympiAD and EMBRACA trial which showed a significant PFS improvement compared to chemotherapy of investigator’s choice (HR 0.58, 95% CI 0.43-0.80 and HR 0.54; 95% CI 0.41-0.71, respectively).
Recently, final OS results from OlympiAD trial were published. No statistically significant OS improvement was observed (HR 0.90, 95%CI 0.66-1.23, in all patients; HR 0.93, 95%CI 0.62-1.43 in TNBC patients) [30]. Of note, the trial was not powered for OS, and crossover after the end of the study can significantly confound OS analysis. Interestingly, olaparib-treated patients who had not received prior chemotherapy in the advanced setting showed a 7.9 months longer median OS compared to control arm, suggesting a larger benefit of olaparib in earlier lines. This hypothesis should be confirmed in further studies [30]. Similarly, the final analysis from the EMBRACA trial showed no significant OS benefit with talazoparib vs standard chemotherapy (HR 0.85, 95%CI 0.67–1.07) [31].
Gradually, the use of PARPi is evolving, and while PARPi monotherapy is being evaluated in HRR-deficient BC beyond gBRCA, new combination strategies with chemotherapy, immunotherapy, antibody-drug conjugates (ADCs), target therapy (such as ATR-inhibitors, BET-inhibitors) and radiotherapy are under evaluation in BC patients with or without a gBRCA mutation [32]. In a phase III study, patients with metastatic gBRCA-mutated TNBC received carboplatin and paclitaxel with or without veliparib. The addition of the PARPi significantly increased PFS (HR 0.71, 95%CI 0.57–0.88), with a durable benefit, compared to controls [33].
Particularly, the combination of PARPi and chemotherapy is being studied in the treatment of BRCA-wild type (WT) BC [34]. It should be considered that up to 10% of gBRCA-WT TNBC have pathogenic mutations leading to homologous recombination deficiency (HRD), resulting thus in a BRCA-like phenotype despite the gBRCA-WT status (BRCAness) [35]. In a phase II window clinical trial enrolling untreated TNBC, HRD was identified even in 69% of patients using a mutational-signature-based assay [36]. Recently, a randomized phase II study of cisplatin with or without veliparib in three groups of metastatic TNBC (gBRCA mutant carriers, gBRCA-WT but BRCA-like and non-BRCA-like) was presented. In the BRCA-like group, the addition of veliparib was associated with significantly improved PFS with a trend towards OS benefit, while the non-BRCA-like group did not benefit from the addition of veliparib [37].
Another strategy under evaluation is the combination of PARPi with immunotherapy. The rational is that the emergence of neoantigens following PARPi-induced DNA-damage can stimulate antitumoral immune response and improve response to immune checkpoint inhibitors (ICIs) [38, 39]. In the I-SPY 2 trial, the addition of olaparib and durvalumab to standard NAC for stage II/III HER2-negative BC was associated with a significantly improved pCR rate in a small TNBC cohort (47% vs 27%) [40].
Despite most recent studies on PARPi aiming to broaden their indications in BC treatment, maintenance data in the advanced setting is lacking and some challenges remain in the evaluation of their long-term safety profile, the interaction in combination with other therapies, and the overcoming of resistance mechanisms.
The ongoing phase III study OlympiA is evaluating olaparib in the adjuvant setting for gBRCA- HER2-negative BC and will shed further light on the role of PARPi in the early setting of gBRCA-BC (NCT02032823).
Immunotherapy
TNBC represents the optimal BC subtype for ICIs, since it is characterized by higher genomic instability compared to other BC subtypes [6, 41]. Moreover, stromal tumor-infiltrating lymphocytes (sTILs) in TNBC have demonstrated a strong prognostic value as well as a predictive value for response to NAC in the early setting [42, 43], while in the advanced setting, there are data for higher benefit of single-agent checkpoint inhibition [44,45,46]. Main randomized clinical trials testing ICI in TNBC are summarized in Table 1.
Immunotherapy alone in TNBC has low response rates, especially in later lines of therapy [56,57,58,59] and combination treatments have demonstrated more activity in metastatic TNBC.
The anti-PD-L1 antibody atezolizumab in combination with nab-paclitaxel has been proven superior to nab-paclitaxel alone in previously untreated, PD-L1-positive, advanced TNBC patients and is currently standard of care, as reported above [51••]. Surprisingly, a recent press-release reporting results from the IMpassion-131 trial about the combination of atezolizumab with paclitaxel in the same setting did not confirm the positive findings of IMpassion-130: further data are awaited to better understand the reason of this discrepancy.
Anti-PD1 antibody pembrolizumab in combination with chemotherapy (taxanes [paclitaxel or nab-paclitaxel] or carboplatin plus gemcitabine) was evaluated in the phase III KEYNOTE-355 trial, where 847 metastatic TNBC patients were randomized to receive first-line therapy with chemotherapy plus pembrolizumab or placebo [54]. The co-primary endpoints were PFS and OS in the PD-L1-positive population (combined positive score [CPS] ≥10 and ≥1) and in the overall population, with a hierarchical testing for PFS. The predefined significance threshold for PFS was met in the CPS≥10 population. OS data were still immature. In Keynote-119, pembrolizumab alone failed to prove superiority to investigator’s choice of chemotherapy in pre-treated metastatic TNBC patients [53]. As an exception, pembrolizumab alone is approved by US Food and Drug Administration (FDA) for patients with treatment-refractory, mismatch repair deficient tumors (<2% of TNBC cases), based on the efficacy results of tumor-agnostic basket trials [60, 61]. Altogether, in the metastatic setting, immunotherapy seems to provide benefit to a subgroup of TNBC patients selected on PD-L1 expression and the benefit seems larger when combined with chemotherapy in first-line. However, many questions related to ICIs in metastatic TNBC, such as more precise predictive biomarkers and comparative data on the optimal chemotherapy partner, remain unanswered.
Ongoing studies (Table 2) are trying to expand the benefits of immunotherapy in TNBC patients, both anticipating its use in earlier disease settings (adjuvant and neoadjuvant) and going beyond PD-L1 positivity (e.g., TILs enrichment, tumor mutational burden [TMB]) [46, 62].
The addition of ICIs to chemotherapy in the neoadjuvant setting has shown conflicting results (Table 1). In the GeparNuevo trial, the addition of durvalumab to NAC did not significantly improve pCR rates in the ITT population, although in the window cohort (induction durvalumab prior to chemotherapy), better pCR rates were attained [49]. Also the addition of atezolizumab to neoadjuvant nab-paclitaxel plus carboplatin showed no pCR improvement, albeit the trial’s primary endpoint was EFS, yet to be reported [48]. Conversely, the adaptive phase 2 trial I-SPY2 met its primary endpoint of improved pCR by adding pembrolizumab to NAC [50]. KEYNOTE-522 confirmed this benefit in phase III setting by demonstrating a significant increase in pCR rates by the addition of pembrolizumab to neoadjuvant platinum-containing taxane-anthracycline regimen (51.2% to 64.8%) with an early trend towards EFS benefit [47•]. The pCR benefit was irrespective of PD-L1 status.
Furthermore, in the post-neoadjuvant and adjuvant setting, ICIs could represent a treatment option for TNBC, which is being explored in ongoing trials (Table 2).
New Target Therapies in TNBC Treatment
Antibody-Drug Conjugates
Novel ADCs are opening new horizons in TNBC. Sacituzumab-govitecan is an ADC composed of a topoisomerase I inhibitor (SN-38), which is an active metabolite of irinotecan, and an anti-Trop2 monoclonal antibody, linked together by a cleavable protein. Trop-2 is a trophoblast cell-surface antigen which is expressed on the surface of many epithelial cancer cells, including TNBC, and its activation induces cell growth. Sacituzumab-govitecan has been evaluated in a phase I/II study in patients with advanced epithelial cancer. Overall, 108 metastatic, heavily pre-treated TNBC patients received sacituzumab-govitecan, with durable objective responses (33.3% objective response rate (ORR) with a median duration of response of 7.7 months) [63••]. Based on this data, the FDA recently granted accelerated approval to sacituzumab-govitecan for pre-treated metastatic TNBC patients. A phase III study (ASCENT study) comparing sacituzumab-govitecan with single-agent chemotherapy of physician’s choice was terminated early because of compelling efficacy and results are expected soon [64]. The availability of this new treatment option has the potential to change the treatment landscape of TNBC, since it is under evaluation as single agent and in combination in several settings in TNBC.
Another ADC being evaluated in TNBC treatment is ladiratuzumab-vedotin, an anti-LIV1 antibody combined with a microtubule-disrupting agent (MMAE) through a cleavable linker [65]. LIV-1 is a protein expressed on several cancer cells, including TNBC. In a phase I/II study of ladiratuzumab-vedotin in combination with pembrolizumab, 32 patients with first-line TNBC were enrolled in the dose-expansion phase. The combination was tolerable and showed encouraging clinical activity (ORR of 54%) [65].
The HER2-targeted ADC trastuzumab-deruxtecan has shown promising signs of efficacy in an early-phase trial of HER2-low BC (defined as HER2 immunohistochemistry 1+, or 2+ without HER2 amplification per ASCO/CAP guidelines) [66, 67]. Approximately 17% of HER2-low BC are TNBC [68]. A phase III trial in HER2-low BC with trastuzumab-deruxtecan vs. chemotherapy of investigator’s choice is ongoing [69].
PI3K/AKT/mTOR Pathway Inhibitors
The PI3K/AKT/mTOR pathway is often activated in TNBC, mainly due to activating mutations of PI3K catalytic subunit PIK3CA, AKT1 or loss of function of PTEN [70, 71]. AKT is a key effector in PI3K/AKT/mTOR pathway and mediates cell proliferation and survival. Capivasertib, a pan-AKT inhibitor, was evaluated in a randomized phase II trial, in combination with paclitaxel, as first-line treatment for patients with metastatic TNBC [72]. Addition of capivasertib resulted in significantly improved PFS and OS, compared to placebo (HR 0.74, 95%CI 0.50-1.08 and HR 0.61, 95%CI 0.37-0.99, respectively) and benefits were more pronounced in patients with PIK3CA/AKT1/PTEN-altered tumors. These results are consistent with those of the LOTUS trial, a phase II study evaluating the AKT inhibitor ipatasertib in combination with paclitaxel as first-line treatment for metastatic TNBC. The trial showed an increase in median PFS (from 4.9 to 6.2 months in the ITT population, and from 4.9 to 9.0 months in the PIK3CA/AKT1/PTEN altered population) and a trend towards improved OS, supporting the role of AKT inhibitors in TNBC [73]. Phase III studies testing capivasertib, ipatasertib, and alpelisib (CAPItello-290 [NCT03997123], IPATunity130 [NCT03337724] and EPIK-B3 [NCT04251533]) in addition to (nab)-paclitaxel for metastatic TNBC are ongoing.
In a phase II trial, neoadjuvant ipatasertib with 12 weekly paclitaxel did not increase pCR rates compared to placebo/paclitaxel [74]. Nonetheless, MRI-assessed responses, a secondary endpoint, favored ipatasertib/paclitaxel, especially in the PIK3CA/AKT1/PTEN-altered population. Importantly, gastrointestinal adverse events (AE), especially diarrhoea, seem to dominate the toxicity profile of AKT inhibitors [72,73,74].
Other drugs targeting the PI3K/AKT/mTOR pathway are being evaluated in TNBC such as mTOR- and dual inhibitors, and combinations with immunotherapy (NCT02616848, NCT04177108).
Cyclin-Dependent Kinase (CDK) Inhibitors
Targeting the cellular machinery responsible for cell cycle regulation has already been proven beneficial in luminal BC, with diverse CDK4/6-inhibitors (CDK4/6i) showing markedly survival benefit in combination with endocrine therapy [75]. In TNBC, the loss of the tumor suppressor retinoblastoma (Rb), an in vitro biomarker of sensitivity to CDK4/6i, is a common event, especially in basal-like TNBC, explaining the observed lower activity of CDK4/6i in vitro in TNBC compared with luminal models [7, 76, 77]. Moreover, targeting CDK4/6 with palbociclib has actually been shown to antagonize the cytotoxic effect of paclitaxel in Rb-positive TNBC cell lines, possibly due to the lower sensitivity to the cytotoxic effect upon tumor cell cycle arrest. While in unselected TNBC, CDK4/6i alone or in combination with chemotherapy does not seem to be a venue worth exploring, there is a phase II trial ongoing with abemaciclib in Rb-positive metastatic TNBC (NCT03130439). Combinations with antiandrogens and PI3K-inhibitors are discussed later.
Trilaciclib, an intravenous CDK4/6i, was tested in a phase II trial where 102 metastatic TNBC patients were randomized to either chemotherapy alone (carboplatin plus gemcitabine) or to two different schedules of the same chemotherapy and trilaciclib [78]. The primary objective was to show an improvement in myelotoxicity-related endpoints in favor of the trilaciclib arms, since the drug can arrest hematopoietic progenitor cells at G0/G1 and thereby preserve them from the cytotoxic effect, which could be translated into an enhanced chemotherapy dose-intensity and anti-tumor immunity. The trial failed to show a superiority for the trilaciclib arms in terms of severe neutropenia incidence and duration. However, there was an important benefit in the secondary OS endpoint (median OS in the chemotherapy arm of 12.6 vs. 20.1 months in the trilaciclib plus chemotherapy arms combined). A potential explanation for this survival benefit might be an enhanced anti-tumor lymphocyte immunity with trilaciclib, as seen by an increment in T cell production of IFN-γ, and this warrants further studies.
With dinaciclib, a small-molecule inhibitor of CDK1/2/5/9, disappointing results were initially reported from a phase I study combining dinaciclib with epirubicin in TNBC [79]. In the preclinical setting, the inhibition of CDK1 in TNBC xenograft models resulted in synthetic lethality and block in tumor dissemination for models overexpressing MYC, an oncogene overexpressed in approximately 70% of TNBC and associated with poor prognosis [80]. Moreover, MYC-driven TNBC models are associated with an increased PD-1 expression on sTILs. Based on these preclinical data, a phase I study was conducted with dinaciclib and pembrolizumab in metastatic TNBC [81]. Preliminary efficacy analysis showed an ORR of 16.7% and a CBR of 46.7%. Interestingly, at an exploratory analysis, MYC expression correlated with treatment response. Further studies are needed to establish the role of MYC as a possible predictive biomarker.
Antiandrogens
Androgen receptor (AR) positivity occurs in about 24% of TNBC and is associated with a lower recurrence risk [82], supporting the hypothesis that AR-driven TNBC represent a distinct subtype. There is significant overlap between AR positivity and LAR TNBC subtype, which is enriched in lobular histology and frequently correlated with PIK3CA and AKT1 alterations [83, 84]. Bicalutamide and enzalutamide have been tested in phase II trials and shown proof-of-efficacy in AR-positive TNBC patients [85, 86]. Enzalutamide is also being evaluated in a phase III trial, both as single agent and combined with paclitaxel vs paclitaxel monotherapy in patients selected by a genomic signature for AR-driven disease [87].
AR-expression in TNBC tends to be associated with expression of Rb, a biomarker of sensitivity to CDK4/6i [88]. This supports the hypothesis of increased efficacy by combining androgen blockade with CDK4/6i as palbociclib. Results from a phase II study combining bicalutamide with palbociclib for AR-positive TNBC showed that this combination was safe and efficacious, with 11/33 patients progression-free at 6 months [89]. Additionally, antiandrogens combined with PIK3CA-inhibitors are under evaluation in AR and PTEN-positive metastatic BC (NCT03207529).
Other Immunotherapeutic Approaches
Purinergic Pathway Antagonists
The purine nucleoside adenosine exerts multiple immunosuppressive functions in the tumor micro-environment [90]. The ecto-enzyme CD73 is responsible for generating adenosine from a by-product of ATP, whilst adenosine receptors (A2R) initiate the adenosine intracellular signaling pathway. It has been shown that CD73 overexpression in TNBC is associated with lower sTILs and worse prognosis, whereas by the same time CD73 and A2R blockade inhibits BC cells growth and migration [91,92,93]. Therefore, compounds targeting the purinergic pathway are currently under clinical development in TNBC: oleclumab, an anti-CD73 monoclonal antibody, given together with durvalumab plus chemotherapy, is being compared with durvalumab plus chemotherapy in 2 phase I/II trials [94, 95]. An oral A2R inhibitor is also being studied in metastatic TNBC (NCT03207867).
Anti-cytotoxic T-Lymphocyte-Associated Protein 4 (CTLA4)
The combination of anti-PD1 nivolumab with anti-CTLA4 ipilimumab is currently being tested for TNBC in two phase II trials (NCT03789110, NCT03668119) [62]. Eligibility is restricted to patients with hypermutated tumors (TMB ≥10 mutations/megabase), a rare event in BC (5% of cases) [96]. These trials are expected to clarify the role of dual ICI and to provide a prospective evaluation of TMC as predictive biomarker in TNBC.
Innate Immune Activators
Imprime-PGG is an intravenously administered, yeast-derived beta-glucan, which is currently being evaluated in combination with ICIs for metastatic TNBC [97]. Imprime-PGG acts as a pathogen-associated molecular pattern activating the innate immune response against tumor cells by enhancing antigen presentation and T cell activation [98]. Imprime-PGG was evaluated in a phase II study (Imprime-1), where it was administered as first-line therapy in combination with pembrolizumab in patients with metastatic TNBC [97]. ORR was 13.6% and median OS was 13.7 months [97].
Angiogenesis Inhibition
By decreasing neovessel permeability and tumor interstitial pressure, antiangiogenic drugs facilitate chemotherapy delivery and exert synergistic effects with various chemotherapy agents [99]. Nevertheless, despite increasing median PFS in metastatic HER2-negative BC, the addition of bevacizumab, a monoclonal antibody against circulating vascular endothelial growth factor (VEGF) to chemotherapy never showed an OS benefit [100]. Together with a low cost-effectiveness and an increased rate of bleeding, thromboembolic and cardiovascular AE, bevacizumab’s approval for HER2-negative metastatic BC was revoked by the FDA in 2010, albeit the drug is still available for combination with paclitaxel as first-line therapy in Europe [99].
Nonetheless, different combinations with antiangiogenic agents may play a future role in the care of TNBC patients. Antiangiogenics have immunomodulatory properties and are able to increase lymphocytic infiltration into the tumor, hereby enhancing antitumor immune responses [101]. A phase II single-arm trial with 57 HER2-negative BC patients has explored the combination of bevacizumab, weekly paclitaxel and nivolumab in first-line [102]. The study met its primary endpoint, showing an ORR of 75.4% (83.3% in 18 patients with TNBC) [103]. A similar trial focusing on TNBC patients is ongoing (NCT04408118).
The angiogenesis pathway deeply interacts with DNA repair mechanisms, since tumor hypoxia induces DNA damage, genomic instability, and, ultimately, cell death. Therefore, antiangiogenics combined with DNA-repair inhibitors and/or ICIs might provide another synergistic approach [104, 105]. In a phase II study, cediranib, a pan-VEGF receptor tyrosine kinase inhibitor, was administered with olaparib in patients with advanced solid tumors including TNBC and resulted in objective responses in 14% of heavily pretreated metastatic TNBC. Toxicity profile was manageable, with gastrointestinal symptoms and hypertension among the most common AE [106].
TRK Inhibitors
Although extremely rare in unselected BC (<1% of cases), neurotrophic tyrosine receptor kinase (NTRK) oncogene fusions are described in ductal TNBC with secretory features and ETV6-NTRK3 fusions are pathognomonic in the rare secretory subtype that can present as TNBC [107]. These fusions can be efficiently targeted by the tropomyosin kinase protein (TRK) inhibitors larotrectinib and entrectinib. [108] In this sense, patients with NTRK fusion-positive TNBC are eligible for treatment with TRK inhibitors, based on efficacy data coming from various tumor-agnostic basket trials [109].
Histone Deacetylase Inhibitors
The acetylation of histone proteins induces the activation of genes mediating cell growth and proliferation, and histone deacetylases (HDAC) are often overexpressed in malignancies [110]. Thus far, HDAC inhibitors have shown limited activity as single agent in BC, and combination strategies are currently being tested. Entinostat, an oral HDAC inhibitor, in combination with atezolizumab was evaluated in a phase II study for patients with advanced TNBC. The combination did not improve PFS compared to placebo and was more toxic [111]. Therefore, the role of HDAC inhibitors in TNBC is yet to be defined.
Other Strategies
Several trials are evaluating further approaches in advanced TNBC (including, but not limited to, interleukin-7, NKTR-214, bispecific antibodies, STAT3-inhibitors, NOTCH-inhibitors, CXCR4-antagonists) whose results are eagerly awaited.
Conclusions
Recent evidence has introduced new therapies which have modified the treatment landscape from chemotherapy as only available treatment to a more personalized approach for TNBC: atezolizumab is now a standard of care for first-line advanced TNBC with PD-L1-positive tumors, PARPi are approved for BRCA-mutated advanced TNBC and recently sacituzumab-govitecan was FDA-approved for previously treated metastatic TNBC. Ongoing studies aim to broaden treatment indications for immunotherapy, PARP inhibitors, and ADC in TNBC, both anticipating their use in earlier disease settings (adjuvant and neoadjuvant) and going beyond the current limitations of PD-L1 positivity and gBRCA mutation for immunotherapy and PARPi, respectively. Several other target therapies are currently being evaluated, reflecting a promising evolution towards a more subtype-based approach in TNBC.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Howlader N, Noone AM, Krapcho M, Miller D, Brest A, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJ CK. Cancer Statistics Review, 1975-2016 - SEER Statistics. based on November 2018 SEER data submission, posted to the SEER web site, April 2019.
Dent R, Trudeau M, Pritchard KI, Hanna WM, Kahn HK, Sawka CA, et al. Triple-negative breast cancer: Clinical features and patterns of recurrence. Clin Cancer Res. 2007;13(15):4429–34. https://doi.org/10.1158/1078-0432.CCR-06-3045.
Caparica R, Lambertini M, de Azambuja E. How I treat metastatic triple-negative breast cancer. ESMO Open. 2019;4(Suppl 2):e000504. https://doi.org/10.1136/esmoopen-2019-000504.
Reis-Filho JS, Tutt ANJ. Triple negative tumours: A critical review. Vol. 52, Histopathology. Histopathology. 2008:108–18. https://doi.org/10.1111/j.1365-2559.2007.02889.x.
Foulkes WD, Smith IE, Reis-Filho JS. Triple-negative breast cancer. Vol. 363, New England Journal of Medicine. Massachussetts Medical Society. 2010:1938–48. https://doi.org/10.1056/NEJMra1001389.
Denkert C, Liedtke C, Tutt A, von Minckwitz G. Molecular alterations in triple-negative breast cancer—the road to new treatment strategies. The Lancet. Lancet Publishing Group. 2017;389:2430–42. https://doi.org/10.1016/S0140-6736(16)32454-0.
Lehmann BD, Bauer JA, Chen X, Sanders ME, Chakravarthy AB, Shyr Y, et al. Identification of human triple-negative breast cancer subtypes and preclinical models for selection of targeted therapies. J Clin Invest. 2011;121(7):2750–67. https://doi.org/10.1172/JCI45014.
Burstein MD, Tsimelzon A, Poage GM, Covington KR, Contreras A, Fuqua SAW, et al. Comprehensive genomic analysis identifies novel subtypes and targets of triple-negative breast cancer. Clin Cancer Res. 2015;21(7):1688–98. https://doi.org/10.1158/1078-0432.CCR-14-0432.
Cardoso F, Kyriakides S, Ohno S, Penault-Llorca F, Poortmans P, Rubio IT, et al. Early breast cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up†. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(8):1194–220. https://doi.org/10.1093/annonc/mdz173.
Cardoso F, Senkus E, Costa A, Papadopoulos E, Aapro M, André F, et al. 4th ESO-ESMO International Consensus Guidelines for Advanced Breast Cancer (ABC 4) †. Ann Oncol. 2018;29(8):1634–57. https://doi.org/10.1093/annonc/mdy192.
Paluch-Shimon S, Pagani O, Partridge AH, Abulkhair O, Cardoso MJ, Dent RA, et al. ESO-ESMO 3rd international consensus guidelines for breast cancer in young women (BCY3). Breast. 2017;35:203–17. https://doi.org/10.1016/j.breast.2017.07.017.
Poggio F, Bruzzone M, Ceppi M, Pondé NF, La Valle G, Del Mastro L, et al. Platinum-based neoadjuvant chemotherapy in triple-negative breast cancer: a systematic review and meta-analysis. Ann Oncol. 2018;29(7):1497–508. https://doi.org/10.1093/annonc/mdy127.
Balic M, Thomssen C, Würstlein R, Gnant M, Harbeck N. St. Gallen/Vienna. A brief summary of the consensus discussion on the optimal primary breast cancer treatment. Vol. 14, Breast Care. S. Karger AG. 2019;2019:103–10. https://doi.org/10.1159/000499931.
Torrisi R, Zuradelli M, Agostinetto E, Masci G, Losurdo A, De Sanctis R, et al. Platinum salts in the treatment of BRCA-associated breast cancer: A true targeted chemotherapy? Vol. 135, Critical Reviews in Oncology/Hematology. Elsevier Ireland Ltd; 2019. p. 66–75. Doi: https://doi.org/10.1016/j.critrevonc.2019.01.016
Zhang J, Yao L, Liu Y, Ouyang T, Li J, Wang T, et al. Impact of the addition of carboplatin to anthracycline-taxane-based neoadjuvant chemotherapy on survival in BRCA1/2-mutated triple-negative breast cancer. Int J cancer. 2020;148:941–9. https://doi.org/10.1002/ijc.33234.
Pandy JGP, Balolong-Garcia JC, Cruz-Ordinario MVB, Que FVF. Triple negative breast cancer and platinum-based systemic treatment: a meta-analysis and systematic review. BMC Cancer. 2019;19(1):1065. https://doi.org/10.1186/s12885-019-6253-5.
Yu K-D, Ye F-G, He M, Fan L, Ma D, Mo M, et al. Effect of Adjuvant Paclitaxel and Carboplatin on Survival in Women With Triple-Negative Breast Cancer: A Phase 3 Randomized Clinical Trial. JAMA Oncol. 2020;6:1390–6. https://doi.org/10.1001/jamaoncol.2020.2965.
•• Masuda N, Lee S-J, Ohtani S, Im Y-H, Lee E-S, Yokota I, et al. Adjuvant Capecitabine for Breast Cancer after Preoperative Chemotherapy. N Engl J Med. 2017;376(22):2147–59. https://doi.org/10.1056/NEJMoa1612645This trial demonstrated an improvement in survival by adding capecitabine in the adjuvant setting for HER2-negative BC with residual disease after neoadjuvant chemotherapy.
van Mackelenbergh M, Seither F, Möbus V, O’Shaugnessy J, Martin M, Joenssuu H, et al. Abstract GS1-07: Effects of capecitabine as part of neo-/adjuvant chemotherapy. A meta-analysis of individual patient data from 12 randomized trials including 15,457 patients. In: Cancer Research. American Association for Cancer Research (AACR); 2020. p. GS1-07-GS1-07.
Denduluri N, Chavez-MacGregor M, Telli ML, Eisen A, Graff SL, Hassett MJ, et al. Selection of Optimal Adjuvant Chemotherapy and Targeted Therapy for Early Breast Cancer: ASCO Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(23):2433–43. https://doi.org/10.1200/JCO.2018.78.8604.
Li J, Yu K, Pang D, Wang C, Jiang J, Yang S, et al. Adjuvant Capecitabine With Docetaxel and Cyclophosphamide Plus Epirubicin for Triple-Negative Breast Cancer (CBCSG010): An Open-Label, Randomized, Multicenter, Phase III Trial. J Clin Oncol. 2020;38(16):JCO.19.02474. https://doi.org/10.1200/JCO.19.02474.
Wang X, Wang S-S, Huang H, Cai L, Peng R-J, Zhao L, et al. Phase III trial of metronomic capecitabine maintenance after standard treatment in operable triple-negative breast cancer (SYSUCC-001). J Clin Oncol. 2020;38(15_suppl):507–507. https://doi.org/10.1200/JCO.2020.38.15_suppl.507.
Couch FJ, Hart SN, Sharma P, Toland AE, Wang X, Miron P, et al. Inherited Mutations in 17 Breast Cancer Susceptibility Genes Among a Large Triple-Negative Breast Cancer Cohort Unselected for Family History of Breast Cancer. J Clin Oncol. 2014;33(4):304–11. https://doi.org/10.1200/JCO.2014.57.1414.
Pothuri B. BRCA1- and BRCA2-related mutations: therapeutic implications in ovarian cancer. Ann Oncol. 2013;24:viii22–7. https://doi.org/10.1093/annonc/mdt307.
Nijman SMB. Synthetic lethality: General principles, utility and detection using genetic screens in human cells. FEBS Letters. Elsevier B.V. 2011;585:1–6. https://doi.org/10.1016/j.febslet.2010.11.024.
Kelley MR, Logsdon D, Fishel ML. Targeting DNA repair pathways for cancer treatment: What’s new? Future Oncology. Future Medicine Ltd. 2014;10:1215–37. https://doi.org/10.2217/fon.14.60.
Ledermann JA, Pujade-Lauraine E. Olaparib as maintenance treatment for patients with platinum-sensitive relapsed ovarian cancer. Therapeutic Advances in Medical Oncology. SAGE Publications Inc. 2019;11. https://doi.org/10.1177/1758835919849753.
•• Robson M, Im S-A, Senkus E, Xu B, Domchek SM, Masuda N, et al. Olaparib for Metastatic Breast Cancer in Patients with a Germline BRCA Mutation. N Engl J Med. 2017;377(6):523–33. https://doi.org/10.1056/NEJMoa1706450This trial led to approval of olaparib for metastatic BC in patient with a germline BRCA mutation.
•• Litton JK, Rugo HS, Ettl J, Hurvitz SA, Gonçalves A, Lee KH, et al. Talazoparib in patients with advanced breast cancer and a germline BRCA mutation. N Engl J Med. 2018;379(8):753–63. https://doi.org/10.1056/NEJMoa1802905This trial led to approval of talazoparib in patients with advanced BC and a germline BRCA mutation.
Robson ME, Tung N, Conte P, Im S-A, Senkus E, Xu B, et al. OlympiAD final overall survival and tolerability results: Olaparib versus chemotherapy treatment of physician’s choice in patients with a germline BRCA mutation and HER2-negative metastatic breast cancer. Ann Oncol. 2019 Apr 1;30(4):558–66. https://doi.org/10.1093/annonc/mdz012.
Litton J, Hurvitz S, Mina L, Rugo H. Talazoparib (TALA) in germline BRCA1/2 (gBRCA1/2)-mutated human epidermal growth factor receptor 2 negative (HER2-) advanced breast cancer (ABC): Final overall survival (OS) results from randomized Phase 3 EMBRACA trial. In: Proceedings of the 111th Annual Meeting of the American Association for Cancer Research; 2020 June 22-24. Philadelphia (PA): AACR; 2020.
Zimmer AS, Gillard M, Lipkowitz S, Lee JM. Update on PARP Inhibitors in Breast Cancer. Current Treatment Options in Oncology. Springer New York LLC. 2018;19. https://doi.org/10.1007/s11864-018-0540-2.
Diéras V, Han HS, Kaufman B, Wildiers H, Friedlander M, Ayoub J-P, et al. Veliparib with carboplatin and paclitaxel in BRCA-mutated advanced breast cancer (BROCADE3): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21:1269–82. https://doi.org/10.1016/S1470-2045(20)30447-2.
Papadimitriou M, Mountzios G, Papadimitriou CA. The role of PARP inhibition in triple-negative breast cancer: Unraveling the wide spectrum of synthetic lethality. Cancer Treat Rev. 2018 Jun 1;67:34–44. https://doi.org/10.1016/j.ctrv.2018.04.010.
Telli ML, Stover DG, Loi S, Aparicio S, Carey LA, Domchek SM, et al. Homologous recombination deficiency and host anti-tumor immunity in triple-negative breast cancer. Breast Cancer Res Treat. 2018;171(1):21–31. https://doi.org/10.1007/s10549-018-4807-x.
Chopra N, Tovey H, Pearson A, Cutts R, Toms C, Proszek P, et al. Homologous recombination DNA repair deficiency and PARP inhibition activity in primary triple negative breast cancer. Nat Commun. 2020;11(1):2662. https://doi.org/10.1038/s41467-020-16142-7.
Sharma P, Rodler E, Barlow WE, Gralow J, Huggins-Puhalla SL, Anders CK, et al. Results of a phase II randomized trial of cisplatin +/- veliparib in metastatic triple-negative breast cancer (TNBC) and/or germline BRCA -associated breast cancer (SWOG S1416). J Clin Oncol. 2020;38(15_suppl):1001–1001.
Mouw KW, Goldberg MS, Konstantinopoulos PA, D’Andrea AD. DNA damage and repair biomarkers of immunotherapy response. Cancer Discovery. American Association for Cancer Research Inc. 2017;7:675–93. https://doi.org/10.1158/2159-8290.CD-17-0226.
Hellmann MD, Nathanson T, Rizvi H, Creelan BC, Sanchez-Vega F, Ahuja A, et al. Genomic Features of Response to Combination Immunotherapy in Patients with Advanced Non-Small-Cell Lung Cancer. Cancer Cell. 2018;33(5):843-852.e4. https://doi.org/10.1016/j.ccell.2018.03.018.
Pusztai L, et al. Evaluation of durvalumab in combination with olaparib and paclitaxel in high-risk HER2 negative stage II/III breast cancer: Results from the I-SPY 2 TRIAL. In: Proceedings of the 111th Annual Meeting of the American Association for Cancer Research; 2020 June 22-24. Philadelphia (PA): AACR. p. 2020.
Budczies J, Bockmayr M, Denkert C, Klauschen F, Lennerz JK, Györffy B, et al. Classical pathology and mutational load of breast cancer - integration of two worlds. J Pathol Clin Res. 2015 Jul 20;1(4):225–38. https://doi.org/10.1002/cjp2.25.
Gao G, Wang Z, Qu X, Zhang Z. Prognostic value of tumor-infiltrating lymphocytes in patients with triple-negative breast cancer: a systematic review and meta-analysis. BMC Cancer. 2020;20(1):179. https://doi.org/10.1186/s12885-020-6668-z.
Loi S, Adams S, Schmid P, Cortés J, Cescon DW, Winer EP, et al. Relationship between tumor infiltrating lymphocyte (TIL) levels and response to pembrolizumab (pembro) in metastatic triple-negative breast cancer (mTNBC): Results from KEYNOTE-086. Ann Oncol. 2017;28:v608. https://doi.org/10.1093/annonc/mdx440.005.
Adams S, Goldstein LJ, Sparano JA, Demaria S, Badve SS. Tumor infiltrating lymphocytes (TILs) improve prognosis in patients with triple negative breast cancer (TNBC). Oncoimmunology. 2015 Sep;4(9):e985930. https://doi.org/10.4161/2162402X.2014.985930.
Fuchs TL, Pearson A, Pickett J, Diakos C, Dewar R, Chan D, et al. Why pathologists and oncologists should know about tumour-infiltrating lymphocytes (TILs) in triple-negative breast cancer: an Australian experience of 139 cases. Pathology. 2020 Jun;52:515–21. https://doi.org/10.1016/j.pathol.2020.04.004.
Loi S, Winer E, Lipatov O, Im S-A, Goncalves A, Cortes J, et al. Abstract PD5-03: Relationship between tumor-infiltrating lymphocytes (TILs) and outcomes in the KEYNOTE-119 study of pembrolizumab vs chemotherapy for previously treated metastatic triple-negative breast cancer (mTNBC). Cancer Res. 2020;80(4 Supplement):PD5-03 LP-PD5-03. https://doi.org/10.1158/1538-7445.SABCS19-PD5-03.
• Schmid P, Cortes J, Pusztai L, McArthur H, Kümmel S, Bergh J, et al. Pembrolizumab for early triple-negative breast cancer. N Engl J Med. 2020;382(9):810–21. https://doi.org/10.1056/NEJMoa1910549This trial showed a significant increase in pathological complete response with the addition of pembrolizumab to neoadjuvant chemotherapy for TNBC patients.
Gianni L, Huang C-S, Egle D, Bermejo B, Zamagni C, Thill M, et al. Abstract GS3-04: Pathologic complete response (pCR) to neoadjuvant treatment with or without atezolizumab in triple negative, early high-risk and locally advanced breast cancer. NeoTRIPaPDL1 Michelangelo randomized study. AACR. 2020.
Loibl S, Untch M, Burchardi N, Huober J, Sinn BV, Blohmer J-U, et al. A randomised phase II study investigating durvalumab in addition to an anthracycline taxane-based neoadjuvant therapy in early triple-negative breast cancer: clinical results and biomarker analysis of GeparNuevo study. Ann Oncol. 2019 Aug 1;30(8):1279–88. https://doi.org/10.1093/annonc/mdz158.
Nanda R, Liu MC, Yau C, Shatsky R, Pusztai L, Wallace A, et al. Effect of Pembrolizumab Plus Neoadjuvant Chemotherapy on Pathologic Complete Response in Women With Early-Stage Breast Cancer: An Analysis of the Ongoing Phase 2 Adaptively Randomized I-SPY2 Trial. JAMA Oncol. 2020 May 1;6(5):676–84. https://doi.org/10.1001/jamaoncol.2019.6650.
•• Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab and nab-paclitaxel in advanced triple-negative breast cancer. N Engl J Med. 2018;379(22):2108–21. https://doi.org/10.1056/NEJMoa1809615This trial led to approval of atezolizumab and nab-paclitaxel as first-line of treatment in advanced, PD-L1-positive TNBC patients.
Schmid P, Rugo HS, Adams S, Schneeweiss A, Barrios CH, Iwata H, et al. Atezolizumab plus nab-paclitaxel as first-line treatment for unresectable, locally advanced or metastatic triple-negative breast cancer (IMpassion130): updated efficacy results from a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2020;21(1):44–59. https://doi.org/10.1016/S1470-2045(19)30689-8.
Cortés J, Lipatov O, Im S-A, Gonc¸alves A, Lee KS, Schmid P, et al. KEYNOTE-119: Phase III study of pembrolizumab (pembro) versus single-agent chemotherapy (chemo) for metastatic triple negative breast cancer (mTNBC). Annals of Oncology. 2019;30(suppl_5):v851–934. https://doi.org/10.1093/annonc/mdz394.
Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. KEYNOTE-355: Randomized, double-blind, phase III study of pembrolizumab + chemotherapy versus placebo + chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer. J Clin Oncol. 2020;38(15_suppl):1000. https://doi.org/10.1200/JCO.2020.38.15_suppl.1000.
Dalenc F, Garberis I, Filleron T, Lusque A, Bachelot T, Arnedos M, et al. Abstract GS3-02: Durvalumab compared to maintenance chemotherapy in patients with metastatic breast cancer: Results from phase II randomized trial SAFIR02-IMMUNO. AACR. 2020. https://doi.org/10.1158/1538-7445.SABCS19-GS3-02.
Dirix LY, Takacs I, Jerusalem G, Nikolinakos P, Arkenau HT, Forero-Torres A, et al. Avelumab, an anti-PD-L1 antibody, in patients with locally advanced or metastatic breast cancer: A phase 1b JAVELIN solid tumor study. Breast Cancer Res Treat. 2018;167(3):671–86. https://doi.org/10.1007/s10549-017-4537-5.
Adams S, Schmid P, Rugo HS, Winer EP, Loirat D, Awada A, et al. Pembrolizumab monotherapy for previously treated metastatic triple-negative breast cancer: cohort A of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):397–404. https://doi.org/10.1093/annonc/mdy517.
Emens LA, Cruz C, Eder JP, Braiteh F, Chung C, Tolaney SM, et al. Long-term Clinical Outcomes and Biomarker Analyses of Atezolizumab Therapy for Patients with Metastatic Triple-Negative Breast Cancer: A Phase 1 Study. JAMA Oncol. 2019;5(1):74–82. https://doi.org/10.1001/jamaoncol.2018.4224.
Adams S, Loi S, Toppmeyer D, Cescon DW, De Laurentiis M, Nanda R, et al. Pembrolizumab monotherapy for previously untreated, PD-L1-positive, metastatic triple-negative breast cancer: cohort B of the phase II KEYNOTE-086 study. Ann Oncol. 2019;30(3):405–11. https://doi.org/10.1093/annonc/mdy518.
Lemery S, Keegan P, Pazdur R. First FDA Approval Agnostic of Cancer Site — When a Biomarker Defines the Indication. N Engl J Med. 2017 Oct 11;377(15):1409–12. https://doi.org/10.1056/NEJMp1709968.
Cheng AS, Leung SCY, Gao D, Burugu S, Anurag M, Ellis MJ, et al. Mismatch repair protein loss in breast cancer: clinicopathological associations in a large British Columbia cohort. Breast Cancer Res Treat. 2020;179(1):3–10. https://doi.org/10.1007/s10549-019-05438-y.
Barroso-Sousa R, Trippa L, Lange P, Andrews C, McArthur HL, Haley BB, et al. Nimbus: A phase II study of nivolumab plus ipilimumab in metastatic hypermutated HER2-negative breast cancer. J Clin Oncol. 2019;37(15_suppl):TPS1115–TPS1115. https://doi.org/10.1200/JCO.2019.37.15_suppl.TPS1115.
•• Bardia A, Mayer IA, Vahdat LT, Tolaney SM, Isakoff SJ, Diamond JR, et al. Sacituzumab Govitecan-hziy in Refractory Metastatic Triple-Negative Breast Cancer. N Engl J Med. 2019;380(8):741–51. https://doi.org/10.1056/NEJMoa1814213This trial led to approval of sacituzumab govitecan in refractory metastatic TNBC patients.
ASCENT-Study of Sacituzumab Govitecan in Refractory/Relapsed Triple-Negative Breast Cancer - ClinicalTrials.gov. https://clinicaltrials.gov/ct2/show/NCT02574455
Han H (Heather), Diab S, Alemany C, Basho R, Brown-Glaberman U, Meisel J, et al. Abstract PD1-06: Open label phase 1b/2 study of ladiratuzumab vedotin in combination with pembrolizumab for first-line treatment of patients with unresectable locally-advanced or metastatic triple-negative breast cancer. In: Cancer Research. American Association for Cancer Research (AACR); 2020. p. PD1-06-PD1-06. https://doi.org/10.1158/1538-7445.SABCS19-PD1-06.
Modi S, Saura C, Yamashita T, Park YH, Kim SB, Tamura K, et al. Trastuzumab deruxtecan in previously treated HER2-positive breast cancer. N Engl J Med. 2020;382(7):610–21. https://doi.org/10.1056/NEJMoa1914510.
Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, et al. Human Epidermal Growth Factor Receptor 2 Testing in Breast Cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. Arch Pathol Lab Med. 2018;142(11):1364–82. https://doi.org/10.5858/arpa.2018-0902-SA.
Fehrenbacher L, Cecchini RS, Geyer CE, Rastogi P, Costantino JP, Atkins JN, et al. NSABP B-47/NRG Oncology Phase III Randomized Trial Comparing Adjuvant Chemotherapy With or Without Trastuzumab in High-Risk Invasive Breast Cancer Negative for HER2 by FISH and With IHC 1+ or 2. J Clin Oncol. 2020;38(5):444–53. https://doi.org/10.1200/JCO.19.01455.
Modi S, Ohtani S, Lee C, Wang Y, Saxena K, Cameron DA. Abstract OT1-07-02: A phase 3, multicenter, randomized, open-label trial of [fam-] trastuzumab deruxtecan (T-DXd; DS-8201a) vs investigator’s choice in HER2-low breast cancer (DESTINY-Breast04). Cancer Res. 2020;80(4 Supplement):OT1-07-02 LP-OT1-07–02. https://doi.org/10.1158/1538-7445.SABCS19-OT1-07-02.
Cossu-Rocca P, Orrù S, Muroni MR, Sanges F, Sotgiu G, Ena S, et al. Analysis of PIK3CA Mutations and Activation Pathways in Triple Negative Breast Cancer. PLoS One. 2015;10(11):e0141763. https://doi.org/10.1371/journal.pone.0141763.
Costa RLB, Han HS, Gradishar WJ. Targeting the PI3K/AKT/mTOR pathway in triple-negative breast cancer: a review. Breast Cancer Res Treat. 2018;169(3):397–406. https://doi.org/10.1007/s10549-018-4697-y.
Schmid P, Abraham J, Chan S, Wheatley D, Brunt AM, Nemsadze G, et al. Capivasertib Plus Paclitaxel Versus Placebo Plus Paclitaxel As First-Line Therapy for Metastatic Triple-Negative Breast Cancer: The PAKT Trial. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(5):423–33. https://doi.org/10.1200/JCO.19.00368.
Dent R, Oliveira M, Isakoff SJ, Im S-A, Espié M, Blau S, et al. 139O Final results of the double-blind placebo (PBO)-controlled randomised phase II LOTUS trial of first-line ipatasertib (IPAT) + paclitaxel (PAC) for inoperable locally advanced/metastatic triple-negative breast cancer (mTNBC). Ann Oncol. 2020;31:S64–S5. https://doi.org/10.1016/j.annonc.2020.03.239.
Oliveira M, Saura C, Nuciforo P, Calvo I, Andersen J, Passos-Coelho JL, et al. FAIRLANE, a double-blind placebo-controlled randomized phase II trial of neoadjuvant ipatasertib plus paclitaxel for early triple-negative breast cancer. Ann Oncol. 2019;30(8):1289–97. https://doi.org/10.1093/annonc/mdz177.
Spring LM, Wander SA, Andre F, Moy B, Turner NC, Bardia A. Cyclin-dependent kinase 4 and 6 inhibitors for hormone receptor-positive breast cancer: past, present, and future. Lancet. 2020;395(10226):817–27. https://doi.org/10.1016/S0140-6736(20)30165-3.
Finn RS, Dering J, Conklin D, Kalous O, Cohen DJ, Desai AJ, et al. PD 0332991, a selective cyclin D kinase 4/6 inhibitor, preferentially inhibits proliferation of luminal estrogen receptor-positive human breast cancer cell lines in vitro. Breast Cancer Res. 2009;11(5):R77. https://doi.org/10.1186/bcr2419.
Herschkowitz JI, He X, Fan C, Perou CM. The functional loss of the retinoblastoma tumour suppressor is a common event in basal-like and luminal B breast carcinomas. Breast Cancer Res. 2008;10(5):R75. https://doi.org/10.1186/bcr2142.
Tan AR, Wright GS, Thummala AR, Danso MA, Popovic L, Pluard TJ, et al. Trilaciclib plus chemotherapy versus chemotherapy alone in patients with metastatic triple-negative breast cancer: a multicentre, randomised, open-label, phase 2 trial. Lancet Oncol. 2019;20(11):1587–601. https://doi.org/10.1016/S1470-2045(19)30616-3.
Mitri Z, Karakas C, Wei C, Briones B, Simmons H, Ibrahim N, et al. A phase 1 study with dose expansion of the CDK inhibitor dinaciclib (SCH 727965) in combination with epirubicin in patients with metastatic triple negative breast cancer. Invest New Drugs. 2015;33(4):890–4. https://doi.org/10.1007/s10637-015-0244-4.
Horiuchi D, Kusdra L, Huskey NE, Chandriani S, Lenburg ME, Gonzalez-Angulo AM, et al. MYC pathway activation in triple-negative breast cancer is synthetic lethal with CDK inhibition. J Exp Med. 2012;209(4):679–96. https://doi.org/10.1084/jem.20111512.
Chien AJ, Rahmaputri S, Dittrich HF, Majure MC, Rugo HS, Melisko ME, et al. A phase Ib trial of the cyclin-dependent kinase inhibitor dinaciclib (dina) in combination with pembrolizumab (P) in patients with advanced triple-negative breast cancer (TNBC). J Clin Oncol. 2019;37(15_suppl):1072. https://doi.org/10.1200/JCO.2019.37.15_suppl.1072.
Wang C, Pan B, Zhu H, Zhou Y, Mao F, Lin Y, et al. Prognostic value of androgen receptor in triple negative breast cancer: A meta-analysis. Oncotarget. 2016;7(29):46482–91. https://doi.org/10.18632/oncotarget.10208.
Bareche Y, Venet D, Ignatiadis M, Aftimos P, Piccart M, Rothe F, et al. Unravelling triple-negative breast cancer molecular heterogeneity using an integrative multiomic analysis. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(4):895–902. https://doi.org/10.1093/annonc/mdy024.
Pascual J, Turner NC. Targeting the PI3-kinase pathway in triple-negative breast cancer. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(7):1051–60. https://doi.org/10.1093/annonc/mdz133.
Gucalp A, Tolaney S, Isakoff SJ, Ingle JN, Liu MC, Carey LA, et al. Phase II trial of bicalutamide in patients with androgen receptor-positive, estrogen receptor-negative metastatic Breast Cancer. Clin Cancer Res. 2013;19(19):5505–12. https://doi.org/10.1158/1078-0432.CCR-12-3327.
Traina TA, Miller K, Yardley DA, Eakle J, Schwartzberg LS, O’Shaughnessy J, et al. Enzalutamide for the Treatment of Androgen Receptor-Expressing Triple-Negative Breast Cancer. J Clin Oncol. 2018;36(9):884–90. https://doi.org/10.1200/JCO.2016.71.3495.
Dent R, Schmid P, Cortes J, Kim S-B, Andre F, Abramson V, et al. Abstract OT3-02-02: ENDEAR: A randomized international phase 3 study comparing the efficacy and safety of enzalutamide in combination with paclitaxel chemotherapy or as monotherapy vs placebo with paclitaxel in patients with advanced diagnostic-positive triple-negative breast cancer. In: Cancer Research. American Association for Cancer Research (AACR); 2017. p. OT3-02-02-OT3-02–02. https://doi.org/10.1158/1538-7445.SABCS16-OT3-02-02.
Patel JM, Goss A, Garber JE, Torous V, Richardson ET, Haviland MJ, et al. Retinoblastoma protein expression and its predictors in triple-negative breast cancer. npj Breast Cancer. 2020;6(1):19. https://doi.org/10.1038/s41523-020-0160-4.
Gucalp A, Boyle LA, Alano T, Arumov A, Gounder MM, Patil S, et al. Phase II trial of bicalutamide in combination with palbociclib for the treatment of androgen receptor (+) metastatic breast cancer. J Clin Oncol. 2020;38(15_suppl):1017. https://doi.org/10.1200/JCO.2020.38.15_suppl.1017.
Arab S, Hadjati J. Adenosine Blockage in Tumor Microenvironment and Improvement of Cancer Immunotherapy. Immune Netw. 2019;19(4):e23–e23. https://doi.org/10.4110/in.2019.19.e23.
Buisseret L, Pommey S, Allard B, Garaud S, Bergeron M, Cousineau I, et al. Clinical significance of CD73 in triple-negative breast cancer: multiplex analysis of a phase III clinical trial. Ann Oncol Off J Eur Soc Med Oncol. 2018;29(4):1056–62. https://doi.org/10.1093/annonc/mdx730.
Zhou X, Zhi X, Zhou P, Chen S, Zhao F, Shao Z, et al. Effects of ecto-5’-nucleotidase on human breast cancer cell growth in vitro and in vivo. Oncol Rep. 2007;17(6):1341–6. https://doi.org/10.3892/or.17.6.1341.
Beavis PA, Divisekera U, Paget C, Chow MT, John LB, Devaud C, et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc Natl Acad Sci U S A. 2013;110(36):14711–6. https://doi.org/10.1073/pnas.1308209110.
Maurer C, Eiger D, Velghe C, Aftimos PG, Maetens M, Gaye J, et al. 195TiP - SYNERGY: Phase I and randomized phase II trial to investigate the addition of the anti-CD73 antibody oleclumab to durvalumab, paclitaxel and carboplatin for previously untreated, locally recurrent inoperable or metastatic triple-negative breast c. Ann Oncol. 2019;30:iii62. https://doi.org/10.1093/annonc/mdz100.046.
Schmid P, Nunes AT, Lall R, D', Cruz C, Grinsted L, et al. Abstract OT3-01-01: BEGONIA: Phase Ib/II open-label, platform study of safety and efficacy of durvalumab, paclitaxel and other novel oncology therapy agents as first-line (1L) therapy in patients with metastatic triple negative breast cancer (mTNBC). Cancer Res. 2019;79(4 Supplement):OT3-01-01 LP-OT3-01–01. https://doi.org/10.1158/1538-7445.SABCS18-OT3-01-01.
Barroso-Sousa R, Jain E, Cohen O, Kim D, Buendia-Buendia J, Winer E, et al. Prevalence and mutational determinants of high tumor mutation burden in breast cancer. Ann Oncol. 2020 Mar 1;31(3):387–94. https://doi.org/10.1016/j.annonc.2019.11.010.
O’Day S, Borges VF, Chmielowski B, Rao RD, Abu-Khalaf MM, Stopeck A, et al. An open label, multicenter phase II study combining imprime PGG (PGG) with pembrolizumab (P) in previously treated metastatic triple-negative breast cancer (mTNBC). J Clin Oncol. 2019;37(15_suppl):2550. https://doi.org/10.1200/JCO.2019.37.15_suppl.2550.
Chan ASH, Jonas AB, Qiu X, Ottoson NR, Walsh RM, Gorden KB, et al. Imprime PGG-Mediated Anti-Cancer Immune Activation Requires Immune Complex Formation. PLoS One. 2016;11(11):e0165909. https://doi.org/10.1371/journal.pone.0165909.
Montero AJ, Escobar M, Lopes G, Glück S, Vogel C. Bevacizumab in the treatment of metastatic breast cancer: friend or foe? Curr Oncol Rep. 2012;14(1):1–11. https://doi.org/10.1007/s11912-011-0202-z.
Valachis A, Polyzos NP, Patsopoulos NA, Georgoulias V, Mavroudis D, Mauri D. Bevacizumab in metastatic breast cancer: a meta-analysis of randomized controlled trials. Breast Cancer Res Treat. 2010;122(1):1–7. https://doi.org/10.1007/s10549-009-0727-0.
de Aguiar RB, de Moraes JZ. Exploring the Immunological Mechanisms Underlying the Anti-vascular Endothelial Growth Factor Activity in Tumors. Front Immunol. 2019;10:1023. https://doi.org/10.3389/fimmu.2019.01023.
Ozaki Y, Mukohara T, Tsurutani J, Takahashi M, Matsumoto K, Futamura M, et al. Abstract PD1-03: A multicenter phase II study evaluating the efficacy of nivolumab plus paclitaxel plus bevacizumab triple-combination therapy as a first-line treatment in patients with HER2-negative metastatic breast cancer: WJOG9917B NEWBEAT trial. In: Cancer Research. American Association for Cancer Research (AACR); 2020. p. PD1-03-PD1-03. https://doi.org/10.1158/1538-7445.SABCS19-PD1-03.
Miles DW, Diéras V, Cortés J, Duenne A-A, Yi J, O’Shaughnessy J. First-line bevacizumab in combination with chemotherapy for HER2-negative metastatic breast cancer: pooled and subgroup analyses of data from 2447 patients. Ann Oncol. 2013;24(11):2773–80. https://doi.org/10.1093/annonc/mdt276.
Zimmer AS, Nichols E, Cimino-Mathews A, Peer C, Cao L, Lee M-J, et al. A phase I study of the PD-L1 inhibitor, durvalumab, in combination with a PARP inhibitor, olaparib, and a VEGFR1-3 inhibitor, cediranib, in recurrent women’s cancers with biomarker analyses. J Immunother cancer. 2019;7(1):197. https://doi.org/10.1186/s40425-019-0680-3.
Hegan DC, Lu Y, Stachelek GC, Crosby ME, Bindra RS, Glazer PM. Inhibition of poly(ADP-ribose) polymerase down-regulates BRCA1 and RAD51 in a pathway mediated by E2F4 and p130. Proc Natl Acad Sci U S A. 2010;107(5):2201–6. https://doi.org/10.1073/pnas.0904783107.
Hafez N, Soliman HH, Fu S, Gelmon KA, Abdul Razak AR, Munster PN, et al. Preliminary efficacy data of triple-negative breast cancer cohort of NCI 9881 study: A phase II study of cediranib in combination with olaparib in advanced solid tumors. J Clin Oncol. 2020;38(15_suppl):1077. https://doi.org/10.1200/JCO.2020.38.15_suppl.1077.
Del Castillo M, Chibon F, Arnould L, Croce S, Ribeiro A, Perot G, et al. Secretory Breast Carcinoma: A Histopathologic and Genomic Spectrum Characterized by a Joint Specific ETV6-NTRK3 Gene Fusion. Am J Surg Pathol. 2015;39(11):1458–67. https://doi.org/10.1097/PAS.0000000000000487.
Ross J, Chung J, Elvin J, Vergilio J-A, Ramkissoon S, Suh J, et al. Abstract P2-09-15: NTRK fusions in breast cancer: Clinical, pathologic and genomic findings. In: Cancer Research. American Association for Cancer Research (AACR); 2018. p. P2-09-15-P2-09–15. https://doi.org/10.1158/1538-7445.SABCS17-P2-09-15.
Drilon A. TRK inhibitors in TRK fusion-positive cancers. Ann Oncol Off J Eur Soc Med Oncol. 2019;30(Suppl_8):viii23–30. https://doi.org/10.1093/annonc/mdz282.
Garmpis N, Damaskos C, Garmpi A, Kalampokas E, Kalampokas T, Spartalis E, et al. Histone Deacetylases as New Therapeutic Targets in Triple-negative Breast Cancer: Progress and Promises. Cancer Genomics Proteomics. 2017;14(5):299–313. https://doi.org/10.21873/cgp.20041.
O’Shaughnessy J, Moroose RL, Babu S, Baramidze K, Chan D, Leitner SP, et al. Results of ENCORE 602 (TRIO025), a phase II, randomized, placebo-controlled, double-blinded, multicenter study of atezolizumab with or without entinostat in patients with advanced triple-negative breast cancer (aTNBC). J Clin Oncol. 2020;38(15_suppl):1014. https://doi.org/10.1200/JCO.2020.38.15_suppl.1014.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Elisa Agostinetto has no potential conflicts of interest.
Daniel Eiger: funding for his ESMO Fellowship (2018-2019) from Novartis. Speaker honoraria from Janssen.
Kevin Punie: Honoraria for advisory/consultancy roles for AstraZeneca, Eli Lilly, Novartis, Pfizer, Pierre Fabre, Hoffmann/La Roche, Teva and Vifor Pharma (paid to institution); speaker fees for Eli Lilly, Mundi Pharma, Novartis, Pfizer and Hoffmann/La Roche (paid to institution); research funding from Pfizer and Sanofi (paid to institution); travel support from AstraZeneca, Novartis, Pfizer, PharmaMar and Hoffmann/La Roche.
Evandro de Azambuja: Honoraria and advisory board from Roche/GNE, Novartis, Seattle Genetics and Zodiacs; Travel grants from Roche/GNE and GSK/Novartis; Research grant to my institution from Roche/GNE, AstraZeneca, GSK/Novartis, and Servier.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the author.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Breast Cancer
Rights and permissions
About this article
Cite this article
Agostinetto, E., Eiger, D., Punie, K. et al. Emerging Therapeutics for Patients with Triple-Negative Breast Cancer. Curr Oncol Rep 23, 57 (2021). https://doi.org/10.1007/s11912-021-01038-6
Accepted:
Published:
DOI: https://doi.org/10.1007/s11912-021-01038-6