Abstract
The benefit of endocrine therapy has always been limited by the eventual development of acquired resistance. For the first time, clinical research has identified a therapeutic agent, everolimus, that targets the mammalian target of rapamycin (mTOR), which in combination with the aromatase inhibitor exemestane can substantially reduce the risk of disease progression and seemingly circumvent endocrine resistance. The magnitude of the benefit represents a quantum shift in how we should use endocrine therapy in future, and potentially defines a new standard of care in this setting.
Similar content being viewed by others
Background
It has been unclear what the optimal management is for postmenopausal women with oestrogen receptor (ER)-positive advanced breast cancer that has developed resistance to non-steroidal aromatase inhibitors (NSAIs) [1]. Clinical studies have shown that endocrine agents with different mechanisms of action, such as the steroid aromatase inactivator exemestane or the steroidal selective ER down-regulator fulvestrant, can induce responses in this setting. In a prior randomised phase III study in this setting (EFECT) no significant difference was observed between fulvestrant and exemestane, with a median progression-free survival of only 3 months [2].
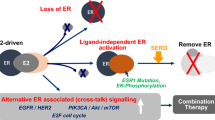
Pre-clinical studies to investigate mechanisms of resistance to oestrogen deprivation have demonstrated persistence of an active ER pathway [3]. A number of intracellular signalling pathways may 'cross-talk' and activate ER, including the human epidermal growth factor receptor (HER) family pathway [4], and the phos-phatidylinositol 3-kinase (PI3K)/Akt/mammalian target of rapamycin (mTOR) pathway [5]. This has led to much interest in the concept of combining various growth factor receptor antagonists or signalling inhibitors with aromatase inhibitors to enhance endocrine responsiveness and delay, or even reverse resistance. To date, attempts to combine aromatase inhibitors with the epidermal growth factor receptor tyrosine kinase inhibitor (TKI) gefitinib or the HER2-targeted TKI lapatinib in ER-positive HER2-negative breast cancer have been disappointing in unselected patients [6–9], with success only seen when there is known dual target expression (that is, ER+ HER2+) [9, 10].
Significance of BOLERO-2
The success of the recent BOLERO-2 study, therefore, represents a significant development in this field [11]. In this phase III randomised trial, 724 patients with ER-positive advanced breast cancer were enrolled, all of whom had evidence of recurrence or progression while receiving prior NSAIs. Of particular note, 84% of patients had evidence of prior hormone sensitivity, such that BOLERO-2 addressed the treatment of ER-positive breast cancer that had developed acquired endocrine resistance to NSAIs. The response to exemestane alone as the control arm was very similar to the data seen in the previous EFECT study [2], namely a median progression-free survival (centrally assessed) of only 4.1 months. In contrast, those treated with the combination of everolimus and exemestane had a median progression-free survival of 10.6 months. The objective response rate was significantly better for the combination (7% versus 0.4%, P < 0.001), although there were a greater number of grade 3/4 adverse events, in particular stomatitis (8% versus 1%).
Not only was the magnitude of the clinical benefit seen for the addition of everolimus very substantial, but this trial, together with the related phase II TAMRAD study, correctly identified the best group of patients to target with the combination [12], namely those with acquired endocrine resistance following prior hormonal responsiveness. Pre-clinical experiments had already told us that PI3K/Akt/mTOR intracellular signalling can become activated during long-term oestrogen deprivation [3], a situation that may be replicated in a treated ER-positive breast cancer that develops acquired resistance to long-term aromatase inhibitor therapy. It is likely, therefore, that these patients have ER-positive tumour cells that, during long-term NSAI therapy, develop survival path-ways driven by PI3K signalling, and as such have become primed to respond to a combination of an mTOR inhibitor with exemestane.
So why did the previous first-line study of letrozole combined with the mTOR inhibitor temsirolimus in ER-positive advanced breast cancer, where 56% patients had no prior exposure to endocrine therapy, fail to demonstrate additional benefit [13]? The absence of the activated pathway in many untreated hormone-sensitive ER-positive breast cancers probably means that the addition of an mTOR inhibitor at that stage is highly unlikely to produce greater anticancer effects over an aromatase inhibitor alone. Moreover, blockage of the pathway from the outset may just allow other intracellular signalling adaptations to occur over time. This was well illustrated in pre-clinical experiments with trastuzumab and letrozole in an MCF7 HER2-negative xenograft model where co-treatment up front failed to improve on tumour control compared with letrozole alone, and yet by contrast co-treatment at the time of relapse on letrozole demonstrated that the addition of trastuzumab was highly effective in overcoming endocrine resistance once it was established [14]. The lesson is clear in the first-line setting - unless the tumour cells have switched the signalling pathway on, combined therapy will not work. In contrast, at the time of relapse on endocrine therapy tumour cells may have become primed to respond better to combined therapy.
Implications for clinical practice
It is quite likely that the combination of exemestane and everolimus will become a new approved standard of care in this advanced breast cancer setting given the magnitude of the additional clinical benefit seen in BOLERO-2. The key issue for clinical practice will be whether clinicians will select the most appropriate patients for this therapy, and in particular choose only those with prior hormone-sensitive breast cancer that has developed acquired resistance to NSAIs. In my view, it would be inappropriate to translate the findings from BOLERO-2 into a new first-line treatment option for all ER-positive patients, regardless of prior exposure to NSAIs. The negative first-line studies cited above indicate that very few (if any) untreated ER-positive tumours may benefit from the combination upfront - the caveat to this, however, is that there will probably be a few untreated ER-positive tumours that may already have an activated pathway that could cause 'de novo' endocrine resistance, and these cases could respond very well to the combination. In the neo-adjuvant study of letrozole plus everolimus, some patients had ER-positive tumours with activated PIK3CA mutations, and in these tumours the combination of letrozole plus everolimus had a significantly greater anti-proliferative effect than letrozole alone [15]. More research on biomarker selection is needed to confirm these findings in untreated ER-positive breast cancer. In the meantime, however, BOLERO-2 has given us new hope that we can overcome endocrine resistance in breast cancer.
Abbreviations
- ER:
-
oestrogen receptor
- HER:
-
human epidermal growth factor receptor
- mTOR:
-
mammalian target of rapamycin
- NSAI:
-
non-steroidal aromatase inhibitor
- PI3K:
-
phosphatidylinositol 3-kinase
- TKI:
-
tyrosine kinase inhibitor.
References
Ring A, Dowsett M: Mechanisms of tamoxifen resistance. Endocr Relat Cancer. 2004, 11: 643-658. 10.1677/erc.1.00776.
Chia S, Gradishar W, Mauriac L, Bines J, Amant F, Federico M, Fein L, Romieu G, Buzdar A, Robertson JF, Brufsky A, Possinger K, Rennie P, Sapunar F, Lowe E, Piccart M: Double-blind, randomised placebo controlled trial of fulvestrant compared with exemestane after prior non-steroidal aromatase inhibitor therapy in postmenopausal women with hormone receptor positive advanced breast cancer: results form EFECT. J Clin Oncol. 2008, 26: 1664-1670. 10.1200/JCO.2007.13.5822.
Martin LA, Farmer I, Johnston SR, Ali S, Marshall C, Dowsett M: Enhanced estrogen receptor (ER) alpha, ERBB2, and MAPK signal transduction pathways operate during the adaptation of MCF-7 cells to long term estrogen deprivation. J Biol Chem. 2003, 278: 30458-30468. 10.1074/jbc.M305226200.
Nicholson RI, McClelland RA, Robertson JF, Gee JM: Involvement of steroid hormone and growth factor cross-talk in endocrine response in breast cancer. Endocr Relat Cancer. 1999, 6: 373-387. 10.1677/erc.0.0060373.
Campbell RA, Bhat-Nakshatri P, Patel NM, Constantinidou D, Ali S, Nakshatri H: Phosphatidylinositol 3-kinase/AKT-mediated activation of estrogen receptor alpha: a new model for anti-estrogen resistance. J Biol Chem. 2001, 276: 9817-9824. 10.1074/jbc.M010840200.
Smith IE, Walsh G, Skene A, Llombart A, Mayordomo JI, Detre S, Salter J, Clark E, Magill P, Dowsett M: A phase II placebo-controlled trial of neo-adjuvant anastrozole alone or with gefitinib in early breast cancer. J Clin Oncol. 2007, 25: 3816-3822. 10.1200/JCO.2006.09.6578.
Osborne CK, Neven P, Dirix LY, Mackey JR, Robert J, Underhill C, Schiff R, Gutierrez C, Migliaccio I, Anagnostou VK, Rimm DL, Magill P, Sellers M: Gefitinib or placebo in combination with tamoxifen in patients with hormone receptor-positive metastatic breast cancer: a randomized phase II study. Clin Cancer Res. 2011, 17: 1147-1159. 10.1158/1078-0432.CCR-10-1869.
Cristofanilli M, Valero V, Mangalik A, Royce M, Rabinowitz I, Arena FP, Kroener JF, Curcio E, Watkins C, Bacus S, Cora EM, Anderson E, Magill PJ: Phase II, randomized trial to compare anastrozole combined with gefitinib or placebo in postmenopausal women with hormone receptor-positive metastatic breast cancer. Clin Cancer Res. 2010, 16: 1904-1914. 10.1158/1078-0432.CCR-09-2282.
Johnston S, Pippen J, Pivot X, Lichinitser M, Sadeghi S, Dieras V, Gomez HL, Romieu G, Manikhas A, Kennedy MJ, Press MF, Maltzman J, Florance A, O'Rourke L, Oliva C, Stein S, Pegram M: Lapatinib combined with letrozole versus letrozole and placebo as first-line therapy for postmenopausal hormone-receptor-positive metastatic breast cancer. J Clin Oncol. 2009, 27: 5538-5846. 10.1200/JCO.2009.23.3734.
Kaufman B, Mackey JR, Clemens MR, Bapsy PP, Vaid A, Wardley A, Tjulandin S, Jahn M, Lehle M, Feyereislova A, Révil C, Jones A: Trastuzumab plus anastrozole versus anastrozole alone for the treatment of postmenopausal women with human epidermal growth factor receptor 2-positive, hormone receptor-positive metastatic breast cancer: results form the randomized TAnDEM study. J Clin Oncol. 2009, 27: 5529-5537. 10.1200/JCO.2008.20.6847.
Baselga J, Campone M, Piccart M, Burris HA 3rd, Rugo HS, Sahmoud T, Noguchi S, Gnant M, Pritchard KI, Lebrun F, Beck JT, Ito Y, Yardley D, Deleu I, Perez A, Bachelot T, Vittori L, Xu Z, Mukhopadhyay P, Lebwohl D, Hortobagyi GN: Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N Engl J Med. 2012, 366: 520-529. 10.1056/NEJMoa1109653.
Bachelot T, Bourgier C, Cropet C, Guastalla J-P, Ferrero J-M, Leger-Falandry C, Soulie P, Eymard J-C, Debled M, Spaeth D, Legouffe E, Delozier T, El Kouri C, Chidiac J: TAMRAD: a GINECO randomized phase II trial of everolimus in combination with tamoxifen versus tamoxifen alone in patients (pts) with hormone-receptor positive, HER2 negative metastatic breast cancer (MBC) with prior exposure to aromatase inhibitors (AI). Cancer Res. 2010, 70 (Suppl 2): Abstract S1-6
CChow LWC, Sun Y, Jassem J, Baselga J, Hayes DF, Wolff AC, Hachemi S, Cincotta M, Yu BW, Kong S, Moore L: Phase 3 study of temsirolimus with letrozole or letrozole alone in postmenopausal women with locally advanced or metastatic breast cancer. Breast Cancer Res Treat. 2006, 100 (Suppl 1): Abs 6091
Sabnis G, Schavowitz A, Goloubeva O, Macedo LF, Brodie A: Trastuzumab reverses letrozole resistance and amplifies the sensitivity of breast cancer cells to estrogen. Cancer Res. 2009, 69: 1416-1428. 10.1158/0008-5472.CAN-08-0857.
Baselga J, Semiglazov V, van Dam P, Manikhas A, Bellet M, Mayordomo J, Campone M, Kubista E, Greil R, Bianchi G, Steinseifer J, Molloy B, Tokaji E, Gardner H, Phillips P, Stumm M, Lane HA, Dixon JM, Jonat W, Rugo HS: Phase II randomised study of neo-adjuvant everolimus plus letrozole compared with placebo plus letrozole in patients with estrogen receptor-positive breast cancer. J Clin Oncol. 2009, 27: 2630-2637. 10.1200/JCO.2008.18.8391.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Johnston, S.R. BOLERO-2 - will this change practice in advanced breast cancer?. Breast Cancer Res 14, 311 (2012). https://doi.org/10.1186/bcr3126
Published:
DOI: https://doi.org/10.1186/bcr3126