Abstract
Discerning between primary brain tumor progression and treatment-related effect is a significant issue and a major challenge in neuro-oncology. The difficulty in differentiating tumor progression from treatment-related effects has important implications for treatment decisions and prognosis, as well as for clinical trial design and results. Conventional MRI is widely used to assess disease status, but cannot reliably distinguish between tumor progression and treatment-related effects. Several advanced imaging techniques are promising, but have yet to be prospectively validated for this use. This review explores two treatment-related effects, pseudoprogression and radiation necrosis, as well as the concept of pseudoresponse, and highlights several advanced imaging modalities and the evidence supporting their use in differentiating tumor progression from treatment-related effect.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Patients with brain tumors often develop new or worsening contrast-enhancing lesions on routine follow-up imaging, which may reflect tumor recurrence, treatment effect, or a combination of both. Discerning between tumor recurrence and treatment effect is a clinically significant issue and a major challenge in neuro-oncology. Treatment-related effects likely exist within a spectrum, with “pseudoprogression” reflecting subacute and often transient injury, and “radiation necrosis” reflecting later and more permanent damage. The difficulty in differentiating tumor progression from treatment-related effects has implications for individual patient treatment decisions and prognosis, as well as for clinical trial design and interpretation of results. This issue has become more pronounced since the incorporation of the concurrent use of temozolomide (TMZ) chemotherapy with radiotherapy into the standard of care for glioblastoma (GBM) [1, 2]. Although a tissue sampling procedure is the gold standard for differentiating tumor progression from treatment-related effects, it is associated with surgical risk, significant cost, and may be open to misinterpretation because of sampling error. Conventional MRI is widely used to assess disease status, but cannot reliably distinguish between tumor progression and treatment-related effects. Several advanced non-invasive imaging techniques are promising, but have yet to be prospectively validated to discern tumor recurrence from treatment-related effects [1], and are not incorporated into the current response assessment criteria [3•]. An opposing phenomenon, “pseudoresponse,” may occur in the setting of anti-angiogenic therapy, and mimics positive tumor response to treatment.
Management of Newly-Diagnosed Glioblastoma
The standard of care for patients with newly-diagnosed GBM consists of safe, maximum tumor resection followed by radiotherapy (RT) with concurrent and adjuvant TMZ chemotherapy. This protocol was established by a relatively recent landmark trial which demonstrated the superiority of chemoradiotherapy (chemoRT) compared to radiotherapy alone, in patients with newly-diagnosed GBM [4]. Although no strict guidelines exist [1, 5], it is common practice for patients to undergo a “baseline” brain MRI within 48 h following surgery, and then a routine follow-up brain MRI scan about 4 weeks following completion of chemoRT, just prior to the anticipated initiation of adjuvant chemotherapy, to assess for treatment response, and to establish a new “baseline.” Subsequently, patients are scanned every 4 – 6 weeks to assess tumor status.
Treatment Response Criteria
One of the more difficult tasks in neuro-oncology is the interpretation of routine follow-up MRI scans, particularly the initial post-chemoRT scan. Conventional MRI cannot reliably differentiate tumor progression from treatment-related effects [1], as both entities destabilize the blood–brain barrier (BBB), resulting in nonspecific contrast enhancement and/or pericavitary non-enhancing T2/fluid attenuated inversion recovery (FLAIR) hyperintensity [6]. Further complicating the situation, lesions depicted on MRI often represent a mixture of both tumor recurrence and treatment-related changes. The gold standard for diagnosing glioma progression is histopathologic confirmation of active tumor on biopsied tissue samples [1]. However, biopsy is not always ideal, given the risk of surgical morbidity, significant cost, and potential for inaccurate diagnosis due to sampling error or presence of both disease and treatment effect.
Accurate identification of progressive disease (PD) and pseudoprogression (PsP) is crucial, as it determines important clinical management decisions. Early discontinuation of adjuvant (post-RT) TMZ chemotherapy due to PsP that is mistaken for PD may potentially negatively impact survival, as this may result in discontinuation of therapy that is actually working [1, 14, 15]. In fact, those patients with PsP may have a better prognosis. On the other hand, failure to recognize tumor progression may result in the inappropriate continuation of ineffective treatment. Further, the results of clinical trials may be skewed if patients with PsP are erroneously enrolled in protocols for tumor recurrence [1, 16]. As a consequence, most clinical trials exclude patients worsening within 12 weeks of completing chemoRT [17]. For the majority of cases of subacute imaging changes, cautious observation with early follow-up MRI is recommended. Adjuvant TMZ is typically continued, with consideration of a trial of dexamethasone if the patient is symptomatic [6]. Surgical resection can be considered for diagnostic and/or therapeutic purposes, with the additional caveat that early treatment-related effect symptoms are often transient.
The classic MacDonald response criteria define tumor progression as a greater than 25 % increase in enhancement on T1-weighted post-contrast MRI. However, both PD and treatment-related effects may appear as an increase in T1 post-contrast enhancement and T2/FLAIR peritumoral/pericavitary hyperintensity [3•, 7, 8], and so are frequently indistinguishable on structural MRI [9]. Therefore, post-treatment post-contrast MRI enhancement is not a reliable surrogate of treatment response [3•, 10]. The intensification of standard treatment to include chemotherapy concurrently with RT further complicates the interpretation of MRI findings, as treatment-related effects may occur more frequently and earlier with concurrent chemoRT, versus RT alone [11–13]. Conversely, the use of anti-angiogenic therapy, frequently used in the recurrent setting, is associated with a decrease in enhancement on T1 post-contrast MRI that does not necessarily signify a treatment response.
The Response Assessment in Neuro-Oncology (RANO) criteria provide updated - but not yet validated - guidelines for assessing treatment response, and takes into account T2/FLAIR MRI changes in the assessment of both progression and response [14]. Additionally, in the 12-week period following completion of chemoRT, progression can only be defined in the setting of new enhancement beyond the 80 % isodose line or by unequivocal histopathologic confirmation of active tumor.
Pseudo-Progression
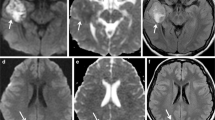
Pseudo-progression (PsP) is a subacute treatment-related effect with MRI features mimicking tumor progression. PsP typically develops within 3 months of completion of chemoRT, and may persist up to 6 months after completion of treatment [3•]. The presence or absence of symptoms does not differentiate PD from PsP, although several authors report PD to be more likely associated with symptoms than PsP [1]. For instance, Taal et al. found PD to be associated with symptoms in 67 % of patients vs. 33 % of patients with PsP (p = 0.094) [13]. PsP is usually diagnosed retrospectively, based on subsequent, spontaneous imaging improvement or stabilization without intervention [9]. Advanced MRI sequences such as perfusion MRI and MR spectroscopy may be helpful in differentiating treatment-related effect versus true PD (Fig. 1 and Fig. 2); however, formal criteria with these imaging techniques have not been established or validated. PsP may result from an inflammatory reaction [9], and likely reflects transient changes in BBB permeability resulting in edema and contrast enhancement without frank necrosis.
Pseudoprogression. MRI of the brain obtained 4 weeks after the completion of concurrent chemoradiation in a 56 year old male patient with glioblastoma. a) Axial T1 post contrast image shows nodular enhancement along the medial border of the resection cavity in the left temporal lobe. b) The corresponding ADC image demonstrates no restricted diffusion. c) MR perfusion (DSC) shows no increased in rCBV. d) MR spectroscopy shows a large lactate peak with marked decrease in other metabolites. These changes typically reflect post-treatment changes, in this case pseudoprogression, rather than true tumor progression
There is considerable variability in the incidence of PsP reported in retrospective studies. This is due to inconsistent definitions of PsP, the difficulty in differentiating PD from PsP [3•], the relatively recent widespread availability of MRI, and the somewhat recent incorporation of TMZ into the standard-of-care, which appears to have a radiosensitization effect [12]. PsP has been reported in 14 – 31 % of patients with treated malignant glioma [11, 12, 15–17]. In the largest retrospective series to date, Young et al. noted 93 (29 %) of 321 patients to have initial post-chemoRT MRI scans with new or increased enhancing lesions [15]. Of these 93 patients, 67.7 % were determined to have PD, and 32 % to have PsP.
Certain molecular markers may be associated with the development of PsP. Brandes et al. found that 91 % (21 of 23) of patients with MGMT promoter methylated GBM and early MRI changes had PsP [9]. Unfortunately, methylation status seems to be unreliable in predicting PsP or PD in an individual patient [15]. Nonetheless, PsP has been suggested as an indicator of better prognosis, an observation that may be explained by its association with MGMT promoter methylation, a known positive prognostic marker in GBM [1, 18] and was independently associated with improved survival (p = 0.045) [1]. Other markers such as P53 and Ki67 show a weaker association with PsP. Isocitrate dehydrogenase (IDH) 1 mutation is yet another potential factor [3•, 19].
Radiation Necrosis
Radiation necrosis is similar, but not synonymous with PsP [15]. Radiation necrosis is defined as a severe, post-treatment local tissue reaction with disruption of the BBB, with evidence of edema, and necrosis (typically absent in PsP), with or without mass effect on MRI. While PsP is a subacute effect, classically, RT necrosis is a “late” effect, arising 3 – 12 months after treatment, and has even been reported years later. The clinical course of RT necrosis is quite variable, ranging from an asymptomatic presentation to progressive focal deficits and signs of increased intracranial pressure that in severe cases may be fatal. Some cases may require medical management with dexamethasone or surgery for symptom control, while others may spontaneously recover [1]. In refractory cases, a trial of bevacizumab, a monoclonal anti-vascular endothelial growth factor (VEGF) antibody, may be helpful, and its use is supported by class 1 data [20–23].
RT necrosis was reported to occur in 5 – 25 % of patients in the pre-Stupp era [24]. As with PsP, these numbers may underestimate the current incidence. Ruben et al., in the largest series to date, described 426 glioma patients, with an incidence of RT necrosis of 4.9 % [25]. The occurrence of RT necrosis appears to be directly related to the RT field and RT dose [11]. A threshold of 54 Gy has been suggested [26]. Other risk factors include high dose fractions (i.e., >2.5 Gy/day), hyperfractionation (i.e., two fractions of 1 – 3 Gy/day), interstitial brachytherapy, stereotactic radiosurgery, reirradiation, and RT plus chemotherapy, including TMZ [11].
Pseudoprogression vs. Radiation Necrosis
There is no consensus regarding how these two entities are related. PsP and RT necrosis may reflect loosely defined time points along a pathologic continuum [3•, 6] (Fig. 2) of radiation damage. Differentiating the two entities by time from completion of RT is somewhat arbitrary [1], and some authors consider them to be discrete processes [6]. Although pseudoprogression has been noted to progress to RT necrosis in some cases [11, 13], there is evidence of a dichotomy between PsP and RT necrosis [1]. This view is supported by evidence that PsP is the result of injury to tumor, while RT necrosis is the result of injury to normal tissue. Kruser asserts the defining difference between these entities is that PsP is clinically diagnosed retrospectively by its transient features, while RT necrosis is histopathologically diagnosed and is typically permanent. Further, PsP is self-limited and characterized by subacute enhancement [6] secondary to a combination of treatment effect on residual tumor cells and transient breakdown of the BBB [6]. RT necrosis, in contrast, is a later occurrence with histologic findings of demyelination, vascular abnormalities, and tissue necrosis [6].
Tumor progression. MRI of the brain obtained in a 62 year old patient with temporal lobe glioblastoma, status post-surgery, concurrent chemoradiation, and six cycles of adjuvant temozolomide, reveals a new lesion in the left thalamus. a) T2/FLAIR image shows hyperintensity in the left thalamus, b) with slight enhancement noted on corresponding T1 post-contrast image. c) There is a minimal increase in perfusion (permeability) as depicted by DCE, and d) DSC demonstrates an increase in perfusion (rCBV >2). e and f) MR spectroscopy reveals an increased choline-to-creatinine ratio (Cho:Cr >2). Together, these findings suggest this lesion represents a high-grade glioma such as glioblastoma
Imaging Features and Modalities
Conventional (Morphological/Structural) Imaging
Structural imaging in the form of conventional MRI is the standard imaging modality for assessing treatment response. Although associated with high sensitivity, its use is limited in discerning PsP from PD, as post-treatment contrast enhancement is non-specific (Fig. 1a, 2a, b).
Kumar et al. described certain conventional MRI features that favored the diagnosis of treatment-related effects, including conversion of a lesion from non-enhancing to enhancing following RT, a lesion distant from the primary tumor resection site, corpus callosum, or periventricular white matter involvement, “Swiss cheese” and “soap bubble” contrast-enhancement patterns [27]. However, other studies have either failed to validate these findings or reported contradictory results. Relatively small sample sizes may explain the inconsistencies, as may the confounding effects of additional treatment such as immunotherapy and gene therapy, which may also produce PsP, as well as antiangiogenic therapy, which may produce pseudoresponse (discussed below) [28•]. Of the 11 conventional MRI signs evaluated by Young et al [15]. only subependymal enhancement was predictive of PD (p = 0.001). Unfortunately, this sign is of low utility in the majority of patients, given factors including low sensitivity and low negative predictive value.
A combination of MRI features may offer more statistical power and thus better diagnostic accuracy in discerning treatment-related effects from PD [28•]. Mullins et al. evaluated 27 patients, and found that combining two conventional signs, involvement of the corpus callosum and multiple enhancing lesions, was useful (p = 0.02), as was combining three signs, corpus callosum involvement, multiple enhancing lesions, and crossing of the midline (p = 0.04) or subependymal spread (p = 0.01). However, they noted a lack of significance for subependymal spread alone (p = 0.26).
Advanced Imaging Techniques and Functional Imaging
There is great interest in developing better non-invasive methods to differentiate tumor from treatment-related effects, and several advanced imaging techniques are under investigation. Although some have shown promising results, none have been validated in prospective clinical trials.
Functional imaging provides important physiologic information that supplements the structural information gleaned from conventional imaging.
Diffusion Imaging
Diffusion weighted imaging (DWI) measures the degree of Brownian water diffusion within tissue, and allows calculation of the apparent diffusion coefficient (ADC), which represents the magnitude of water diffusion at the cellular level [1, 28•]. Tumor recurrence is associated with areas of high cellularity, restricting water mobility, and therefore decreased ADC, while RT necrosis is associated with increased water mobility and an increase in ADC [28•, 29] (Fig. 1).
Potential limitations to the usefulness of ADC include variation in diffusion weighting (the b-value) and the effects of processes such as gliosis. Edema, infiltration, and proliferation may also play a role in confounding the data. There appear to be limitations to the use of ADC when evaluating lesions containing both tumor and necrosis. ADC histogram analysis may provide additional information to clarify these mixed lesions [28•]. The sensitivity and specificity of DWI has not yet been fully determined, although limited evidence suggests the specificity may be less than with MRS [30].
Diffusion tensor imaging (DTI), a sophisticated version of DWI that measures the directionality of water diffusion in the white matter [31] and within each voxel in terms of fractional anisotrophy (FA), may also have potential. However, although FA may help differentiate among tumor grades, it may inconsistently differentiate between recurrence and necrosis [28•].
Perfusion Imaging
In brain tumors, magnetic resonance perfusion (MRP) can measure relative cerebral blood volume (rCBV) and evaluate vascularity and hemodynamics [6]. MRP rCBV findings during chemoRT may be predictive of survival in glioma [9] and post-chemoRT MRP rCBV findings may further help differentiate PD from PsP. The two most widely used methods of MRP, dynamic susceptibility contrast (DSC) MRI, and dynamic contrast enhanced (DCE) MRI, are discussed below.
DSC MRI
DSC MRI utilizes the T2*-weighted MRI signal drop caused by susceptibility effect of gadolinium-based contrast in brain tissue; the typical hemodynamic parameter include rCBV, relative peak height (rPH), and percentage of signal-intensity recovery (PSR) [28•]. Typically, a higher rCBV value [28•] and hyperperfusion is found in recurrence, due to increased metabolic activity and neo-angiogenesis from increased (VEGF) expression [32–34]. A lower rCBV value [28•], hypoperfusion, and ischemia-related changes from occlusive vasculopathy are associated with treatment-induced necrosis and pseudoprogression [35]. A caution to interpreting rCBV values is that rapidly growing tumor may exceed its blood supply and result in necrosis or hypoperfusion [6] (Fig. 1c and Fig. 2d).
There are several other limitations to consider. Values have differed between studies and optimal thresholds must be determined and validated prospectively [1] and the difficulty of interpreting mixed findings must be addressed [36, 37]. Another issue is the leaking of contrast into interstitial fluid, which may lead to underestimation of rCBV [38]. Ferumoxytol [39, 40], an alternative to gadolinium contrast, may distinguish between PD and PsP and be a useful prognostic tool [40]. Other limitations include cost and the availability of MRP in the community [36, 37].
DCE MRI
DCE MRI is a T1-based technique that reflects the overall perfusion, vascular permeability, and extracellular volume in tumors. There are few reports describing the application of the vascular permeability (K-trans) parameter, which is higher in tumor progression than treatment related changes, to differentiating PD from PsP; however, the quantification of hemodynamic parameters in DCE MRI is complex and prone to error. Further, DCE MRI is associated with poor temporal resolution and a limited area of lesion coverage [28•]. However, there are benefits to DCE MRI over DSC MRI, including better spatial resolution, which may provide an advantage for differentiating mixed lesion cases (Fig. 2d)
Magnetic Resonance Spectroscopy
Magnetic resonance spectroscopy (MRS) detects different metabolites in tissue [1], such as N-acetylaspartate (NAA), choline (Cho), creatinine (Cr), lipid, and lactate. MRS has been used to grade tumors (correlating with histologic outcomes) [28•], and may help discriminate PD from treatment-related effects based on the ratios of NAA, Cho and Cr [11, 28•]. High-grade gliomas demonstrate elevated Cho (due to increased cell membrane phospholipids)-to-Cr (Cho:Cr ratio >2) and decreased NAA; this is not seen in normal white matter or lesions related to treatment [1]. RT necrosis is associated with elevated lipid and lactate peaks [1], decreased NAA, and variable changes to Cho and Cr over time [6, 28•] (Fig. 1d and Fig. 2e, f)
Early studies utilizing single-voxel technique make findings difficult to interpret unless the lesion consisted of pure tumor or pure radiation necrosis. Multi-voxel techniques may improve sensitivity, specificity, and diagnostic accuracy [1]. Further, these multi-voxel techniques may capture abnormal spectra beyond the area of enhancement and may help delineate extent of infiltration, information which may be helpful in planning RT. Limitations for standard clinical practice include various technical factors making interpretation difficult, long scan time, cost and limited insurance coverage [28•].
Nuclear Medicine Imaging
Nuclear medicine utilizes radiopharmaceuticals – weakly radioactive compounds – to ascertain physiological properties of tissues. The rationale for using nuclear medicine technology to differentiate treatment-related effect from PD is that the increased metabolism of active tumor progression will result in higher uptake of tracer compared to uptake in treatment necrosis [28•]. The two types of nuclear medicine studies used for imaging brain lesions are positron emission tomography (PET) and single photon emission CT (SPECT), discussed below.
Positron Emission Tomography (PET)
PET imaging allows for evaluation of regional metabolic activity based on the degree of radiotracer uptake [28•]. Initial studies of 18F-labeled fluoro-deoxy-glucose (FDG)-PET, the most commonly used PET tracer, reported sensitivity and specificity in the range of 80 – 100 % for the differentiation of treatment-related changes and PD, but did not correlate with a histopathologic diagnosis [6]. More recent studies, correlating FDG-PET findings with histopathology, have been disappointing, with sensitivity and specificity as low as 40 % and 22 %, respectively [6]. FDG-PET likely has limited use in the brain due in part to the high glucose utilization of normal brain that results in high background activity [1], making interpretation difficult. Further, both high-grade glioma and inflammatory lesions such as treatment-related necrosis can demonstrate increased FDG-PET activity [1].
Such limitations led to the search for novel PET tracers with lower background brain activity [1], that may more accurately discern PD from PsP [28•, 41, 42]. One such tracer, 11C-MET, an amino acid tracer, may discriminate PD with better accuracy than FDG-PET, and may reliably determine tumor infiltration into normal tissue [43–45]. However, 11C-MET may be limited by its increased accumulation in necrotic tissue [28•], and by a relatively short half-life [6]. Other amino acid tracers under investigation include O-2-18F-fluoroethyl-L-tyrosine (18F-FET-PET), 3,4-dihydroxy-6-18F-fluoro-L-phenylalanine (18F-FDOPA), and 3-deoxy-3-18F-fluorothymidine (18F-FLT-PET) [6]. FLT-PET is DNA-based and appears to be a more specific marker of proliferation [6].
Other limitations of PET include confounding processes such as status epilepticus, which can increase glucose metabolism in the brain, relatively low spatial resolution that limits sensitivity, and the clinical risks of gamma radiation exposure [28•]. The combination of PET with CT may help to localize lesions better, discern normal brain from tumor better, and reduce scan time, although it does expose the patient to ionizing radiation and does not have adequate resolution for tissue discrimination. PET/MR is promising and is being developed [28•].
Multi-Modality Imaging
Single functional imaging modalities may not provide the diagnostic power necessary to differentiate between PsP and PD. In the scenario of mixed lesions of recurrence and treatment necrosis, relative abundances of tumor and necrosis may skew data and lead to erroneous interpretations.
Multi-modality functional imaging protocols have proven more successful in the quest to differentiate PD from PsP. One study reported that the ability to detect brain tumors increased from 68 % to 97 % when structural MRI was used along with FET-PET and MRS [46]. Another study reported strong discriminatory power with the use of MRS plus DWI [47]. Prat et al. suggested MRS and MRP as the most promising combination [48] for detecting recurrence, and discouraged the use of a single modality. Matsusue implemented a multiparametric protocol of diffusion imaging, perfusion imaging and MRS, and reported accuracy for differentiating PsP from PD significantly better than any single modality [49] (Figs. 1 and 2) This approach may be potentially limited by the low availability of scanners, high operative costs, and lack of coverage by insurance providers [28•].
Quantitative Approaches – Morphometric/Morphological Analysis
Radiographic images are typically interpreted through 2D quantitative and subjective visual inspection, which may lend to inter-observer variability and concern that granular differences between PD and treatment-related effects will be missed [28•]. Quantitative MRI research can extend from 3D-volumetric assessments to the sampling of image intensities in different tumor regions [50, 51]. Advanced approaches using morphologic analyses of features noted on structural and functional imaging is largely new territory in regards to differentiating treatment-related effects from PD, although it has been applied to noninvasive brain tumor grading and prognostication [52–56] (and Joshi, ABS Classification of Brain Cancer Using Artificial Neural Network, 2010, Verma, 135* [28•]). A quantitative approach could address many of the limitations of earlier studies attempting to distinguish treatment-related effects and PD, and provide additional useful information [28•].
Bevacizumab and Other Antiangiogenic Agents
Several antiangiogenic agents are in use or under investigation for the treatment of GBM, a highly vascularized tumor. VEGF has a role in endothelial cell survival, proliferation, invasion and migration, which affect tumor progression and angiogenesis [57•, 58]. Bevacizumab, the prototypical anti-angiogenic agent, is a recombinant monoclonal antibody that prevents the proliferation of endothelial cells and the formation of new blood vessels by inhibiting vascular endothelial growth factor (VEGF) [59].
Although bevacizumab is FDA-approved for the treatment of recurrent GBM, there are several concerns associated with its use. Bevacizumab has been shown to increase progression-free survival, but not overall survival. Further, GBM may respond to antiangiogenic agents with increased tumor invasiveness and vessel cooption, potentially resulting in a more aggressive tumor phenotype [60–62]. Moreover, only a portion of patients respond to antiangiogenic therapy, and “response” is not clearly defined [59]. It has been suggested that the responses seen in bevacizumab-treated patients may be due to improvement of vessel permeability rather than to true tumor response [20].
Bevacizumab and other anti-angiogenics add a layer of complexity to the interpretation of MRIs, given the concept of “pseudoresponse” [59]. Pseudoresponse is an improvement of contrast-enhancing lesions on MRI, despite a lack of actual tumor response. Bevacizumab may normalize tumor vasculature and stabilize the BBB, rapidly decreasing TI contrast enhancement and peritumoral edema, without necessarily decreasing tumor size or providing an anti-tumor effect [57•]. With bevacizumab treatment, GBM appears more likely to progress as non-enhancing tumor [57•], reflected as an increase in T2/FLAIR abnormality, without a concomitant increase in contrast enhancement.
While the importance of T2/FLAIR changes is increasingly recognized, the identification of other imaging techniques is under investigation. Recent research has shown DWI MRI and perfusion MRI CBV to be predictive MRI biomarkers for tumor recurrence and response to therapy in the setting of anti-angiogenesis modulators [63]. ADC values may be helpful as an early predictor of treatment failure by suggesting an increase of non-enhancing infiltrative tumor growth. For instance, decreased ADC in non-enhancing areas of the lesion suggests progressive disease, despite a decreased volume of contrast enhancing lesions (pseudoresponse). Hyperperfusion volume (HPV) can predict treatment response independent of contrast enhancement changes. FLT-PET may also be a predictive marker for recurrent tumor in patients treated with anti-VEGF therapies [64].
Conclusion
Distinguishing post-treatment effects from PD on routine follow-up MRI scan for patients with glioma is a key issue and a major challenge in neuro-oncology. Clinical awareness of this predicament is imperative, as it affects the implementation or discontinuation of treatments. Further, the possibility of PsP mimicking PD must be considered in clinical trial design, as the spontaneous improvement would otherwise be interpreted at treatment response, leading to falsely positive clinical trials. A second issue is uniformly defining PsP versus RT necrosis. A third area of investigation is the prognostic significance of PsP and the association with MGMT and possibly other markers. While biopsy is the gold standard for diagnosing PD from treatment-related effects, there is associated surgical risk and monetary cost. Although structural imaging is widely used to assess treatment response, it is unsatisfactory in determining the diagnosis in a timely manner. The presence or absence of symptoms is also not particularly helpful, as symptoms may be present in both PsP and PD. Novel, non-invasive, advanced imaging biomarkers are promising, but prospective evaluations are needed. Multiple modalities are suggested to improve accuracy of predictions. Anti-angiogenic therapies such as bevacizumab, and other treatment regimens such as immunotherapy and gene therapy, may further confound MRI response evaluation as they may result in “pseudoresponse” or PsP, respectively. Continued research exploring imaging biomarkers is necessary to reliably differentiate tumor progression from treatment-related changes.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Kruser TJ, Mehta MP, Robins HI. Pseudoprogression after glioma therapy: a comprehensive review. Expert Rev Neurother. 2013;13(4):389–403.
Peca C et al. Early clinical and neuroradiological worsening after radiotherapy and concomitant temozolomide in patients with glioblastoma: tumour progression or radionecrosis? Clin Neurol Neurosurg. 2009;111(4):331–4.
Linhares P et al. Early pseudoprogression following chemoradiotherapy in glioblastoma patients: the value of RANO evaluation. J Oncol. 2013;2013:690585. A study and discussion of pseudoprogression in the context of tumor response criteria.
Stupp R et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med. 2005;352(10):987–96.
Sanghera P et al. The concepts, diagnosis and management of early imaging changes after therapy for glioblastomas. Clin Oncol (R Coll Radiol). 2012;24(3):216–27.
Siu A et al. Radiation necrosis following treatment of high grade glioma–a review of the literature and current understanding. Acta Neurochir (Wien). 2012;154(2):191–201. discussion 201.
Sanghera P et al. Pseudoprogression following chemoradiotherapy for glioblastoma multiforme. Can J Neurol Sci. 2010;37(1):36–42.
Roldan GB et al. Population-based study of pseudoprogression after chemoradiotherapy in GBM. Can J Neurol Sci. 2009;36(5):617–22.
Brandes AA et al. MGMT promoter methylation status can predict the incidence and outcome of pseudoprogression after concomitant radiochemotherapy in newly diagnosed glioblastoma patients. J Clin Oncol. 2008;26(13):2192–7.
Hygino da Cruz Jr LC. Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. AJNR Am J Neuroradiol. 2011;32(11):1978–85.
Brandsma D et al. Clinical features, mechanisms, and management of pseudoprogression in malignant gliomas. Lancet Oncol. 2008;9(5):453–61.
Chamberlain MC et al. Early necrosis following concurrent Temodar and radiotherapy in patients with glioblastoma. J Neurooncol. 2007;82(1):81–3.
Taal W, et al. The incidence of pseudo-progression in a cohort of malignant glioma patients treated with chemo-radiation with temozolomide. J Clin Oncol, 2007. 25: (18_suppl 2009).
Wen PY et al. Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol. 2010;28(11):1963–72.
Young RJ et al. Potential utility of conventional MRI signs in diagnosing pseudoprogression in glioblastoma. Neurology. 2011;76(22):1918–24.
de Wit MC et al. Immediate post-radiotherapy changes in malignant glioma can mimic tumor progression. Neurology. 2004;63(3):535–7.
Taal W et al. Incidence of early pseudo-progression in a cohort of malignant glioma patients treated with chemoirradiation with temozolomide. Cancer. 2008;113(2):405–10.
Hegi ME et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352(10):997–1003.
Motegi H et al. IDH1 mutation as a potential novel biomarker for distinguishing pseudoprogression from true progression in patients with glioblastoma treated with temozolomide and radiotherapy. Brain Tumor Pathol. 2013;30(2):67–72.
Gonzalez J et al. Effect of bevacizumab on radiation necrosis of the brain. Int J Radiat Oncol Biol Phys. 2007;67(2):323–6.
Torcuator R et al. Initial experience with bevacizumab treatment for biopsy confirmed cerebral radiation necrosis. J Neurooncol. 2009;94(1):63–8.
Wong ET et al. Bevacizumab reverses cerebral radiation necrosis. J Clin Oncol. 2008;26(34):5649–50.
Levin VA et al. Randomized double-blind placebo-controlled trial of bevacizumab therapy for radiation necrosis of the central nervous system. Int J Radiat Oncol Biol Phys. 2011;79(5):1487–95.
Shah AH et al. Discriminating radiation necrosis from tumor progression in gliomas: a systematic review what is the best imaging modality? J Neurooncol. 2013;112(2):141–52.
Ruben JD et al. Cerebral radiation necrosis: incidence, outcomes, and risk factors with emphasis on radiation parameters and chemotherapy. Int J Radiat Oncol Biol Phys. 2006;65(2):499–508.
Marks JE et al. Cerebral radionecrosis: incidence and risk in relation to dose, time, fractionation and volume. Int J Radiat Oncol Biol Phys. 1981;7(2):243–52.
Kumar AJ et al. Malignant gliomas: MR imaging spectrum of radiation therapy- and chemotherapy-induced necrosis of the brain after treatment. Radiology. 2000;217(2):377–84.
Verma N et al. Differentiating tumor recurrence from treatment necrosis: a review of neuro-oncologic imaging strategies. Neuro Oncol. 2013;15(5):515–34. A discussion of structural and functional imaging modalities as they relate to the differentiation of tumor recurrence from treatment-related changes.
Sundgren PC et al. Differentiation of recurrent brain tumor versus radiation injury using diffusion tensor imaging in patients with new contrast-enhancing lesions. Magn Reson Imaging. 2006;24(9):1131–42.
Rock JP et al. Associations among magnetic resonance spectroscopy, apparent diffusion coefficients, and image-guided histopathology with special attention to radiation necrosis. Neurosurgery. 2004;54(5):1111–7. discussion 1117–9.
Le Bihan D et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13(4):534–46.
Aronen HJ, Perkio J. Dynamic susceptibility contrast MRI of gliomas. Neuroimaging Clin N Am. 2002;12(4):501–23.
Covarrubias DJ, Rosen BR, Lev MH. Dynamic magnetic resonance perfusion imaging of brain tumors. Oncologist. 2004;9(5):528–37.
Jain RK. Tumor angiogenesis and accessibility: role of vascular endothelial growth factor. Semin Oncol. 2002;29(6 Suppl 16):3–9.
Ellika SK et al. Role of perfusion CT in glioma grading and comparison with conventional MR imaging features. AJNR Am J Neuroradiol. 2007;28(10):1981–7.
Jain RK. Normalizing tumor vasculature with anti-angiogenic therapy: a new paradigm for combination therapy. Nat Med. 2001;7(9):987–9.
Jain RK, Tong RT, Munn LL. Effect of vascular normalization by antiangiogenic therapy on interstitial hypertension, peritumor edema, and lymphatic metastasis: insights from a mathematical model. Cancer Res. 2007;67(6):2729–35.
Gahramanov S et al. Improved perfusion MR imaging assessment of intracerebral tumor blood volume and antiangiogenic therapy efficacy in a rat model with ferumoxytol. Radiology. 2011;261(3):796–804.
Gahramanov S et al. Potential for differentiation of pseudoprogression from true tumor progression with dynamic susceptibility-weighted contrast-enhanced magnetic resonance imaging using ferumoxytol vs. gadoteridol: a pilot study. Int J Radiat Oncol Biol Phys. 2011;79(2):514–23.
Gahramanov S et al. Pseudoprogression of glioblastoma after chemo- and radiation therapy: diagnosis by using dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging with ferumoxytol versus gadoteridol and correlation with survival. Radiology. 2013;266(3):842–52.
Tsuyuguchi N et al. Methionine positron emission tomography for differentiation of recurrent brain tumor and radiation necrosis after stereotactic radiosurgery–in malignant glioma. Ann Nucl Med. 2004;18(4):291–6.
Rachinger W et al. Positron emission tomography with O-(2-[18F]fluoroethyl)-l-tyrosine versus magnetic resonance imaging in the diagnosis of recurrent gliomas. Neurosurgery. 2005;57(3):505–11. discussion 505–11.
Li DL et al. (1)(1)C-methionine and (1)(8)F-fluorodeoxyglucose positron emission tomography/CT in the evaluation of patients with suspected primary and residual/recurrent gliomas. Chin Med J (Engl). 2012;125(1):91–6.
Van Laere K et al. Direct comparison of 18F-FDG and 11C-methionine PET in suspected recurrence of glioma: sensitivity, inter-observer variability and prognostic value. Eur J Nucl Med Mol Imaging. 2005;32(1):39–51.
Chung JK et al. Usefulness of 11C-methionine PET in the evaluation of brain lesions that are hypo- or isometabolic on 18F-FDG PET. Eur J Nucl Med Mol Imaging. 2002;29(2):176–82.
Floeth FW et al. Multimodal metabolic imaging of cerebral gliomas: positron emission tomography with [18F]fluoroethyl-L-tyrosine and magnetic resonance spectroscopy. J Neurosurg. 2005;102(2):318–27.
Zeng QS et al. Distinction between recurrent glioma and radiation injury using magnetic resonance spectroscopy in combination with diffusion-weighted imaging. Int J Radiat Oncol Biol Phys. 2007;68(1):151–8.
Prat R et al. Relative value of magnetic resonance spectroscopy, magnetic resonance perfusion, and 2-(18F) fluoro-2-deoxy-D-glucose positron emission tomography for detection of recurrence or grade increase in gliomas. J Clin Neurosci. 2010;17(1):50–3.
Matsusue E et al. Distinction between glioma progression and post-radiation change by combined physiologic MR imaging. Neuroradiology. 2010;52(4):297–306.
Verma R et al. Multiparametric tissue characterization of brain neoplasms and their recurrence using pattern classification of MR images. Acad Radiol. 2008;15(8):966–77.
Hu X et al. Support vector machine multiparametric MRI identification of pseudoprogression from tumor recurrence in patients with resected glioblastoma. J Magn Reson Imaging. 2011;33(2):296–305.
Blanchet L et al. Discrimination between metastasis and glioblastoma multiforme based on morphometric analysis of MR images. AJNR Am J Neuroradiol. 2011;32(1):67–73.
Georgiadis P et al. Improving brain tumor characterization on MRI by probabilistic neural networks and non-linear transformation of textural features. Comput Methods Programs Biomed. 2008;89(1):24–32.
Georgiadis P et al. Enhancing the discrimination accuracy between metastases, gliomas and meningiomas on brain MRI by volumetric textural features and ensemble pattern recognition methods. Magn Reson Imaging. 2009;27(1):120–30.
Zacharaki EI et al. Classification of brain tumor type and grade using MRI texture and shape in a machine learning scheme. Magn Reson Med. 2009;62(6):1609–18.
Kassner A, Thornhill RE. Texture analysis: a review of neurologic MR imaging applications. AJNR Am J Neuroradiol. 2010;31(5):809–16.
Thompson EM, Frenkel EP, Neuwelt EA. The paradoxical effect of bevacizumab in the therapy of malignant gliomas. Neurology. 2011;76(1):87–93. A discussion of tumor progression in the context of antiangiogenic agents.
Wang Y et al. Biological activity of bevacizumab, a humanized anti-VEGF antibody in vitro. Angiogenesis. 2004;7(4):335–45.
Pope WB, Young JR, Ellingson BM. Advances in MRI assessment of gliomas and response to anti-VEGF therapy. Curr Neurol Neurosci Rep. 2011;11(3):336–44.
Du R et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13(3):206–20.
Rubenstein JL et al. Anti-VEGF antibody treatment of glioblastoma prolongs survival but results in increased vascular cooption. Neoplasia. 2000;2(4):306–14.
Kunkel P et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2. Cancer Res. 2001;61(18):6624–8.
Sawlani RN et al. Glioblastoma: a method for predicting response to antiangiogenic chemotherapy by using MR perfusion imaging–pilot study. Radiology. 2010;255(2):622–8.
Chen W et al. Predicting treatment response of malignant gliomas to bevacizumab and irinotecan by imaging proliferation with [18F] fluorothymidine positron emission tomography: a pilot study. J Clin Oncol. 2007;25(30):4714–21.
Compliance with Ethics Guidelines
Conflict of Interest
Barbara O’Brien and Rivka R. Colen declare that they have no conflict of interest
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neuro-oncology
Rights and permissions
About this article
Cite this article
O’Brien, B.J., Colen, R.R. Post-Treatment Imaging Changes in Primary Brain Tumors. Curr Oncol Rep 16, 397 (2014). https://doi.org/10.1007/s11912-014-0397-x
Published:
DOI: https://doi.org/10.1007/s11912-014-0397-x