Abstract
Purpose of Review
Magnetic resonance neurography (MRN) is being increasingly used as a problem-solving tool for diagnosis and management of peripheral neuropathies. This review is aimed at summarizing important technological advances, including MR pulse sequence and surface coil developments, which have facilitated MRN’s use in clinical practice.
Recent Findings
The most recent research in MRN focuses on its clinical applications, with concomitant development of three-dimensional, parallel imaging and vascular suppression techniques that facilitate higher spatial resolution and depiction of small nerve branches arising from the brachial and lumbosacral plexi as well as fascicular abnormalities of more distal extremity nerves. Quantitative diffusion tensor imaging (DTI) has been studied as a tool to detect microstructural abnormalities of peripheral nerves and more precisely define grades of nerve injury but will require additional investigation to determine its role in daily clinical practice.
Summary
MRN continues to evolve due to technological improvements and awareness by the medical community of its capabilities. Additional technological developments related to surface coil designs and vascular suppression techniques will be needed to move the field forward.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
High-resolution peripheral nerve magnetic resonance imaging (MRI), or MR neurography (MRN), is a continuously evolving field due to technological progress and increased awareness of MR capabilities both by radiologists and referring physicians. The term “MR neurography,” akin to MR angiography, was first used by Howe et al. in the early 1990s to describe the application of diffusion-weighted and fat-suppresed pulse sequences to delineate peripheral nerves from surrounding tissues [1]. The term now connotes any combination of qualitative or quantitative techniques, most adapted from those developed in other imaging subspecialities (neuro, abdominal, cardiac), to evaluate peripheral nerve pathology. In this review article, we highlight some important technical advancements and provide a few suggestions for optimizing MR neurography, based on our experience.
Hardware Development
Technologic development in MRN has been supported by widespread access to high-field, 3.0 T magnets and more efficient, multichannel surface coils placed around a body part that enable parallel imaging, a technique that exploits redundant coil sensitivities to accelerate imaging. Increased speed affords the ability to either increase the total imaged volume or augment through-place (thinner slices) or in-plane (smaller voxels) spatial resolution within a single acquisition. As most peripheral nerves in the human body are < 1 cm in diameter and many < 1 mm, spatial resolution becomes paramount to detect not only long-segment morphologic and signal intensity changes of the entire nerve but also more focal, fascicular changes that can be seen with both inflammatory and traumatic neuropathies [2].
Surface coils can be broadly categorized as “rigid” and “non-rigid,” or flexible. MRN has benefitted from the design of more flexible surface coils, including the ability to wrap a coil on itself without destructive interference (i.e., signal dropout). Increased flexibility has enabled placing coils closer to the skin surface, particularly in anatomical regions that vary in contour—e.g., the neck, shoulder, and axillary regions for evaluation of the brachial plexus and its branches and the inner thigh/gluteal region for evaluation of the sacral plexus and pudendal nerve. Positioning the coil closer to the skin, and therefore region of interest, affords higher signal intensity and potential for improved image quality.
For example, one challenge (among many) in brachial plexus MRI is acquiring sufficient signal-to-noise ratio (SNR) in the region of the infra-clavicular plexus and its terminal branches. Traditional coil arrangements include a rigid neurovascular array (Fig. 1a), which affords adequate SNR to evaluate the supraclavicular plexus (i.e., roots, trunks) but fails to provide sufficient SNR to evaluate the infra-clavicular plexus and terminal branches. We have instead found the combination of two flexible coils (Fig. 1b) secured over the neck/shoulder region to provide adequate SNR in both the supra- and infra-clavicular regions, although positioning can be challenging and potential for destructive interference between overlapping regions of both coils exists. The use of AIR™ coil technology, which allows for extreme coil flexibility while diminishing the potential for signal interference of overlapping elements, has enabled creation of a prototype, 64-channel brachial plexus receive coil, for use with a GE Signa Premier MRI system (Fig. 2a) [3]. This new brachial plexus coil provides flexibility to accommodate a wide range of body habiti, from the 5th percentile female to the 99 percentile male, and supports up to a three-fold acceleration factor. While still in development, this prototype coil covers the cervical, shoulder, axillary, and arm regions with high fidelity by wrapping coil elements tightly around these regions. The advantage of this coil is that it encompasses a large imaging volume, often required in brachial plexus studies, while still supporting high spatial resolution (Fig. 2b).
A “bilateral” brachial plexus prototype coil (General Electric Healthcare) is wrapped around a mannequin (a), for illustration purposes. Coronal maximum intensity projections (MIPs) from 3D short tau inversion recovery (STIR) pulse sequences obtained with this prototype coil demonstrate its ability to obtain imaging of the (b) bilateral plexi (field of view (FOV) = 40 cm) as well as the (c) unilateral right plexus (22-cm FOV), with adequate spatial resolution, in the same setting. An oblique sagittal T2-weighted Dixon fat suppressed sequence (d) with a 16-cm FOV, could also be obtained with clear demonstration of the extraforaminal C5-T1 roots
Software Development
Qualitative Imaging
By convention, MRN employs heavily T2-weighted fat-suppressed sequences to depict pathologic signal changes of the nerve, typically represented by increased T2 signal intensity that is thought to reflect edema at the site of injury [4]. The addition of fat suppression to the T2-weighted sequence facilitates resetting of the dynamic contrast range, thus increasing conspicuity of both normal and pathologic nerves as they course through intra- and inter-muscular, and subcutaneous, fat planes. MRN has benefitted from improvements in Dixon fat water separation techniques, which in the authors’ experience provide the most reliable combination of fat suppression and optimal SNR, compared with other techniques such as inversion recovery and conventional chemical fat suppression, to delineate nerve pathology and denervation edema patterns of muscle.
Two-dimensional (2D) sequences are relied upon to obtain high in-plane spatial resolution to detect fascicular abnormalities that are common in neuralgic amyotrophy (Parsonage-Turner syndrome) (Fig. 3) [5•]. Development of three-dimensional (3D) sequences has enabled the ability to obtain isotropic acquisitions, which can then be reformatted into arbitrary planes (curved multiplanar reformats) at the radiologist’s workstation, without distortion, along the longitudinal course of the nerves (Fig. 4) [6,7,8]. Longitudinal views are helpful both for the radiologist to detect subtle morphologic changes and are important for convenient demonstration of pathology to referring physicians, particularly for pre-operative planning. 3D sequences, however, have yet to replace 2D sequences in MR neurography as they do not afford the same, in-plane spatial resolution due to time constraints (to maintain adequate SNR), despite advanced parallel imaging techniques to reduce lengthy scan times.
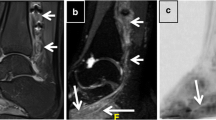
Twenty-four-year-old man with recurrent bouts of Parsonage-Turner syndrome (prior right long thoracic neuropathy), now with pronation weakness of the right hand. a Axial gradient echo fat-suppressed sequence through the distal right arm demonstrates selective enlargement and signal hyperintensity of an anteromedially positioned fascicular bundle (arrow) of the median nerve (oval) (magnified inset, bottom right). b Curved, longitudinal multiplanar reformatted image from the same sequence in (a) demonstrates multiple severe intrinsic constrictions of this same bundle. c Axial T2-weighted fast spin echo fat-suppressed image of the proximal right forearm demonstrates denervation edema pattern of the pronator teres (PT) and flexor carpi radialis (FCR) muscles
Curved multiplanar reformatted image of the right brachial plexus from a 3D short tau inversion recovery (STIR) pulse sequence following intravenous gadolinium demonstrates clear depiction of the supra- and infra-clavicular portions of the plexus, with complete vascular suppression. Also note a beaded appearance, indicating severe intrinsic constrictions, of the suprascapular nerve (bracket) in this patient with Parsonage-Turner syndrome. Note glenohumeral joint fluid (GH) for reference
As previously mentioned, brachial plexus MRI is inherently challenging due to anatomic coverage requirements. However, it is further complicated by the potential for respiratory-induced motion artifact. One technique we have successfully adopted (from abdominal/cardiac MRI) to reduce respiratory motion artifact and improve image quality is prospective respiratory gating, with a bellows wrapped around the patient’s torso [9]. The bellows detect end-expiration of the respiratory cycle, coinciding with relative stasis of the chest wall. However, as the technique adds additional time and its benefit is limited in patients with an irregular respiratory rhythm, future techniques to minimize respiratory motion are needed.
Vascular suppression is also a critical component in brachial plexus MRI to reliably differentiate small-caliber but clinically relevant branch nerves (e.g., suprascapular, long thoracic) from adjacent veins and arteries. Non-contrast vascular suppression techniques that have been implemented, however, are unable to suppress signal from slow-flowing blood in neck and pelvic veins. The most commonly used vascular suppression techniques—3D motion-sensitized driven equilibrium (flow-saturation preparation) and 3D diffusion-weighted reversed fast imaging with steady state precession (3D DW-PSIF) sequences—also suffer from lengthy acquisition time, motion artifact, and field inhomogeneity susceptibility [10, 11•, 12]. The use of intravenous (IV) gadolinium combined with a short tau inversion recovery (STIR) pulse in MRN has been recently shown to effectively provide simultaneous fat and vascular suppression [13, 14]. In our experience, a 3D STIR sequence with IV gadolinium is superior to both non-contrast 3D STIR and MSDE sequences for vascular suppression in the region of the brachial plexus as well as visualization of branch nerves (suprascapular, axillary, long thoracic) of the plexus (Fig. 4) [15].
Quantitative Imaging
One major shortcoming of MRN is its overall qualitative nature and inability to reliably quantify degree of nerve injury and regeneration, particularly when the nerve remains in continuity (e.g., stretch or compression injury). The most common quantitative approach to evaluate peripheral nerves is diffusion-weighted imaging (DWI) [16]. The longitudinal fiber orientation of peripheral nerves makes them uniquely suited for DWI and in particular diffusion tensor imaging (DTI), which assesses both the directionality and magnitude of diffusion. In general, when nerves are injured, they lose their inherent anisotropy (i.e., preferential movement of water molecules in a particular direction), and this can be expressed as a scalar measurement, i.e., fractional anisotropy (FA). Peripheral nerves pose unique challenges for DWI including proclivity to distortion, aliasing, and requirement for high spatial resolution; some of these can be addressed with techniques developed for the brain but others will likely require specific development for the periphery to move DWI from the research to clinical realm [16]. Diffusion tractography, extrapolated from diffusion data, is an imaging tool that can depict the precise, 3D spatial relationship of extrinsic and intrinsic masses, most commonly peripheral nerve sheath tumors, and adjacent nerves [8]. DTI-based tractography is the most widely used tractography method and has shown promise in pre-operative planning for tumor excision (Fig. 5) [17].
Sagittal Dixon fat suppression (a) image of the left forearm demonstrates a peripheral nerve sheath tumor arising from the median nerve. Tractography images, acquired from a diffusion tensor imaging sequence, are fused to the sagittal (b) and (c) axial (PD) images and demonstrate the relationship of nerve fibers relative to the mass, which may be useful for pre-surgical planning. (This figure appeared in the Spring 2018, ISMRM Edition (Volume 23) of SIGNA Pulse of MR, republished with permission from GE Healthcare)
MRN complements electrodiagnostic testing in the work-up of peripheral neuropathies. In particular, MRN provides important information regarding the presence of muscle denervation, typically manifested as increased T2-weighted signal intensity (thought to reflect increased blood volume and extracellular fluid) in the acute/subacute stage and fatty infiltration in the chronic stage. Quantitative T2 mapping technique techniques are able to generate T2 relaxation values that reflect tissue biostructure and free water content and in a preliminary study have correlated with electromyography grades of denervation [18]. Quantitative Dixon fat/water separation techniques, in addition to T2 mapping, have shown a correlation between muscle fat quantification and disease progression and functional status [19,20,21]. We anticipate that fat quantification techniques can be applied as well in MRN to determine the extent of denervation.
Present and Future MRN Optimization
Maximizing diagnostic yield of MRN exams requires not only employing the most advanced technology but also careful planning, monitoring, and interpretation by radiologists with specific expertise in the field. At our institution, all MRN exams are protocoled in advanced by a radiologist after reviewing clinical notes, electrodiagnostic exams, and relevant prior imaging. Exams are acquired by technologists with experience in performing MRN and monitored by radiologists. Real-time monitoring allows the radiologist, as necessary, to both alter the focus of the exam and obtain non-traditional acquisition planes that target particular nerves. For example, if the stated clinical concern is scapular winging of unclear etiology, the trapezius and serratus anterior can first be evaluated for signs of muscle denervation. The radiologist can then tailor the MRI, which may involve a completely different surface coil setup, to evaluate either the spinal accessory nerve (neck to upper shoulder) or long thoracic nerve (lower neck to chest wall) depending on which muscle is involved.
Conclusions
Our everyday experience, supported by others’, is that MRN strongly influences diagnosis and management of peripheral neuropathies, and we expect its role to expand with improvements in high-field MRI (≥ 7 T) and increased awareness by the scientific community of its capability [22, 23, 24•]. Artificial intelligence will likely abet MRN’s development by improving image quality, via denoising and artifact correction algorithms, and support quantitative techniques, via automated peripheral nerve segmentation, that shed light on peripheral nerve and muscle physiology [25, 26].
References
Papers of particular interest, published recently, have been highlighted as: • Of importance
Howe FA, Filler AG, Bell BA, Griffiths JR. Magnetic resonance neurography. Magn Reson Med. 1992;28:328–38.
Heinen C, Dömer P, Schmidt T, Kewitz B, Janssen-Biehnhold U, Kretschmer T. Fascicular ratio pilot study: high-resolution neurosonography-a possible tool for quantitative assessment of traumatic peripheral nerve lesions before and after nerve surgery. Neurosurgery. 2018;85:415–422.
McGee KP, Stormont RS, Lindsay SA, Taracila V, Savitskij D, Robb F, et al. Characterization and evaluation of a flexible MRI receive coil array for radiation therapy MR treatment planning using highly decoupled RF circuits. Phys Med Biol. 2018;63:08NT02.
Bordalo-Rodrigues M. Magnetic resonance neurography in musculoskeletal disorders. Magn Reson Imaging Clin N Am. 2018;26:615–30.
• Sneag DB, Rancy SK, Wolfe SW, Lee SC, Kalia V, Lee SK, et al. Brachial plexitis or neuritis? MRI features of lesion distribution in parsonage-turner syndrome. Muscle Nerve. 2018;58:359–66 This study demonstrates that the prevailing imaging findings in Parsonage-Turner syndrome are intrinsic constrictions of peripheral nerves distal to the brachial plexus proper.
Chhabra A, Rozen S, Scott K. Three-dimensional MR neurography of the lumbosacral plexus. Semin Musculoskelet Radiol. 2015;19:149–59.
Chhabra A, Thawait GK, Soldatos T, Thakkar RS, Del Grande F, Chalian M, et al. High-resolution 3T MR neurography of the brachial plexus and its branches, with emphasis on 3D imaging. AJNR Am J Neuroradiol. 2013;34:486–97.
Fritz J, Ahlawat S. High-resolution three-dimensional and cinematic rendering MR neurography. Radiology. 2018;288:25.
Sneag DB, Mendapara P, Zhu JC, Lee SC, Lin B, Curlin J, et al. Prospective respiratory triggering improves high resolution brachial plexus magnetic resonance image quality. J Magn Reson Imaging. 2019;49:1723–9.
Yoneyama M, Takahara T, Kwee TC, Nakamura M, Tabuchi T. Rapid high resolution MR neurography with a diffusion-weighted pre-pulse. Magn Reson Med Sci. 2013;12:111–9.
• Cervantes B, Kirschke JS, Klupp E, Kooijman H, Börnert P, Haase A, et al. Orthogonally combined motion- and diffusion-sensitized driven equilibrium (OC-MDSDE) preparation for vessel signal suppression in 3D turbo spin echo imaging of peripheral nerves in the extremities. Magn Reson Med. 2017;79:407–15 This article describes a non-contrast technique for vascular suppression in MRN.
Chhabra A, Subhawong TK, Bizzell C, Flammang A, Soldatos T. 3T MR neurography using three-dimensional diffusion-weighted PSIF: technical issues and advantages. Skelet Radiol. 2011;40:1355–60.
Chen WC, Tsai YH, Weng HH, Wang SC, Liu HL, Peng SL, et al. Value of enhancement technique in 3D-T2-STIR images of the brachial plexus. J Comput Assist Tomogr. 2014;38:335–9.
Wang L, Niu Y, Kong X, Yu Q, Kong X, Lv Y, et al. The application of paramagnetic contrast-based T2 effect to 3D heavily T2W high-resolution MR imaging of the brachial plexus and its branches. Eur J Radiol. 2016;85:578–84.
Sneag DB, Curlin J, Shin J, Fung M, Lin B, Daniels SP. High-resolution brachial plexus imaging using 3-D short tau inversion recovery (CUBESTIR) with IV gadolinium for vascular suppression. International Society for Magnetic Resonance in Medicine Annual Meeting. May 14, 2019.
Noguerol M, Barousse R, Socolovsky M, Luna A. Quantitative magnetic resonance (MR) neurography for evaluation of peripheral nerves and plexus injuries. Quant Imaging Med Surg. 2017;7:398–421.
Cage TA, Yuh EL, Hou SW, Birk H, Simon NG, Noss R, et al. Visualization of nerve fibers and their relationship to peripheral nerve tumors by diffusion tensor imaging. Neurosurg Focus. 2015;39:E16.
Shah P, Argentieri E, Koff MF, Sneag DB. Quantitative evaluation of T2 signal intensity for the assessment of muscle denervation. ISMRM 25th Scientific Meeting & Exhibition. Honolulu, HI. April 22–27, 2017.
Burakiewicz J, Sinclair CDJ, Fischer D, Walter GA, Kan HE, Hollingsworth KG. Quantifying fat replacement of muscle by quantitative MRI in muscular dystrophy. J Neurol. 2017;264(10):2053–67.
Heskamp L, van Nimwegen M, Ploegmakers MJ, Bassez G, Deux JF, Cumming SA, et al. Lower extremity muscle pathology in myotonic dystrophy type 1 assessed by quantitative MRI. Neurology. 2019;92:e2803–14.
Yin L, Xie ZY, Xu HY, Zheng SS, Wang ZX, Xiao JX, et al. T2 mapping and fat quantification of thigh muscles in children with Duchenne muscular dystrophy. Curr Med Sci. 2019;39:138–45.
Chhabra A, Belzberg AJ, Rosson GD, Thawait GK, Chalian M, Farahani SJ, et al. Impact of high resolution 3 tesla MR neurography (MRN) on diagnostic thinking and therapeutic patient management. Eur Radiol. 2016;26:1235–44.
Fisher S, Wadhwa V, Manthuruthil C, Cheng J, Chhabra A. Clinical impact of magnetic resonance neurography in patients with brachial plexus neuropathies. Br J Radiol. 2016;89:20160503.
• Yoon D, Biswal S, Rutt B, Lutz A, Hargreaves B. Feasibility of 7T MRI for imaging fascicular structures of peripheral nerves. Muscle Nerve. 2018;57:494–8 This article describes the role of 7T for evaluating fascicular architecture of peripheral nerves.
Shin J, Curlin J, Tan ET, Fung M, Sneag DB. Denoising of diffusion MRI improves peripheral nerve conspicuity and reproducibility. International Society for Magnetic Resonance in Medicine Annual Meeting. Montreal, Canada. May 13, 2019.
Balsiger F, Steindel C, Arn M, Wagner B, Grunder L, El-Koussy M, et al. Segmentation of peripheral nerves from magnetic resonance neurography: a fully-automatic, deep learning-based approach. Front Neurol. 2018;9:777.
Acknowledgments
The authors would like to thank Drs. Fraser Robb and Yun-Jeong Stickle from GE Healthcare, Inc., in Aurora, OH, for designing and building the 64-channel prototype brachial plexus coil mentioned in this article.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Darryl B. Sneag and Sophie Queler report the Hospital for Special Surgery has an institutional research agreement with GE Healthcare.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
This article is part of the Topical Collection on Nerve and Muscle
Rights and permissions
About this article
Cite this article
Sneag, D.B., Queler, S. Technological Advancements in Magnetic Resonance Neurography. Curr Neurol Neurosci Rep 19, 75 (2019). https://doi.org/10.1007/s11910-019-0996-x
Published:
DOI: https://doi.org/10.1007/s11910-019-0996-x