Abstract
Endocrine therapy (ET) with aromatase inhibitors (AIs) has become the standard of care for postmenopausal women with hormone-receptor–positive (HR+) advanced breast cancer (ABC); however, progression following initial treatment remains a major clinical challenge given the large patient population, many of whom develop progressive disease. There is an unmet need for treatment strategies that can overcome endocrine resistance. Growth factor-mediated signaling pathways, such as the phosphatidylinositol-3-kinase/protein kinase B/mammalian target of rapamycin (PI3K/AKT/mTOR) pathway, contribute to estrogen-independent growth that may lead to endocrine resistance. Preclinical studies have demonstrated that the use of mTOR inhibitors, such as everolimus and temsirolimus, is a promising strategy to potentially enhance endocrine sensitivity in ABC. This review will focus on the current ET options for women with HR+ ABC who have progressed on prior AI therapy, the role of mTOR-mediated signaling in breast cancer, and the clinical evidence supporting the use of mTOR inhibitors.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Up to 75 % of invasive breast cancers (BCs) are classified as hormone-receptor–positive (HR+; defined as estrogen-receptor–positive and progesterone-receptor–positive [ER+/PgR+]) [1]. Endocrine therapy (ET), particularly with aromatase inhibitors (AIs) such as anastrozole, letrozole, and exemestane, has become the standard of care for patients with HR+ advanced breast cancer (ABC); however, endocrine resistance remains a major clinical challenge. Up to 50 % of patients with HR+ ABC have primary resistance and do not respond to initial ET [2]. In almost all women, even those responding to initial therapy, acquired or secondary resistance will lead to disease progression despite ET. Signaling through growth factor receptor pathways and second messengers that can activate estrogen receptor (ER)-mediated cellular processes are emerging as important causes of endocrine resistance. This review will focus on the current ET options for patients with HR+ ABC who have progressed on prior AI therapy, the role of mammalian target of rapamycin (mTOR)-mediated signaling in BC, and the clinical evidence supporting the use of mTOR inhibitors such as everolimus.
Current Treatment Options for HR+ ABC Following Progression on AI Therapy
International guidelines state that women with HR+ ABC progressing on initial ET may benefit from sequential use of ET [3•, 4•]. Chemotherapy is only recommended after three consecutive ET regimens with no clinical benefit, or in the presence of visceral crisis [4•]. Several ET switching approaches have been described [5]. Patients who have progressed on nonsteroidal AIs (eg, anastrozole and letrozole) may be switched to the steroidal AI exemestane, or to an ER down-regulator such as fulvestrant. Regardless of the sequence used, duration of benefit from second-line ET after an AI tends to be short (approximately 3–5 months).
Clinical trials of ET for ABC have mostly evaluated efficacy in patients pretreated with tamoxifen. Limited studies have evaluated ET for patients with ABC who have progressed on prior AI therapy (Table 1) [6–8]. The EFECT trial evaluated treatment with fulvestrant 250 mg/month (n = 351) versus exemestane 25 mg/day (n = 342) in postmenopausal women (PMW) with HR+ ABC who had progressed on nonsteroidal AI therapy in the adjuvant or first-line metastatic setting [6]. This trial failed to meet the primary endpoint, superiority of fulvestrant versus exemestane to extend time to progression (TTP); however, based on a retrospective non-inferiority analysis at a median follow-up of 13.0 months, TTP was 3.7 months in both arms (P = 0.653). Objective response rate (ORR) and clinical benefit rate (CBR) were also similar between arms. In the CONFIRM trial, PMW with HR+ ABC who had progressed on previous ET in the adjuvant or first-line metastatic setting were randomized to high-dose (500 mg/month; n = 362) or low-dose (250 mg/month; n = 374) fulvestrant [7]. For 42.5 % of patients, the last ET before fulvestrant treatment was an AI. Overall, progression-free survival (PFS) was 1 month longer in the 500-mg fulvestrant arm compared with the 250-mg fulvestrant arm (6.5 versus 5.5 months, respectively; P = 0.006). Subgroup analyses demonstrated that patients who last received an AI derived less PFS benefit from high-dose fulvestrant than patients who last received antiestrogen therapy. Patients with visceral involvement derived less benefit than patients without. High-dose fulvestrant was not associated with an increase in ORR or CBR compared with low-dose fulvestrant. The SoFEA trial evaluated combination treatment with fulvestrant 250 mg/month plus anastrozole 1 mg/day versus fulvestrant 250 mg/month or exemestane 25 mg/day (n = 250 for all) in PMW with HR+ ABC who had progressed on previous nonsteroidal AI therapy in the adjuvant or first-line metastatic setting [8]. Median PFS was similar in the combination arm (4.4 months) compared with fulvestrant alone (4.8 months; P = 0.98), and was also similar in the fulvestrant-alone arm (4.8 months) compared with exemestane alone (3.4 months; P = 0.56). Overall survival (OS) and ORR were also similar between groups. These results demonstrate that treatment with fulvestrant or exemestane provides between 3 and 5 months of clinical benefit in patients with advanced disease, who have progressed on prior AI therapy. There is no demonstrated benefit to adding an AI to fulvestrant (ie, concomitant use of two agents that target the same endocrine signaling pathway) in this setting. Therefore, additional treatment strategies are needed for women with HR+ ABC who have progressed on prior AI therapy. Recent evidence suggests that dual inhibition of the ER pathway and compensatory pathways involved in estrogen-independent proliferation is a promising approach [9, 10•].
Role of mTOR in Endocrine Resistance
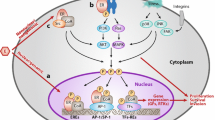
The majority of women with BC (up to 75 %) have ER+ disease, thereby underscoring the importance of managing progressive ABC [1]. Signaling via ER is mediated by the classic ligand-dependent pathway and by the more recently appreciated ligand-independent pathways. Upregulation and activation of receptor tyrosine kinase (RTK)-mediated pathways through ligand-independent activation of ER is one mechanism of endocrine resistance (Fig. 1) [11]. Another mechanism is enhanced downstream signaling via the phosphatidylinositol-3-kinase/protein kinase B/mTOR (PI3K/AKT/mTOR) pathway; mTOR is a serine/threonine protein kinase that regulates cell proliferation, migration, survival, metabolism, and apoptosis by controlling these processes at the translational level [12]. One of the main downstream substrates of mTOR is ribosomal p70 S6 kinase (S6K1), which can phosphorylate ER to induce ligand-independent activation. Although mTOR is placed directly downstream of PI3K/AKT in a linear pathway, AKT may also regulate mTOR through a more complex signaling cascade.
Role of PI3K/AKT/mTOR signaling in estrogen-independent growth in breast cancer. In addition to ER-mediated signaling (right side), other growth factor-mediated signaling pathways (left side) also play a role in ABC. There is crosstalk between ER-mediated and other growth factor-mediated signaling pathways, such as the PI3K/AKT/mTOR pathway. Activation of the PI3K/AKT/mTOR pathway leads to ligand-independent activation of ER. Therefore, combined targeting of both ER and PI3K/AKT/mTOR pathways is a promising treatment approach for HR+ ABC. In addition, crosstalk between the PI3K/AKT/mTOR pathway and signaling through tyrosine kinases including HER2 support a role for mTOR inhibition in enhancing trastuzumab sensitivity and reversing resistance to anti-HER2 therapies in HER2+ BC. ABC, advanced breast cancer; AKT, protein kinase B; BC, breast cancer; ER, estrogen receptor; HER2, human epidermal growth factor receptor; HR +, hormone-receptor–positive; mTOR, mammalian target of rapamycin; PI3K, phosphatidylinositol-3-kinase. Adapted from Osborne CK, et al. [11]
The PI3K/AKT signaling pathway is dysregulated in many human cancers, including BC, leading to upregulation of mTOR-mediated signaling [13]. Somatic mutations in the gene for PI3K (PIK3CA) have been observed in approximately 20–25 % of primary breast tumors, and approximately 9 % of tumors also harbor a gain of PIK3CA gene copy number [14, 15]. Moreover, 15–35 % of patients with BC have reduced expression of phosphatase and tensin homolog deleted on chromosome 10 (PTEN), which is an endogenous inhibitor of the PI3K/AKT pathway [16].
Inhibition of mTOR has the potential to affect the progression of BC by modulating RTK-dependent and estrogen-dependent, as well as estrogen-independent, pathways. Preclinical studies have demonstrated that estrogen-dependent cells cultured long-term in estrogen-depleted medium (mimics resistance to AI therapy) rely on mTOR signaling for growth and are excessively sensitive to its inhibition [17, 18]. Preclinical studies have shown that mTOR inhibition can enhance sensitivity of endocrine-resistant BC cells to ET [19–21].
Clinical Activity of mTOR Inhibitors in ABC
Temsirolimus
Temsirolimus (Torisel®, Wyeth Pharmaceuticals Inc, a subsidiary of Pfizer Inc, Philadelphia, PA) is an intravenous rapamycin derivative that inhibits mTOR [22]. In a Phase 2 study, heavily pretreated patients (N = 109) with locally advanced or metastatic BC were randomized to temsirolimus 75 or 250 mg/week as a 30-minute intravenous infusion [23]. Approximately 38 % of patients had HR+ tumors and 36 % had tumors of unknown receptor status. Ten patients achieved a partial response (PR) with a median time to response of 1.9 months. Objective response rate was 9.2 % and median TTP was 12 weeks. Although efficacy was similar between arms, toxicity was more common with the higher dose of temsirolimus. The most frequent temsirolimus-related adverse events (AEs) were mucositis (70 %), maculopapular rash (51 %), and nausea (43 %). These results suggest that temsirolimus has antitumor activity in heavily pretreated patients with ABC.
Temsirolimus was also evaluated in women with locally advanced or metastatic BC in combination with letrozole in a three-arm, Phase 2 study [24]. Patients (N = 92) were randomized to one of three cohorts: (1) temsirolimus 10 mg/day plus letrozole 2.5 mg/day; (2) intermittent temsirolimus 30 mg daily for 5 days every 2 weeks plus letrozole 2.5 mg/day; or (3) letrozole 2.5 mg/day alone. Patients in cohort 2 had a longer median PFS (11.5, 13.2, and 11.6 months, respectively), and a higher estimated rate of PFS at 16 months (39 %, 45 %, and 27 %, respectively). The most common AEs were: peripheral edema (46 %), asthenia (42 %), and diarrhea (36 %) in cohort 1; asthenia (60 %), diarrhea (43 %), and mucositis (43 %) in cohort 2; and asthenia (55 %), arthralgia (31 %), and nausea (31 %) in cohort 3. These results suggest that intermittent dosing of temsirolimus in combination with letrozole may be more efficacious than letrozole alone [24].
A Phase 3, randomized, double-blind study assessing temsirolimus in combination with letrozole was conducted in PMW with locally advanced or metastatic BC [25]. Patients (N = 1,112) were randomized to intermittent temsirolimus 30 mg (daily for 5 days every 2 weeks) plus letrozole 2.5 mg/day, or placebo plus letrozole 2.5 mg/day. Patients who had received prior ET for advanced or metastatic disease were excluded. Approximately 56 % of patients were ET-naive overall, and the remaining patients had only received ET in the adjuvant setting; therefore, patients were not selected for acquired endocrine resistance. Based on interim results, there were no differences in ORR (27 % for both arms), CBR (45 % versus 46 %, respectively), or median PFS (8.8 versus 8.9 months, respectively; hazard ratio 0.89; P = 0.1803) for the temsirolimus plus letrozole arm versus letrozole alone. The most common grade ≥ 3 AEs were hyperglycemia (4 %), dyspnea, neutropenia, and gamma glutamyltransferase increase (3 % for each) in the combination arm; and dyspnea (3 %), bone pain (2 %), gamma glutamyltransferase increase, and asthenia (1 % for both) in the letrozole alone arm. These results suggest that addition of temsirolimus to letrozole provides no improvement in clinical benefit compared with letrozole alone in PMW with ABC who had not received prior ET.
Everolimus
Everolimus (Afinitor®, Novartis Pharmaceuticals Corporation, East Hanover, NJ) is an oral rapamycin derivative that inhibits mTOR [26]. An initial Phase 1 study evaluating everolimus 5 versus 10 mg/day demonstrated that 10 mg/day was adequately tolerated with promising activity [27]. The National Cancer Institute of Canada conducted a multicenter, noncomparative, randomized, Phase 2 study evaluating two dosing schedules of everolimus (10 mg/day [n = 33] versus 70 mg/week [n = 16]) in minimally pretreated patients with metastatic BC [28]. Approximately 84 % of patients had received prior chemotherapy, 78 % had prior ET, and 59 % had ER+ tumors. Response rate at 8 weeks with daily everolimus was 12 % (95 % confidence interval [CI]: 3.4–28.2 %) versus 0 % (95 % CI: 0.0–20.6 %) for weekly everolimus. More patients progressed on weekly versus daily everolimus (33 % versus 69 %, respectively). Clinical benefit with daily everolimus was greater in patients with ER+ tumors (42 %) compared with ER-negative tumors (15 %). Adverse events were generally more common with daily administration versus weekly; the most frequent grade ≥ 3 AEs included fatigue (5 % in both groups), granulocytopenia and lymphopenia (4 % versus 1 % for both), pneumonitis (3 % versus 0 %), and infection (2 % versus 1 %). These data demonstrate that continuous inhibition of mTOR-mediated signaling with everolimus 10 mg daily provides clinical benefit compared with weekly administration.
A double-blind, Phase 2 study in the neoadjuvant setting evaluated the combination of everolimus and letrozole in PMW with ER+ BC [29]. In this study, 270 patients were randomized to everolimus 10 mg/day plus letrozole 2.5 mg/day or placebo plus letrozole 2.5 mg/day. Response rates by both palpation (68.1 % versus 59.1 %; P = 0.062) and ultrasound (58.0 % versus 47.0 %; P = 0.035) were higher with combination treatment versus letrozole alone, suggesting that concomitant mTOR inhibition can enhance endocrine sensitivity in HR+ BC. Anti-proliferative response, as measured by the proportion of patients with Ki67-positive tumor cells < 1 at Day 15, was significantly higher in the everolimus plus letrozole arm (57 %) compared with letrozole alone (30 %; P < 0.01). The safety profile was consistent with the everolimus monotherapy trial described above. This study laid the groundwork for investigating everolimus in ABC.
The TAMRAD study evaluated everolimus in combination with tamoxifen in PMW with HR+ human epidermal growth factor receptor (HER2)-negative (HER2−) metastatic BC whose disease had progressed on previous AI therapy in the adjuvant or metastatic setting [30•] (Table 2) [30•, 31••]. In this open-label, Phase 2 study, patients were randomized to everolimus 10 mg/day plus tamoxifen 20 mg/day (n = 54) or placebo plus tamoxifen 20 mg/day (n = 57). Crossover was not permitted. Approximately 41 % of patients received prior AI treatment in the adjuvant setting and 67 % in the first-line metastatic setting. The 6-month CBR was 61 % (95 % CI: 47–74 % ) with tamoxifen plus everolimus and 42 % (95 % CI: 29–56 %) with tamoxifen alone (exploratory P = 0.045). With a 2-year median follow-up, TTP increased from 4.5 months in the tamoxifen alone arm to 8.6 months in the everolimus plus tamoxifen arm, corresponding to a 46 % reduction in risk of progression with the combination treatment (hazard ratio 0.54; 95 % CI: 0.36–0.81; exploratory P = 0.002). Risk of death was also reduced by 55 % with combination treatment (hazard ratio 0.45; 95 % CI: 0.24–0.81). Exploratory subgroup analyses demonstrated that patients with secondary hormone resistance benefited more from combination therapy than those with primary resistance, suggesting that patients who previously responded to AI therapy and then become resistant may derive more benefit from everolimus plus tamoxifen. Similar results were also recently reported in a Phase 1/2 study evaluating the combination of sirolimus plus tamoxifen compared with tamoxifen alone [32]. Although results favor treatment of patients with secondary resistance, no convincing evidence to date suggests that patients with primary resistance should be excluded from mTOR inhibitor treatment [33]. In the TAMRAD study, the main AEs in the combination arm versus tamoxifen alone were fatigue (72 % versus 53 %), stomatitis (56 % versus 7 %), rash (44 % versus 7 %), anorexia (43 % versus 18 %), and diarrhea (39 % versus 11 %) [30•].
Everolimus in combination with exemestane was evaluated in the BOLERO-2 (Breast Cancer Trials of Oral Everolimus) international, multicenter, randomized, Phase 3 study [31••, 34••, 35] (Table 2) [30•, 31••]. Postmenopausal women (N = 724) with HR+ HER2− ABC who had recurrence or progression following prior therapy with letrozole or anastrozole were randomized in a 2:1 ratio to receive everolimus (10 mg/day) or matching placebo in combination with open-label exemestane (25 mg/day) [34••]. There was no crossover after disease progression. The majority of patients (77 %) had bone metastases, 56 % had visceral involvement, and 69 % had measurable disease. Approximately 19 % of patients had adjuvant therapy and 26 % had chemotherapy in the advanced setting. The primary endpoint was PFS based on investigator (local radiology) assessment.
At the protocol-defined interim analysis, median PFS was more than doubled in the everolimus plus exemestane arm (6.9 months) compared with exemestane alone (2.8 months; hazard ratio 0.43; P < 0.001) [34••]. At the time of additional PFS analysis (12.5 months’ median follow-up), median PFS by investigator assessment was 7.4 months for the everolimus plus exemestane arm versus 3.2 months for exemestane alone (hazard ratio 0.44; P < 1 × 10−16) [31••]. Based on central assessment, PFS was 11.0 months for the everolimus plus exemestane arm versus 4.1 months for exemestane alone (hazard ratio 0.36; P < 1 × 10−16). Both ORR and CBR were also significantly greater in the combination arm (12.0 % versus 1.3 % and 50.5 % versus 25.5 %, respectively). At 12.5 months’ median follow-up, OS data were immature, with a total of 138 deaths—17.3 % in the combination arm versus 22.6 % in the exemestane alone arm. Additionally, exploratory analyses suggest that everolimus has beneficial effects on bone turnover and cancer progression in bone compared with exemestane alone [36•]. The most common grade 3/4 AEs were stomatitis (8 % versus 1 %), anemia (7 % versus 1 %), hyperglycemia (5 % versus < 1 %), dyspnea (4 % versus 1 %), fatigue (4 % versus 1 %), and pneumonitis (3 % versus 0 %) for combination treatment compared with exemestane alone [31••]. Results from the protocol-defined final PFS analysis were consistent [35].
Safety Profile of mTOR Inhibitors in HR+ ABC
Clinically notable AEs that may be class effects of mTOR inhibitors include stomatitis, noninfectious pneumonitis, infections, and metabolic abnormalities. Temsirolimus and everolimus have been approved for the treatment of advanced renal cell carcinoma (RCC) since 2007 and 2009, respectively; therefore, most experience in the management of these AEs comes from the RCC setting [37, 38]. An expert group recently published guidance on the management of select AEs associated with everolimus treatment in RCC [39•]. The AE profile of sirolimus is similar to that seen with other mTOR inhibitors in ABC [32]. As use of everolimus expands into the BC setting, it is important that physicians understand its safety profile and apply AE management recommendations in their practice.
Stomatitis
Stomatitis is one of the most frequent and potentially dose-limiting mTOR-inhibitor–associated AEs. Grade 3/4 stomatitis was reported in 8 % of patients in BOLERO-2 [40]. The clinical presentation of mTOR-inhibitor–associated stomatitis is distinct from chemotherapy-induced stomatitis, and presents as aphthous-like ulcers characterized by discrete, ovoid, superficial, well-demarcated ulcerations with a grayish-white pseudomembrane [41]. There is no evidence for increased risk of stomatitis with ongoing everolimus treatment. Ulcers typically develop acutely in the first cycle of therapy, and the severity usually peaks within the first few weeks of treatment [42].
Use of specific topical corticosteroids and mouthwashes for the treatment of chemotherapy-induced stomatitis has been reviewed extensively and may also be appropriate for patients with mTOR-inhibitor–associated stomatitis [43, 44]. Evidence-based guidelines recommend good oral hygiene, treatment of anticipated infections, and avoidance of alcohol- containing or peroxide-containing products [39•, 45]. Additionally, patients should be evaluated for herpes and fungal infections, and antiviral and antifungal agents should be prescribed as appropriate [39•]. Everolimus dose modifications may be necessary for grade 2 and 3 stomatitis, and treatment should be discontinued in the event of grade 4 stomatitis [39•].
Noninfectious Pneumonitis
Noninfectious pneumonitis is a class effect of rapamycin derivatives [46, 47]. Approximately 4 % of patients in BOLERO-2 had grade 3/4 pneumonitis [40]. Common radiographic changes seen with mTOR-inhibitor–associated pneumonitis are ground-glass opacities and focal consolidation, mainly in the lower lobes [46]. Patients may be asymptomatic or have nonspecific respiratory signs and symptoms, including cough, dyspnea, hypoxia, and, rarely, pleural effusion [39•]. Fever may also be present, making differential diagnosis even more challenging. Symptomatic cases are usually mild to moderate in severity and are reversible; however, some cases may be severe [48].
In patients with baseline respiratory symptoms or in those with multiple lung metastases, a computed tomography scan, lung function tests, and arterial oxygen saturation are recommended before everolimus treatment [39•]. Temporary everolimus dose interruption and corticosteroids may be necessary for moderate to severe symptoms; however, following resolution of symptoms, everolimus may be reinitiated at a reduced dose. For grade 4, life-threatening noninfectious pneumonitis, everolimus should be permanently discontinued.
Infections
Inhibitors of mTOR have immunosuppressive properties and, therefore, may predispose patients to opportunistic infections and reactivation of previous infections [39•]. Pneumonia and other bacterial infections and invasive fungal infections have been reported in patients treated with everolimus; however, grade 3/4 infections were rare in both temsirolimus and everolimus trials [25, 34••]. A complete medical history should be obtained before starting everolimus, to identify patients at greater risk of developing an infection [39•]. Because of the potential risk for reactivation, patients with an active fungal infection should be comprehensively treated before initiation of everolimus therapy. Importantly, patients with an invasive systemic fungal infection should not be treated with everolimus. If a diagnosis of invasive systemic fungal infection is suspected, everolimus should be promptly and permanently discontinued, and appropriate antifungal treatment initiated. Patients with hepatitis B virus (HBV) infection should be monitored for HBV DNA levels and treated with high-potency and high-genetic–barrier drugs such as entecavir or tenofovir, if necessary.
Metabolic Abnormalities
Mammalian target of rapamycin is an important downstream effector of insulin signaling and subsequent gluconeogenesis, glycogen synthesis, and lipogenesis [49]. Therefore, mTOR inhibition can disrupt these physiologic processes, resulting in metabolic abnormalities such as hyperglycemia and hyperlipidemia. Grade 3/4 hyperglycemia was reported in 4 % and 5 % of patients in the Phase 3 temsirolimus trial and in the BOLERO-2 trial, respectively [25, 40]. It is recommended that fasting serum glucose and lipid levels are monitored and hepatic function tests performed before initiating everolimus and periodically thereafter [39•]. When possible, optimal glycemic and lipid control should be achieved before starting everolimus. Patients with underlying diabetes require careful monitoring and potential modifications to their antihyperglycemic regimen. Mild elevations in glucose and lipid levels can be managed per standard guidelines and do not require treatment interruption [39•, 50]. However, grade 3 metabolic abnormalities may require everolimus dose interruption, and patients with grade 4 metabolic abnormalities should discontinue treatment [39•].
Future Perspectives—The Evolving Role of mTOR Inhibition
There is an unmet need for treatment strategies than can help women with HR+ ABC who have progressed on prior ET (especially AIs). Based on preclinical evidence, the use of mTOR inhibitors is a rational choice to potentially enhance endocrine sensitivity. Results from the Phase 3 BOLERO-2 study demonstrate that everolimus significantly enhances the efficacy of exemestane in patients with HR+ ABC who have progressed after initial AI treatment [31••, 34••, 35]. The benefit seen in BOLERO-2 was clinically meaningful and the safety profile was manageable and consistent with previous experience in other oncology indications.
Yet not all attempts at using mTOR inhibitors for the treatment of HR+ ABC have been successful. Results from the Phase 3 temsirolimus trial suggest that addition of temsirolimus to letrozole provides no improvement in clinical benefit, compared with letrozole alone in PMW with ABC [25]. The disparity between results from the everolimus and temsirolimus Phase 3 trials is not well understood. One potential reason may be the different patient populations evaluated in the two studies. BOLERO-2 enrolled patients who were refractory to AI therapy for ABC, whereas the temsirolimus Phase 3 study included only ET-naive patients. Another potential reason may be the different dosing schedules used in the two studies. BOLERO-2 used daily dosing, whereas the temsirolimus trial used intermittent dosing. Based on insights from pharmacodynamic studies of everolimus in patients with solid tumors, daily administration of everolimus 10 mg completely and continuously inhibits mTOR-dependent downstream signaling [51]. Similar data for intermittent dosing of temsirolimus are not available; however, a recent report suggests that up to 75 mg/day might be needed to elicit tumor responses if administered at an intermittent schedule of 5 days every 2 weeks [52].
Given the importance of PI3K/AKT/mTOR-mediated signaling, inhibition of mTOR may also be effective against BC in general. For example, there is an unmet need for treatment strategies than can overcome resistance to trastuzumab in women with HER2-positive (HER2+) ABC. Up to 30 % of primary breast tumors overexpress HER2 [53]. Despite the survival benefit provided by trastuzumab, almost all patients with HER2+ disease who initially respond to trastuzumab become unresponsive [54]. Recent advances in the management of HER2+ disease (eg, pertuzumab) remain, nonetheless, dependent on continued overexpression of intact HER2 [34••]. Additional strategies are thus needed to address trastuzumab resistance, potentially by targeting other signaling pathways that interact with and may compensate for HER2 (Fig. 1). Preclinical evidence suggests that activation of mTOR caused by loss of PTEN or overexpression of PI3K is associated with trastuzumab resistance [55, 56]. Early phase studies have demonstrated promising activity of everolimus in combination with trastuzumab and chemotherapy in women with HER2+ ABC who have progressed on prior trastuzumab-based therapy [57, 58, 59•]. Two large, Phase 3 studies of everolimus in the HER2+ setting, BOLERO-1 and BOLERO-3, are fully accrued and results are awaited. BOLERO-1 is evaluating the combination of everolimus plus trastuzumab plus paclitaxel versus trastuzumab plus paclitaxel as first-line treatment for women (N = 719) with HER2+ locally advanced or metastatic disease [60]. In the BOLERO-3 study, 572 women with HER2+ locally advanced or metastatic disease who have had prior taxane therapy and are resistant to trastuzumab will be treated with everolimus plus trastuzumab plus vinorelbine versus trastuzumab plus vinorelbine [61]. The primary endpoint of both studies is PFS; secondary endpoints include OS, ORR, and CBR. Interim efficacy and safety results from these trials are expected in the near future.
While data on the expanded use of everolimus in patients with HER2+ disease from the BOLERO-1 and BOLERO-3 studies are eagerly anticipated, currently available data in HR+ ABC are very promising. Based on the BOLERO-2 study and other supportive results, addition of everolimus to ET should be considered a paradigm shift for the treatment of women with HR+ ABC who have progressed on prior ET [9, 10•]. Everolimus, in combination with exemestane, was recently approved by the United States Food and Drug Administration and the European Committee for Medicinal Products for Human Use for the treatment of PMW with HR+, HER2− ABC after failure of treatment with letrozole or anastrozole [26, 62]. This represents a significant milestone in the clinical management of this patient population.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Cleator SJ, Ahamed E, Coombes RC, Palmieri C. A 2009 update on the treatment of patients with hormone receptor-positive breast cancer. Clin Breast Cancer. 2009;9 Suppl 1:S6–S17.
Higgins MJ, Baselga J. Targeted therapies for breast cancer. J Clin Invest. 2011;121:3797–803.
• Cardoso F, Costa A, Norton L, et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast. 2012;21:242–52. The 1st international Consensus Conference for Advanced Breast Cancer (ABC 1) took place in November 2011 in Lisbon, Portugal. This manuscript summarizes the consensus treatment guidelines developed by this international expert panel specifically for the management of ABC.
• National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology: Breast Cancer. Version I. 2012. Available at http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf. Accessed 8 June 2012. The latest version of the National Comprehensive Cancer Network (NCCN) Clinical Practice Guidelines for Breast Cancer recommends considering the addition of everolimus to exemestane in women who fulfill the eligibility criteria of the Phase 3 BOLERO-2 study.
Hurvitz SA, Pietras RJ. Rational management of endocrine resistance in breast cancer: a comprehensive review of estrogen receptor biology, treatment options, and future directions. Cancer. 2008;113:2385–97.
Chia S, Gradishar W, Mauriac L, et al. Double-blind, randomized placebo controlled trial of fulvestrant compared with exemestane after prior nonsteroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor-positive, advanced breast cancer: results from EFECT. J Clin Oncol. 2008;26:1664–70.
Di Leo A, Jerusalem G, Petruzelka L, et al. Results of the CONFIRM phase III trial comparing fulvestrant 250 mg with fulvestrant 500 mg in postmenopausal women with estrogen receptor-positive advanced breast cancer. J Clin Oncol. 2010;28:4594–600.
Johnston S, Kilburn LS, Ellis P, et al. Fulvestrant alone or with concomitant anastrozole vs exemestane following progression on non-steroidal aromatase inhibitor: first results of the SoFEA trial (CRUKE/03/021 & CRUK/09/007) (ISRCTN44195747) [Abstract 2LBA]. Eur J Cancer. 2012;48:2s.
Johnston SR. BOLERO-2 - will this change practice in advanced breast cancer? Breast Cancer Res. 2012;14:311.
• Villarreal-Garza C, Cortes J, Andre F, Verma S. mTOR inhibitors in the management of hormone receptor-positive breast cancer: the latest evidence and future directions. Ann Oncol. 2012; [Epub ahead of print]. Recent review evaluating the use of mTOR inhibitors in the treatment of HR + BC.
Osborne CK, Schiff R. Mechanisms of endocrine resistance in breast cancer. Annu Rev Med. 2011;62:233–47.
Margariti N, Fox SB, Bottini A, Generali D. “Overcoming breast cancer drug resistance with mTOR inhibitors”. Could it be a myth or a real possibility in the short-term future? Breast Cancer Res Treat. 2011;128:599–606.
Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–30.
Wu G, Xing M, Mambo E, et al. Somatic mutation and gain of copy number of PIK3CA in human breast cancer. Breast Cancer Res. 2005;7:R609–16.
Bachman KE, Argani P, Samuels Y, et al. The PIK3CA gene is mutated with high frequency in human breast cancers. Cancer Biol Ther. 2004;3:772–5.
Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–9.
Yue W, Fan P, Wang J, et al. Mechanisms of acquired resistance to endocrine therapy in hormone-dependent breast cancer cells. J Steroid Biochem Mol Biol. 2007;106:102–10.
Miller TW, Hennessy BT, Gonzalez-Angulo AM, et al. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J Clin Invest. 2010;120:2406–13.
de Graffenried LA, Friedrichs WE, Russell DH, et al. Inhibition of mTOR activity restores tamoxifen response in breast cancer cells with aberrant Akt activity. Clin Cancer Res. 2004;10:8059–67.
Boulay A, Rudloff J, Ye J, et al. Dual inhibition of mTOR and estrogen receptor signaling in vitro induces cell death in models of breast cancer. Clin Cancer Res. 2005;11:5319–28.
Beeram M, Tan QT, Tekmal RR, et al. Akt-induced endocrine therapy resistance is reversed by inhibition of mTOR signaling. Ann Oncol. 2007;18:1323–8.
Wyeth Pharmaceuticals Inc, a subsidiary of Pfizer, Inc, Philadelphia, PA. Torisel (temsirolimus) prescribing information. 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022088s014lbl.pdf. Accessed 28 June 2012.
Chan S, Scheulen ME, Johnston S, et al. Phase II study of temsirolimus (CCI-779), a novel inhibitor of mTOR, in heavily pretreated patients with locally advanced or metastatic breast cancer. J Clin Oncol. 2005;23:5314–22.
Baselga J, Roche H, Fumoleau P. Treatment of postmenopausal women with locally advanced or metastatic breast cancer with letrozole alone or in combination with temsirolimus: a randomized, 3-arm, phase 2 study [abstract 1068]. Breast Cancer Res Treat. 2005;94:S62.
Chow LWC, Sun Y, Jassem J, et al. Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer [abstract 6091]. Breast Cancer Res Treat. 2006;100:S286.
Novartis Pharmaceuticals Corporation, East Hanover, NJ. Afinitor (everolimus) prescribing information. 2012. Available at http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022334s016lbl.pdf. Accessed 8 August 2012.
Awada A, Cardoso F, Fontaine C, et al. The oral mTOR inhibitor RAD001 (everolimus) in combination with letrozole in patients with advanced breast cancer: results of a phase I study with pharmacokinetics. Eur J Cancer. 2008;44:84–91.
Ellard SL, Clemons M, Gelmon KA, et al. Randomized phase II study comparing two schedules of everolimus in patients with recurrent/metastatic breast cancer: NCIC Clinical Trials Group IND.163. J Clin Oncol. 2009;27:4536–41.
Baselga J, Semiglazov V, van Dam P, et al. Phase II randomized study of neoadjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009;27:2630–7.
• Bachelot T, Bourgier C, Cropet C, et al. Randomized phase II trial of everolimus in combination with tamoxifen in patients with hormone receptor-positive, human epidermal growth factor receptor 2-negative metastatic breast cancer with prior exposure to aromatase inhibitors: a GINECO study. J Clin Oncol. 2012;30:2718–24. The phase 2 TAMRAD study demonstrated that addition of everolimus to ET improves clinical benefit compared with ET alone in PMW with HR + , HER2 − , AI-resistant metastatic BC.
•• Hortobagyi GN, Piccart M, Rugo H, et al. Everolimus for postmenopausal women with advanced breast cancer: updated results of the BOLERO-2 phase III trial [abstract S3-7]. Cancer Res. 2011;71:105s–6s. Interim PFS analysis from the BOLERO-2 study demonstrated a robust and consistent benefit of everolimus plus exemestane in HR + ABC patients.
Bhattacharyya GS, Biswas J, Singh JK, et al. Reversal of tamoxifen resistance (hormone resistance) by addition of sirolimus (mTOR inhibitor) in metastatic breast cancer [abstract 16LBA]. Presented at the European Multidisciplinary Cancer Congress. Stockholm, Sweden; 2011.
Rugo HS, Keck S. Reversing hormone resistance: have we found the golden key? J Clin Oncol. 2012;30:2707–9.
•• Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012;366:520–9. BOLERO-2 was the first phase 3 study to demonstrate that addition of an mTOR inhibitor, specifically everolimus, to an AI improves clinical benefit compared with AI therapy alone in PMW with HR + , HER2 − , AI-resistant ABC.
Piccart M, Noguchi S, Pritchard KI, et al. Everolimus for postmenopausal women with advanced breast cancer: Updated results of the BOLERO-2 phase III trial [abstract 559]. Presented at the 48th Annual Meeting of the American Society of Clinical Oncology. Chicago, IL; 2012.
• Gnant M, Baselga J, Rugo HS, et al. Effects of everolimus (EVE) on disease progression in bone and bone markers (BM) in patients (pts) with bone metastases (mets) [abstract 512]. Presented at the 48th Annual Meeting of the American Society of Clinical Oncology. Chicago, IL; 2012. Exploratory analyses from the BOLERO-2 study demonstrated that in patients with bone metastases, addition of everolimus decreased markers of bone turnover compared with exemestane alone.
Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356:2271–81.
Motzer RJ, Escudier B, Oudard S, et al. Phase 3 trial of everolimus for metastatic renal cell carcinoma: final results and analysis of prognostic factors. Cancer. 2010;116:4256–65.
• Porta C, Osanto S, Ravaud A, et al. Management of adverse events associated with the use of everolimus in patients with advanced renal cell carcinoma. Eur J Cancer. 2011;47:1287–98. Recommendations from an expert panel for AE management during everolimus treatment for patients with RCC; these recommendations are also applicable to the BC setting.
Afinitor (everolimus) US Prescribing Information July 20. 2012. Available at: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022334s016lbl.pdf. Accessed 23 July 2012.
Sonis S, Treister N, Chawla S, et al. Preliminary characterization of oral lesions associated with inhibitors of mammalian target of rapamycin in cancer patients. Cancer. 2010;116:210–5.
de Oliveira MA, Martins EMF, Wang Q, et al. Clinical presentation and management of mTOR inhibitor-associated stomatitis. Oral Oncol. 2011;47:998–1003.
Stokman MA, Spijkervet FK, Boezen HM, et al. Preventive intervention possibilities in radiotherapy- and chemotherapy-induced oral mucositis: results of meta-analyses. J Dent Res. 2006;85:690–700.
Potting CM, Uitterhoeve R, Op Reimer WS, Van Achterberg T. The effectiveness of commonly used mouthwashes for the prevention of chemotherapy-induced oral mucositis: a systematic review. Eur J Cancer Care (Engl). 2006;15:431–9.
Harris DJ, Eilers J, Harriman A, et al. Putting evidence into practice: evidence-based interventions for the management of oral mucositis. Clin J Oncol Nurs. 2008;12:141–52.
Duran I, Siu LL, Oza AM, et al. Characterisation of the lung toxicity of the cell cycle inhibitor temsirolimus. Eur J Cancer. 2006;42:1875–80.
Cho D, Signoretti S, Regan M, et al. The role of mammalian target of rapamycin inhibitors in the treatment of advanced renal cancer. Clin Cancer Res. 2007;13:758s–63s.
White DA, Camus P, Endo M, et al. Noninfectious pneumonitis after everolimus therapy for advanced renal cell carcinoma. Am J Respir Crit Care Med. 2010;182:396–403.
Zoncu R, Efeyan A, Sabatini DM. mTOR: from growth signal integration to cancer, diabetes and ageing. Nat Rev Mol Cell Biol. 2011;12:21–35.
Nathan DM, Buse JB, Davidson MB, et al. Medical management of hyperglycemia in type 2 diabetes: a consensus algorithm for the initiation and adjustment of therapy: a consensus statement of the American Diabetes Association and the European Association for the Study of Diabetes. Diabetes Care. 2009;32:193–203.
Tabernero J, Rojo F, Calvo E, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–10.
Buckner JC, Forouzesh B, Erlichman C, et al. Phase I, pharmacokinetic study of temsirolimus administered orally to patients with advanced cancer. Invest New Drugs. 2010;28:334–42.
Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–82.
Nahta R, Esteva FJ. HER-2-targeted therapy: lessons learned and future directions. Clin Cancer Res. 2003;9:5078–84.
Nagata Y, Lan KH, Zhou X, et al. PTEN activation contributes to tumor inhibition by trastuzumab, and loss of PTEN predicts trastuzumab resistance in patients. Cancer Cell. 2004;6:117–27.
Berns K, Horlings HM, Hennessy BT, et al. A functional genetic approach identifies the PI3K pathway as a major determinant of trastuzumab resistance in breast cancer. Cancer Cell. 2007;12:395–402.
Andre F, Campone M, O’Regan R, et al. Phase I study of everolimus plus weekly paclitaxel and trastuzumab in patients with metastatic breast cancer pretreated with trastuzumab. J Clin Oncol. 2010;28:5110–5.
Jerusalem G, Fasolo A, Dieras V, et al. Phase I trial of oral mTOR inhibitor everolimus in combination with trastuzumab and vinorelbine in pre-treated patients with HER2-overexpressing metastatic breast cancer. Breast Cancer Res Treat. 2011;125:447–55.
• Morrow PK, Wulf GM, Ensor J, et al. Phase I/II study of trastuzumab in combination with everolimus (RAD001) in patients with HER2-overexpressing metastatic breast cancer who progressed on trastuzumab-based therapy. J Clin Oncol. 2011;29:3126–32. This pooled analysis of 2 phase 1/2 studies suggests that everolimus in combination with trastuzumab provides clinical benefit in patients with trastuzumab-resistant, HER2 + metastatic BC.
Hurvitz SA, Andre F, Burris HA, et al. BOLERO-1: A randomized, phase III, double-blind, placebo-controlled multicenter trial of everolimus in combination with trastuzumab and paclitaxel as first-line therapy in women with HER2-positive (HER2+), locally advanced or metastatic breast cancer (BC) [abstract TPS648]. J Clin Oncol. 2012;30:43s.
Daily everolimus in combination with trastuzumab and vinorelbine in HER2/Neu positive women with locally advanced or metastatic breast cancer (BOLERO-3). Available at http://clinicaltrials.gov/ct2/show/NCT01007942. Accessed 1 May 2012.
Novartis Pharma GmbH, Nuremberg, Germany. Afinitor (everolimus) EPAR - product information. 2012. Available at http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/001038/WC500022814.pdf. Accessed 8 August 2012.
Acknowledgments
Financial support for medical editorial assistance was provided by Novartis Pharmaceuticals Corporation. I thank Payal N. Gandhi, PhD, ProEd Communications, Inc., for her medical editorial assistance with this manuscript.
Disclosure
M. Gnant: consultancy fees (Novartis, Merrion), speaker’s fees (Roche, Amgen, Novartis, Astra Zeneca, GlaxoSmithKline), and unrestricted grant/research funding (Roche, Sanofi–Aventis, GlaxoSmithKline, Novartis, AstraZeneca).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Gnant, M. The Role of Mammalian Target of Rapamycin (mTOR) Inhibition in the Treatment of Advanced Breast Cancer. Curr Oncol Rep 15, 14–23 (2013). https://doi.org/10.1007/s11912-012-0277-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11912-012-0277-1