Abstract
Purpose of the Review
To discuss the diagnostic approach to patients with septic encephalopathy as well as the need for specific neuro-monitoring and the perspectives on future therapeutic approaches in this setting.
Recent Findings
Most of data-concern experimental studies evaluating the pathophysiology of septic encephalopathy. A combination of neurodegenerative pathways with neurovascular injury is the cornerstone for the development of such complication and the long-term neurological sequelae among survivors.
Summary
Septic encephalopathy is a common complication in septic patients. Clinical presentation may range from mild confusion and disorientation to convulsions and deep coma. The diagnosis of septic encephalopathy is made difficult by the lack of any specific clinical and non-clinical feature, in particular among sedated patients in whom neurological examination is unreliable. In spite of the high mortality rate associated with this condition, there is no prophylactic or targeted therapy to reduce or minimize brain damage in septic patients and clinical management is limited to the treatment of the underlying infection.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sepsis is defined as the overwhelming inflammatory response of the host immune system to an infection, which eventually leads to the development of at least one organ dysfunction [1]. The occurrence of sepsis is associated with a mortality rate of 20–25%, which may significantly increase in case of fragile patients (i.e., immunosuppression, older age, or multiple comorbid diseases) or in case of shock (i.e., cardiovascular dysfunction associated with signs of tissue hypoxia) [2]. Additionally, despite the important progress in the diagnosis and treatment of this condition, a high degree of mortality is still noted and several cases are still inadequately managed because of delayed sepsis recognition, inappropriate or delayed initial antibiotic therapy, and poor control of the infectious focus or support of the failing organs [3••].
The better understanding of sepsis pathophysiology has progressively led to a change in the diagnostic approach of this syndrome, which has moved from the evidence of persistent bacteremia as diagnostic criteria to the identification of a systemic inflammatory response syndrome (SIRS) as main feature of this syndrome [4]. Nevertheless, the insufficient specificity and sensitivity of the SIRS criteria to diagnose sepsis has been well recognized in the recent Sepsis-3 definitions [5••], which have based the diagnosis of sepsis on the presence of organ dysfunctions and/or lactate measurements rather than SIRS or microbiological findings.
Because the nervous system is extremely susceptible to different extra-cerebral factors, brain dysfunction is quite common during sepsis [6] and it is not surprising that an “altered mental status” has been introduced among those clinical features that should be used to identify patients at risk of sepsis outside the Intensive Care Unit, such as at the Emergency Department or at the ward [7]. In this review, we intended to provide an overview of the main characteristics of the so-called “septic encephalopathy” and to discuss practical aspects of monitoring and management as well as elucidate unresolved questions and future directions of the research in this field.
Definition
Septic encephalopathy can be defined as a diffuse brain dysfunction occurring in a patient with sepsis without evidence of an intracranial infection and/or without conditions (i.e., metabolic alterations) unrelated to the infectious process that would significantly alter brain function [8]. The diagnosis should therefore exclude structural brain lesions or primary central nervous system diseases, such as cerebrovascular events or meningitis, seizures, drug intoxication, fat embolism syndrome, autoimmune, or inflammatory brain diseases, such as vasculitis or thrombotic microangiopathy, and anoxic brain injury [9, 10]. This definition, which is not unanimously accepted, therefore encompasses a large panel of clinical signs and symptoms and requires additional diagnostic tests to exclude non-infectious causes of encephalopathy; as such, all epidemiological data concerning septic encephalopathy could have been biased by the clinical and complementary tests that have been used for its recognition and are often difficult to compare among studies. Moreover, few data are available on the best definition of this condition for sedated patients, in whom encephalopathy may occur when neurological examination remains unreliable [11].
Pathophysiology
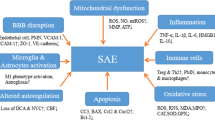
The pathophysiology of septic encephalopathy is still poorly understood; although many mechanisms have been proposed, it is likely to be multifactorial and totally independent from the presence of micro-organisms and/or toxins within the cerebral tissue [12]. The current hypothesis is a combination of direct alterations in brain cells function and signaling with abnormalities of brain perfusion and oxygenation, which can be eventually enhanced by external events, such as renal and/or hepatic dysfunction, disglycemia, fever, the use of neurotropic drugs, or environmental factors (Fig. 1). The combination of these two main mechanisms, i.e., “neurodegenerative” and “neurovascular,” will result in different clinical presentations, according to the severity of sepsis, the presence of previous neurological diseases and patient-related brain susceptibility.
Summary of main potential mechanisms implicated in the development of septic encephalopathy. CVOs = circumventricular organs; NMDA-R = N-methyl-D-aspartate receptors for glutamate; BBB = blood-brain barrier; TLR = toll-like receptors; Ach = acetylcholine; NH4 = ammonium; 5-HT = serotonin; AQP4 = aquaporin-4
Alterations in Brain Cells Function and Signaling
When systemic inflammation occurs, two major pathways allow an immune-brain cross-talk despite the presence of the blood-brain barrier (BBB): the circumventricular organs, located in the midline ventricular system, and the vagus nerve. The circumventricular organs lack a BBB and represent the site where a direct communication between brain and systemic circulation occurs. These organs are located close to neuroendocrine structures, as such corpus pineale and neurohypophysis, or to brainstem centers, such as the area postrema, which could explain the intense dysregulation of the autonomic and hormonal homeostasis during sepsis [13]. The vagus nerve detects visceral inflammation and produces an anti-inflammatory activity through cholinergic pathways. Systemic inflammation also triggers the activation of behavioral, neuroendocrine, and neurovegetative centers, as the afferent vagus signals are relayed to the nucleus tractus solitarius in the brainstem [14]. The results of these phenomena is the activation of microglia, which acquire neurotoxic properties, notably by releasing nitric oxide, cytokines, reactive oxygen species, and glutamate, thereby inducing neuronal death in vulnerable cerebral areas [15].
Systemic inflammation also triggers the local production of cytokines (i.e., “neuroinflammation”), which mediates neuronal dysfunction and, ultimately, cell death. In this setting, TNF-α and IL-6 appear to be the most relevant inflammatory mediators [16, 17]. Significant neuro-inflammation induces neutrophil infiltration of the brain tissue, astrocytes activation through the toll-like receptors (TLRs), over-expression of aquaporin 4, and increased synthesis of prostaglandins and nitric oxide that activate the hypothalamus and the adrenal axis [18, 19•, 20]. These mechanisms result in behavioral alterations, fever and severe neurological impairment because of brain edema and neuronal apoptosis [21].
Several neurotransmitters are also associated with septic encephalopathy, including the dysregulation of the cholinergic and gamma-aminobutyric acid pathways, together with an imbalance in norepinephrine, serotonin and dopamine production or increased ammonium production [22, 23]. Inflammatory is the main determinant of altered cerebral neurotransmission during sepsis [24•]. Deficits in cholinergic function have been postulated to cause delirium and cognitive decline in septic patients [22], although clinical studies showed no benefits from the administration of the cholinesterase inhibitor rivastigmine, compared with placebo, on the occurrence of delirium [25]. Reduced levels of branched-chain amino acids (i.e., leucine, isoleucine, and valine) or extensive muscle proteolysis and reduced hepatic clearance may result into increased brain concentrations of aromatic amino acids (i.e., tyrosine and tryptophan), which can induce brain dysfunction by acting as false neurotransmitters, can reduce cerebral concentrations of norepinephrine, dopamine, and serotonin, or could shut down brain metabolism by decreasing glucose utilization in different brain regions [26, 27••, 28].
Sepsis encephalopathy could also be associated with mitochondrial dysfunction, which can have remarkable consequences on reduced ATP generation and inhibition of mitochondrial respiration, all being responsible for pathological processes in nerve cells and increased neuronal apoptosis [29]. Mitochondrial dysfunction could also contribute to increased intracellular calcium levels, which alter the function of several cytoplasmic enzymes and proteins and may result in impaired learning memory and cognitive function [30].
Abnormalities of Brain Perfusion and Oxygenation
During sepsis, the integrity of the BBB is altered because of several mechanisms inducing alterations in astrocytes, pericytes, and endothelial cells [31•]. In particular, excessive nitric oxide release via the early induction of the endothelial nitric oxide synthase results in a proinflammatory status, causing the activation and dysfunction of cerebrovascular endothelial cells [32]. Also, circulating pro-inflammatory mediators promote the expression of adhesion molecules on brain microvessel endothelial cells that facilitates the passage of neurotoxic factors and of inflammatory cells within the brain tissue [33]. As such, endothelial dysfunction will result into two main pathological findings. The first is the alteration of cerebral microcirculation, which is associated with tissue hypoxia and disturbed cerebral metabolism, in particular when associated with hypotension [34, 35]. The second is the impairment of endothelial cerebral cells to adequately react to external stimuli, such as changes in blood pressure or carbon dioxide, which result in alteration of cerebral autoregulation [36, 37]. The loss of cerebral autoregulation makes the brain more susceptible to tissue hypoperfusion in case of significant changes in mean arterial pressure, in particular in the elderly patients or those with previous cerebrovascular disease [38].
Clinical Presentation and Diagnosis
Neurological dysfunction during septic encephalopathy ranges from mild confusion and lethargy to disturbed cognitive functions and coma [39]. In some cases, patients can also present with muscular rigidity, tremors, or convulsions [40]. Neurological disturbances of this syndrome are similar to the description of delirium, which is often considered as one manifestation of brain dysfunction during sepsis [41]. An accurate diagnosis of septic encephalopathy is often complicated by the concomitant use of sedatives or other systemic disturbances, including liver and kidney failure, hypoglycemia, or severe hypoxemia. Focal involvement of cranial nerves or unilateral symptoms, such as hemiparesis or aphasia, is rare and should bring to additional diagnostic tests to exclude other neurological complications.
Although septic encephalopathy has been considered as a reversible syndrome, mild to moderate neurological symptoms, including memory alterations, depression, anxiety, or cognitive disturbances may persist in up to 40% of patients 1 year after hospital discharge [42••, 43]. According to several studies using brain imaging or experimental post-mortem findings, these long-term cognitive alterations are related to neurodegenerative microglial activation and diffuse ischemic damage, which result in a significant reduction of brain volume and produce the same histopathological changes than chronic neurodegenerative diseases [44, 45].
Because of the variability of the clinical presentation, the diagnosis of septic encephalopathy is challenging for clinicians. After the exclusion of other evident causes of encephalopathy, clinical examination could be integrated with same clinical scales, such as the Glasgow Coma Scale (GCS), the Confusion Assessment Method for the ICU (CAM) scale, the Richmond Agitation Sedation-Scale (RASS) scale or the Full Outline of UnResponsiveness (FOUR) scale [11]. These scales are not specific for the diagnosis of septic encephalopathy, but are very informative to detect subtle changes in awake septic patients who apparently have a normal neurological examination, in particular the CAM-ICU and the FOUR scale for non-intubated and intubated patients; respectively [46, 47]. The analysis of cerebrospinal fluid is not specific for septic encephalopathy and often shows a mild increase in protein concentrations, which may suggest a local inflammation or BBB impairment [48].
Electroencephalography (EEG) is particularly sensitive to detect changes in cortical function during sepsis; slow EEG background can appear even in patients with no clinical abnormalities, while more severe forms of consciousness disturbances are associated with the appearance of delta activity, suppressed patterns, and the lack of background reactivity to external stimuli [49]. Moreover, EEG can detect clinically silent non-convulsive status epilepticus, which occur in up to 20% of comatose septic patients [50]. Septic patients have also a higher risk of long-term seizures than both the general population and other hospitalized patients, suggesting again the presence of permanent neurologic sequelae [51••]. More recently, alterations in EEG variability have been correlated with lower variability of heart rate, which would suggest a common pathways between alterations in cortical activity and autonomic and/or brainstem dysfunction [52•].
There are not specific biomarkers for the diagnosis of septic encephalopathy. Neuron-specific enolase (NSE) and S100β, which are biomarkers for neuronal and glial lesions, respectively, may be increased in septic shock [53, 54•]; also, a decrease in cerebral blood flow or impaired cerebral autoregulation are correlated with increased serum S100β levels among patients with severe sepsis and septic shock [55]. Increased NSE and S100β concentrations have been associated with poor outcome; more importantly, an increase of such biomarkers might help to identify patients with brain lesions on neuroimaging, in particular when sedation prevents the use of clinical examination in such patients [56]. Finally, neuroimaging is often unremarkable in these patients; however, cerebral ischemia can be identified on cerebral magnetic resonance imaging (MRI) in 30% of patients who present with focal neurological signs during sepsis [57, 58]. Also, vasogenic edema and severe leukoencephalopathy could be identified by MRI in those patients suffering from septic shock and presenting with persistent coma, probably related to the loss of autoregulation and microvascular injury [59].
Epidemiology
Because of the lack of a clear definition, septic encephalopathy is often diagnosed by exclusion of other potential causes of encephalopathy. As such, a precise description of the incidence and prevalence of septic encephalopathy is challenging and has been studied in few studies to range between 9 and 71% [9, 60]. Septic encephalopathy represents around 10% of all acute febrile encephalopathies and is one of the most common forms of encephalopathy encountered in critically ill patients [8, 10]. In a large cohort of mechanically ventilated patients, mostly septic or with acute pneumonia, altered mental state was observed in 66% of them at some point during the ICU stay [60]. In a prospective study of 1758 patients admitted to a medical ICU, 217 of them experienced a neurological complication; encephalopathy was present in one third of patients, and SAE was the most frequent etiology [61]. In a series on 69 non-sedated patients with bacteremia, 70% of them had clinical signs of brain dysfunction and half of them showed severe encephalopathy [62]. In a recent large cohort study of septic patients, acute mental state abnormalities were described in more than 50% of them [63••].
How to Monitor
Neuromonitoring of septic patients is potentially of great help for the management and prognostication. Several tools have been proposed for the monitoring of the septic brain and to provide insights into the complex pathophysiology of brain impairment during sepsis; a multimodal monitoring approach is increasingly used in this setting and should be adapted according to the clinical status of the patient (Fig. 2) [64].
Awake Patient
In non-sedated patients, neurological examination, including at least the motor response and the assessment of brainstem reflexes, is the first step for an adequate brain monitoring [11]. However, avoiding cerebral hypoperfusion might also be interesting in such patients. Cerebral oxygenation could be non-invasively evaluated using near-infrared spectroscopy (NIRS) devices, which enables to measure the hemoglobin oxygen saturation of the frontal cortical regions of the brain and can detect major perfusion and circulatory alterations [65]. Also, the correlation between temporal changes in the NIRS-derived cerebral oxygenation and mean arterial blood pressure could be used to assess cerebral autoregulation and be potentially useful for the definition of the optimal MAP target in these patients [65, 66]. Nevertheless, non-invasive cerebral oxygenation measured with NIRS is also influenced by extra-cranial circulation and would assess only anterior circulation [67]. Moreover, few studies on the role of cerebral NIRS in septic patients are available and more data are necessary before recommending this monitoring as a routine procedure.
Similarly, Transcranial Doppler (TCD) is a non-invasive and easily available bedside tool to measure the blood velocity in the main cerebral vessels and could potentially provide indirect information about changes in cerebral blood flow and the status of cerebral autoregulation as well as cerebrovascular reactivity to carbon dioxide (PaCO2) [68]. Some authors have demonstrated that changes in the pulsatility index (PI), an indicator of cerebro-vascular resistances, are associated with clinical symptoms and might have a correlation with the development of delirium [69]. Using TCD, it is also possible to assess the dynamic cerebral autoregulation, which is based on the relationship between arterial blood pressure and cerebral blood flow velocities spontaneous fluctuations [68]. Pfister et al. showed that cerebral autoregulation is often impaired in patients with septic shock, and that high levels of PaCO2 may further compromise cerebral autoregulation in these patients [55]. The main limitation of such approach is related to the interpretation of the PI, which can also be increased because of hypocapnia secondary to hyperventilation, very low diastolic pressure, and pre-existing cerebrovascular disease, which are very common conditions in septic patients. Moreover, the dynamic assessment of cerebral autoregulation using TCD requires few movements of patient’s head to obtain a good recording, which makes this technique effective mostly in sedated patients, and a specific software to rapidly quantify cerebral autoregulation, which is not available in all hospitals.
Sedated or Comatose Patient
In addition to NIRS and TCD, continuous EEG recording would be necessary in most of sedated or comatose septic patients. First of all, EEG recording is extremely sensitive to systemic inflammation and slowering of EEG background or the development of left-right asymmetry might be used to identify neurological deterioration and promptly initiate additional diagnostic tests [49]. Moreover, EEG could detect non-convulsive seizures in up to 20–30% of these patients, which would translate in an immediate administration of anti-epileptic drugs to avoid further brain injury [50, 57, 70]. A quantitative EEG analysis (i.e., the combination of EEG frequency, amplitude, power) could also be used to calculate the burst-suppression ratio (BSR), which could be secondary to excessive sedative regimens and is associated with the occurrence of delirium in these patients [71••].
Although limited data exist on the diagnostic role of serum biomarkers of brain injury in septic patients, elevated serum S100β and/or NSE concentrations were significantly higher in patients with brain dysfunction and predicted the presence of cerebral lesions on neuroimaging [56]. Considering the difficulties to transport septic patients on mechanical ventilation or extra-corporeal therapies, S100β or NSE assessment could be used as a trigger for neuroimaging in this setting. If neuroimaging is decided, CT scan should be the first-line exam to exclude the presence of intracranial pathology, in particular in case of seizures or in patients with focal neurological signs [72]. Nevertheless, MRI is more sensitive to detect microvascular injury, embolic events, the development of brain edema, or of the posterior reversible encephalopathy syndrome (PRES) [57].
Finally, concomitant drugs or metabolic alterations can also contribute to the neurological deterioration in septic patients and should be monitored whenever possible. In particular, monitoring the depth of sedation and the use of alternative drugs to benzodiazepines may reduce the risk of delirium; therapeutic drug monitoring of β-lactam concentrations could detect drug overdosing, which is associated with a significant risk of neuro-worsening; avoiding hyper- and hypo-glycemia may protect neuronal cells from apoptosis; avoiding significant changes in urea levels at the initiation of renal replacement therapy can reduce the risk of the dialysis disequilibrium syndrome and of brain edema; monitoring the degree of anticoagulation may reduce the risk of intracerebral bleeding [73••, 74••, 75, 76].
Any Implications for Treatment?
Despite the advances in the understanding of the pathophysiology and treatment of sepsis, an effective treatment for septic encephalopathy is not available. The cornerstone of the management of this condition relays on the early treatment of the septic conditions with the support of failing organs. Nevertheless, some practical issue should be considered in such patients. First, early withdrawal of sedative treatment can be helpful to reduce the risk of delirium and to enable an early neurological assessment. In particular, benzodiazepines should be avoided. Other interventions that have been used to reduce the risk of delirium might also be helpful for septic encephalopathy, such as aid to orientation (i.e., clock and photos in the room), adequate lighting, early mobilization, avoiding sleep deprivation, and noise reduction, although these have to be better demonstrated. Prophylactic use of antiepileptic drugs is not recommended, but early EEG monitoring should be implemented in comatose or sedated patients to detect seizures and provide therapy only when necessary. Optimization of cerebral perfusion using NIRS or TCD is an intriguing approach, although adequate targets of therapy using these tools are still lacking.
In human studies, α-agonist agent, such as dexmedetomidine, has proven to have neuroprotective effects in septic patients who had more delirium-free days and lower 28-day mortality when compared to patients treated with lorazepam [77]. In addition, dexmedetomidine exerts its positive effects through the inhibition of neuronal apoptosis and the reduction in the sepsis-associated inflammatory response as well [78].
Antipsychotic drugs may effectively treat some symptoms of hyperactive encephalopathy, such as agitation or hallucinations, but are poorly effective in hypoactive status. Although promising [79], recombinant-activated protein C is no more available in the therapeutic armamentarium and cannot be administered in septic patients. Experimental data suggest a potential role for other drugs, such as minocycline, intravenous immunoglobulins or statins, although no human data are available yet [80,81,82].
Conclusions
Septic encephalopathy is characterized by extracranial infection and disturbed mental state without any direct causes of brain injury except of the systemic inflammatory status related to sepsis. Although septic encephalopathy is common among critically ill patients, it is largely underestimated and often not diagnosed. Together with clinical evaluation of brain function, a combination of NIRS, TCD, EEG, biomarkers of brain injury and neuroimaging could be used to better assess the degree of brain dysfunction in such patients as well as potentially help clinicians to adjust therapy. The pathophysiology is complex and still poorly understood, so that treatment remains mainly symptomatic. Adequate therapy of the underlying sepsis syndrome and supportive intensive care is necessary. The occurrence of septic encephalopathy is associated with cognitive impairment in a large proportion of survivors. Therefore, increasing efforts to better characterize the pathophysiology, clinical course and effective therapies of septic encephalopathy are the main fields of research in the next future.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Vandijck DM, Reynvoet E, Blot SI, Vandecasteele E, Hoste EA. Severe infection, sepsis and acute kidney injury. Acta Clin Belg Suppl. 2007;2:332–6.
Thursky K, Lingaratnam S, Jayarajan J, Haeusler GM, Teh B, Tew M, et al. Implementation of a whole of hospital sepsis clinical pathway in a cancer hospital: impact on sepsis management, outcomes and costs. BMJ Open Qual. 2018;7(3):e000355.
•• Coopersmith CM, De Backer D, Deutschman CS, Ferrer R, Lat I, Machado FR, Martin GS, Martin-Loeches I, Nunnally ME, Antonelli M, Evans LE, Hellman J, Jog S, Kesecioglu J, Levy MM, Rhodes A. Surviving sepsis campaign: research priorities for sepsis and septic shock. Intensive Care Med 2018. Recent review article summarizing the priorities for research in sepsis.
Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. 1992;101(6):1644–55.
•• Singer M, Deutschman CS, Seymour CW, Shankar-Hari M, Annane D, Bauer M, et al. The third international consensus definitions for sepsis and septic shock (sepsis-3). JAMA. 2016;315(8):801–10 Recent definitions of sepsis according to an international panel of experts and analysis of large US databases.
Eidelman LA, Putterman D, Putterman C, Sprung CL. The spectrum of septic encephalopathy. Definitions, etiologies, and mortalities. JAMA. 1996;275(6):470–3.
Seymour CW, Liu VX, Iwashyna TJ, Brunkhorst FM, Rea TD, Scherag A, et al. Assessment of clinical criteria for sepsis: for the third international consensus definitions for sepsis and septic shock (Sepsis-3). JAMA. 2016;315(8):762–74.
Wilson JX, Young GB. Sepsis-associated encephalopathy: evolving concepts. Can J Neurol Sci. 2003;30:98–105.
Young GB, Bolton CF, Austin TW, Archibald YM, Gonder J, Wells GA. The encephalopathy associated with sepsis illness. Clin Invest Med. 1990;13:297–304.
Davies NWS, Sharief MK, Howard RS. Infection-associated encephalopathies - their investigation, diagnosis and treatment. J Neurol. 2006;253:833–45.
Sharshar T, Citerio G, Andrews PJ, Chieregato A, Latronico N, Menon DK, et al. Neurological examination of critically ill patients: a pragmatic approach. Report of an ESICM expert panel. Intensive Care Med. 2014;40(4):484–95.
Siami S, Annane D, Sharshar T. The encephalopathy in sepsis. Crit Care Clin. 2008;24:67–82.
Wuerfel E, Infante-Duarte C, Glumm R, Wuerfel JT. Gadofluorine M-enhanced MRI shows involvement of circumventricular organs in neuroinflammation. J Neuroinflammation. 2010;7:70.
Borovikova LV, Ivanova S, Zhang M, Yang H, Botchkina GI, Watkins LR, et al. Vagus nerve stimulation attenuates the systemic inflammatory response to endotoxin. Nature. 2000;405:458–62.
Sharshar T, Gray F, Lorin de la Grandmaison G, Hopkinson NS, Ross E, Dorandeu A, et al. Apoptosis of neurons in cardiovascular autonomic centres triggered by inducible nitric oxide synthase after death from septic shock. Lancet. 2003;362:1799–805.
Alexander JJ, Jacob A, Cunningham P, Hensley L, Quigg RJ. TNF is a key mediator of septic encephalopathy acting through its receptor, TNF receptor-1. Neurochem Int. 2008;52:447–56.
Chong DL, Sriskandan S. Pro-inflammatory mechanisms in sepsis. Contrib Microbiol. 2011;17:86–107.
Rorato R, Menezes AM, Giusti-Paiva A, de Castro M, Antunes-Rodrigues J, Elias LL. Prostaglandin mediates endotoxaemia-induced hypophagia by activation of pro-opiomelanocortin and corticotrophin-releasing factor neurons in rats. Exp Physiol. 2009;94(3):371–9.
• Rump K, Adamzik M. Function of aquaporins in sepsis: a systematic review. Cell Biosci. 2018;8:10 Interesting and completed review on the role of aquaporins in sepsis, including septic encephalopathy.
Takatani Y, Ono K, Suzuki H, Inaba M, Sawada M, Matsuda N. Inducible nitric oxide synthase during the late phase of sepsis is associated with hypothermia and immune cell migration. Lab Investig. 2018;98(5):629–39.
Ning Q, Liu Z, Wang X, Zhang R, Zhang J, Yang M, et al. Neurodegenerative changes and neuroapoptosis induced by systemic lipopolysaccharide administration are reversed by dexmedetomidine treatment in mice. Neurol Res. 2017;39(4):357–66.
Semmler A, Frisch C, Debeir T, Ramanathan M, Okulla T, Klockgether T, et al. Long-term cognitive impairment, neuronal loss and reduced cortical cholinergic innervation after recovery from sepsis in a rodent model. Exp Neurol. 2007;204:733–40.
Kadoi Y, Saito S. An alteration in the gamma-aminobutyric acid receptor system in experimentally induced septic shock in rats. Crit Care Med. 1996;24:298–305.
• Zhai Q, Lai D, Cui P, Zhou R, Chen Q, Hou J, et al. Selective activation of basal forebrain cholinergic neurons attenuates polymicrobial sepsis-induced inflammation via the cholinergic anti-inflammatory pathway. Crit Care Med. 2017;45(10):e1075–82 Experimental studies showing the involvement of cholinergic pathways in the immunomodulation within the cerebral tissue during sepsis.
van Eijk MM, Roes KC, Honing ML, Kuiper MA, Karakus A, van der Jagt M, et al. Effect of rivastigmine as an adjunct to usual care with haloperidol on duration of delirium and mortality in critically ill patients: a multicentre, double-blind, placebo-controlled randomised trial. Lancet. 2010;376(9755):1829–37.
Freund HR, Muggia-Sullam M, LaFrance R, Holroyde J, Fischer JE. Regional brain amino acid and neurotransmitter derangements during abdominal sepsis and septic encephalopathy in the rat. The effect of amino acid infusions. Arch Surg. 1986;121:209–16.
•• Dahl RH, Berg RMG, Taudorf S, Bailey DM, Lundby C, Larsen FS, et al. A reassessment of the blood-brain barrier transport of large neutral amino acids during acute systemic inflammation in humans. Clin Physiol Funct Imaging. 2018;38(4):656–62 Clinical study describing the role of the amino acids metabolism during acute inflammatory status according to the blood-brain barrier integrity.
Berg RM, Taudorf S, Bailey DM, Lundby C, Larsen FS, Pedersen BK, et al. Cerebral net exchange of large neutral amino acids after lipopolysaccharide infusion in healthy humans. Crit Care. 2010;14(1):R16.
Zhao YZ, Gao ZY, Ma LQ, Zhuang YY, Guan FL. Research on biogenesis of mitochondria in astrocytes in sepsis-associated encephalopathy models. Eur Rev Med Pharmacol Sci. 2017;21(17):3924–34.
Zhan RZ, Fujiwara N, Shimoji K. Regionally different elevation of intracellular free calcium in hippocampus of septic rat brain. Shock. 1996;6:293–7.
• Dhaya I, Griton M, Raffard G, Amri M, Hiba B, Konsman JP. Bacterial lipopolysaccharide-induced systemic inflammation alters perfusion of white matter-rich regions without altering flow in brain-irrigating arteries: relationship to blood-brain barrier breakdown? J Neuroimmunol. 2018;314:67–80 Experimental study suggesting a role for impairment of the blood-brain barrier in the alteration of white matter perfusion, in relationship with microvascular dysfunction.
Rodrigues SF, Granger DN. Blood cells and endothelial barrier function. Tissue Barriers. 2015;3(1–2):e978720.
He H, Geng T, Chen P, Wang M, Hu J, Kang L, et al. NK cells promote neutrophil recruitment in the brain during sepsis-induced neuroinflammation. Sci Rep. 2016;6:27711.
Taccone FS, Su F, Pierrakos C, He X, James S, Dewitte O, et al. Cerebral microcirculation is impaired during sepsis: an experimental study. Crit Care. 2010;14(4):R140.
Taccone FS, Su F, De Deyne C, Abdellhai A, Pierrakos C, He X, et al. Sepsis is associated with altered cerebral microcirculation and tissue hypoxia in experimental peritonitis. Crit Care Med. 2014;42(2):e114–22.
Schramm P, Klein KU, Falkenberg L, Berres M, Closhen D, Werhahn KJ, et al. Impaired cerebrovascular autoregulation in patients with severe sepsis and sepsis-associated delirium. Crit Care. 2012;16(5):R181.
Taccone FS, Castanares-Zapatero D, Peres-Bota D, Vincent JL, Berre’ J, Melot C. Cerebral autoregulation is influenced by carbon dioxide levels in patients with septic shock. Neurocrit Care. 2010;12(1):35–42.
Taccone FS, Scolletta S, Franchi F, Donadello K, Oddo M. Brain perfusion in sepsis. Curr Vasc Pharmacol. 2013;11(2):170–86.
Ebersoldt M, Sharshar T, Annane D. Sepsis-associated delirium. Intensive Care Med. 2007;33:941–50.
Leon A, Lepousé C, Floch T, Graftieaux JP. Brain injury during severe sepsis. Ann Fr Anesth Reanim. 2006;25:863–7.
Peterson JF, Pun BT, Dittus RS, Thomason JWW, Jackson JC, Shintani AK, et al. Delirium and its motoric subtypes: a study of 614 critically ill patients. J Am Geriatr Soc. 2006;54:479–84.
•• Barichello T, Sayana P, Giridharan VV, Arumanayagam AS, Narendran B, Della Giustina A, Petronilho F, Quevedo J, Dal-Pizzol F. Long-term cognitive outcomes after sepsis: a translational systematic review. Mol Neurobiol 2018. Recent systematic review dealing with the occurrence of long-term cognitive dysfunction in both experimental and clinical studies.
Semmler A, Widmann CN, Okulla T, Urbach H, Kaiser M, Widman G, et al. Persistent cognitive impairment, hippocampal atrophy and EEG changes in sepsis survivors. J Neurol Neurosurg Psychiatry. 2013;84:62–70.
Gunther ML, Morandi A, Krauskopf E, Pandharipande P, Girard TD, Jackson JC, et al. The association between brain volumes, delirium duration, and cognitive outcomes in intensive care unit survivors: the VISIONS cohort magnetic resonance imaging study. Crit Care Med. 2012;40:2022–32.
Wang LM, Wu Q, Kirk RA, Horn KP, Ebada Salem AH, Hoffman JM, et al. Lipopolysaccharide endotoxemia induces amyloid-β and p-tau formation in the rat brain. Am J Nucl Med Mol Imaging. 2018;8(2):86–99.
Ely EW, Inouye SK, Bernard GR, Gordon S, Francis J, May L, et al. Delirium in mechanically ventilated patients: validity and reliability of the confusion assessment method for the intensive care unit (CAM-ICU). JAMA. 2001;286:2703–10.
Riker RR, Fugate JE, Participants in the International Multi-disciplinary Consensus Conference on Multimodality Monitoring. Clinical monitoring scales in acute brain injury: assessment of coma, pain, agitation, and delirium. Neurocrit Care. 2014;21(Suppl 2):S27–37.
Venkatesh B, Scott P, Ziegenfuss M. Cerebrospinal fluid in critical illness. Crit Care Resusc. 2000;2(1):42–54.
Hosokawa K, Gaspard N, Su F, Oddo M, Vincent JL, Taccone FS. Clinical neurophysiological assessment of sepsis-associated brain dysfunction: a systematic review. Crit Care. 2014;18:674.
Oddo M, Carrera E, Claassen J, SA M, LJ H. Continuous electroencephalography in the medical intensive care unit. Crit Care Med. 2009;37:2051–6.
•• Reznik ME, Merkler AE, Mahta A, Murthy SB, Claassen J, Kamel H. Long-term risk of seizures in adult survivors of sepsis. Neurology. 2017;89(14):1476–82 Large cohort study showing that survivors of sepsis face a significantly higher long-term risk of seizures than other patients.
• Admiraal MM, Gilmore EJ, Van Putten MJAM, Zaveri HP, Hirsch LJ, Gaspard N. Disruption of brain-heart coupling in sepsis. J Clin Neurophysiol. 2017;34(5):413–20 Physiologic study associating the loss of EEG reactivity to the dysfunction of the autonomic system, which was evaluated by the assessment of heart rate varibility.
Piazza O, Cotena S, De Robertis E, Caranci F, Tufano R. Sepsis associated encephalopathy studied by MRI and cerebral spinal fluid S100B measurement. Neurochem Res. 2009;34:1289–92.
• Anderson BJ, Reilly JP, Shashaty MGS, Palakshappa JA, Wysoczanski A, Dunn TG, et al. Admission plasma levels of the neuronal injury marker neuron-specific enolase are associated with mortality and delirium in sepsis. J Crit Care. 2016;36:18–23 Observational study showing a potential prognostic role for biomarkers of brain injury in septic patient.
Pfister D, Siegemund M, Dell-Kuster S, Smielewski P, Rüegg S, Strebel SP, et al. Cerebral perfusion in sepsis-associated delirium. Crit Care. 2008;12:R63.
Nguyen DN, Spapen H, Su F, Schiettecatte J, Shi L, Hachimi-Idrissi S, et al. Elevated serum levels of S-100β protein and neuron-specific enolase are associated with brain injury in patients with severe sepsis and septic shock. Crit Care Med. 2006;34:1967–74.
Polito A, Eischwald F, Maho ALL, Polito A, Azabou E, Annane D, et al. Pattern of brain injury in the acute setting of human septic shock. Crit Care. 2013;17:R204.
Finelli PF, Uphoff DF. Magnetic resonance imaging abnormalities with septic encephalopathy. J Neurol Neurosurg Psychiatry. 2004;75:1189–91.
Sharshar T, Carlier R, Bernard F, Guidoux C, Brouland JP, Nardi O, et al. Brain lesions in septic shock: a magnetic resonance imaging study. Intensive Care Med. 2007;33:798–806.
Ely EW, Shintani A, Truman B, Speroff T, Gordon SM, Harrell FE Jr, et al. Delirium as a predictor of mortality in mechanically ventilated patients in the intensive care unit. JAMA. 2004;291:1753–62.
Bleck TP, Smith MC, Pierre-Louis SJ, Jares JJ, Murray J, Hansen CA. Neurologic complications of critical medical illness. Crit Care Med. 1993;21:98–103.
Sprung CL, Peduzzi PN, Shatney CH, Schein RM, Wilson MF, Sheagren JN, et al. Impact of encephalopathy on mortality in sepsis syndrome. Crit Care Med. 1990;18:474–9.
•• Sonneville R, de Montmollin E, Poujade J, Garrouste-Orgeas M, Souweine B, Darmon M, et al. Potentially modifiable factors contributing to sepsis-associated encephalopathy. Intensive Care Med. 2017;43(8):1075–84 Recent large cohort study investigating the predictors of brain dysfunction in septic patients.
Oddo M, Taccone FS. How to monitor the brain in septic patients? Minerva Anestesiol. 2015;81:776–88.
Vasko A, Siro P, Laszlo I, Szatmari S, Molnar L, Fulesdi B, et al. Assessment of cerebral tissue oxygen saturation in septic patients during acetazolamide provocation - a near infrared spectroscopy study. Acta Physiol Hung. 2014;101:32–9.
Donnelly J, Aries MJ, Czosnyka M. Further understanding of cerebral autoregulation at the bedside: possible implications for future therapy. Expert Rev Neurother. 2015;15:169–85.
Minati L, Kress IU, Visani E, Medford N, Critchley HD. Intra- and extra-cranial effects of transient blood pressure changes on brain near-infrared spectroscopy (NIRS) measurements. J Neurosci Methods. 2011;197(2):283–8.
Robba C, Cardim D, Sekhon M, Budohoski K, Czosnyka M. Transcranial doppler: a stethoscope for the brain-neurocritical care use. J Neurosci Res. 2018;96(4):720–30.
Pierrakos C, Antoine A, Velissaris D, Michaux I, Bulpa P, Evrard P, et al. Transcranial doppler assessment of cerebral perfusion in critically ill septic patients: a pilot study. Ann Intensive Care. 2013;3:28.
Foreman B, Claassen J, Khaled KA, Jirsch J, Alschuler DM, JohnWittman J, et al. Generalized periodic discharges in the critically ill: a case-control study of 200 patients. Neurology. 2012;79:1951–60.
•• Andresen JM, Girard TD, Pandharipande PP, Davidson MA, Ely EW, Watson PL. Burst suppression on processed electroencephalography as a predictor of postcoma delirium in mechanically ventilated ICU patients. Crit Care Med. 2014;42(10):2244–51 Observational study suggesting an association between the depth of anesthesia, which was assessed by the burst suppression rate on EEG, and the occurrence of delirium in ventilated critically ill patients.
Luitse MJ, van Asch CJ, Klijn CJ. Deep coma and diffuse white matter abnormalities caused by sepsis-associated encephalopathy. Lancet. 2013;381(9884):2222.
•• Beumier M, Casu GS, Hites M, Wolff F, Cotton F, Vincent JL, et al. Elevated Beta-lactam concentrations are associ- ated with neurological deterioration in ICU septic patients. Minerva Anestesiol. 2015;81:497–506 Large cohort study suggesting a role for elevated through β-lactam concentrations and the occurrence of neurological deterioration in septic patients.
Pandharipande P, Cotton BA, Shintani A, Thompson J, Pun BT, Morris JA Jr, et al. Prevalence and risk factors for development of delirium in surgical and trauma intensive care unit patients. J Trauma. 2008;65:34–41.
Polito A, Brouland JP, Porcher R, Sonneville R, Siami S, Stevens RD, et al. Hyperglycaemia and apoptosis of micro-glial cells in human septic shock. Crit Care. 2011;15:R131.
Bagshaw SM, Peets AD, Hameed M, Boiteau PJ, Laupland KB, Doig CJ. Dialysis disequilibrium syndrome: brain death following hemodialysis for metabolic acidosis and acute renal failure–a case report. BMC Nephrol. 2004;5:9.
Pandharipande PP, Sanders RD, Girard TD, McGrane S, Thompson JL, Shintani AK, et al. Effect of dexmedetomidine versus lorazepam on outcome in patients with sepsis: an a priori-designed analysis of the MENDS randomized controlled trial. Crit Care. 2010;14:R38.
Zhang X, Yan F, Feng J, Qian H, Cheng Z, Yang Q, et al. Dexmedetomidine inhibits inflammatory reaction in the hippocampus of septic rats by suppressing NF-κB pathway. PLoS One. 2018;13(5):e0196897.
Spapen H, Nguyen DN, Troubleyn J, Huyghens L, Schiettecatte J. Drotrecogin alfa (activated) may attenuate severe sepsis-associated encephalopathy in clinical septic shock. Crit Care. 2010;14:R54. https://doi.org/10.1186/cc8947.
Reis PA, Alexandre PCB, D'Avila JC, Siqueira LD, Antunes B, Estato V, et al. Statins prevent cognitive impairment after sepsis by reverting neuroinflammation, and microcirculatory/endothelial dysfunction. Brain Behav Immun. 2017;60:293–303.
Esen F, Senturk E, Ozcan PE, Ahishali B, Arican N, Orhan N, et al. Intravenous immunoglobulins prevent the breakdown of the blood-brain barrier in experimentally induced sepsis. Crit Care Med. 2012;40(4):1214–20.
Hoshino K, Hayakawa M, Morimoto Y. Minocycline prevents the impairment of hippocampal long-term potentiation in the septic mouse. Shock. 2017;48(2):209–14.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Chiara Robba, Ilaria Alice Crippa, and Fabio Silvio Taccone each declare no potential conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Critical Care
Rights and permissions
About this article
Cite this article
Robba, C., Crippa, I.A. & Taccone, F.S. Septic Encephalopathy. Curr Neurol Neurosci Rep 18, 82 (2018). https://doi.org/10.1007/s11910-018-0895-6
Published:
DOI: https://doi.org/10.1007/s11910-018-0895-6