Abstract
Tourette syndrome (TS) is a childhood onset neurologic disorder with manifestations including multiple motor and phonic tics, and in most cases a variety of behavioral comorbidities such as attention deficit hyperactivity disorder, obsessive compulsive disorder, and other impulse control disorders. Although it is considered a hereditary disorder, likely modified by environmental factors, genetic studies have yet to uncover relevant causative genes and there is no animal model that mimics the broad clinical phenomenology of TS. There has been a marked increase in the number of neurophysiological, neuroimaging, and other studies on TS. The findings from these studies, however, have been difficult to interpret because of small sample sizes, variability of symptoms across patients, and comorbidities. Although anti-dopaminergic drugs are the most widely used medications in the treatment of TS, there has been increasing interest in other drugs, behavioral therapies, and surgical approaches including deep brain stimulation. Herein, we review the current literature and discuss the complexities of TS and the challenges in understanding its pathophysiology and in selecting the most appropriate treatment. We also offer an expert’s view of where the field of TS may be headed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tourette syndrome (TS) is a complex, childhood-onset, neurodevelopmental disorder characterized by motor and phonic tics and a variety of behavioral manifestations, particularly obsessive compulsive disorder (OCD) and attention deficit hyperactivity disorder (ADHD). Jean-Martin Charcot, widely considered the founder of modern clinical neurology, gave Georges Albert Gilles de la Tourette, one of his pupils, credit for recognizing the disorder by naming it TS [1]. In his 1885 paper, Tourette provided a clear description of nine patients suffering from a “malady of tics” [2]. Although the syndrome had previously been described in case reports by Seprenger and Heinrich in 1498, by Itard in 1825 [3], by Trousseau in 1873 [4], and by Hughlings Jackson in 1884 [5], Tourette recognized the clinical features, including motor and phonic tics, echolalia (repeating what others say), echopraxia (mimicking other’s actions), coprolalia (shouting of obscenities or profanities), and its hereditary nature. TS is a complex entity presenting challenges for both the clinician and the researcher. The complexity is highlighted by the variations in individual clinical manifestations, the fluctuations in severity and frequency of symptoms, and the common behavioral comorbidities. Although the pathogenesis of TS is still not fully understood, it is now widely recognized that TS likely results from a multitude of genetic and environmental factors. Since its original description, an increasing number of publications have drawn attention to diagnostic, genetic, imaging, physiological, and therapeutic discoveries related to TS [6]. Many unanswered questions and research challenges, however, still remain. In this review, we present an overview of the clinical features, the neurobiology, the pathogenesis, and treatment options for TS. We will offer expert insights into where TS research and care is headed over the next decade.
Clinical Features
The clinical hallmark of TS is sudden, repetitive movements (motor tics) and vocalizations and other sounds (phonic tics). These tics have varying degrees of intensity and frequency and may be of short or long duration. The frequency, intensity, and course of tics can be quite variable in individual patients and across patient populations [7, 8]. Tics are subclassified into simple and complex categories. Simple motor tics involve individual muscles or groups of muscles, whereas complex tics consist of more coordinated and sequenced movements, which may in some cases be socially inappropriate [9]. Common simple motor tics include eye blinking, head, neck, or limb jerking, sustained mouth opening, or shoulder rotation, whereas examples of complex motor tics include touching, hitting, gyrating, bending, copropraxia (gesturing and touching of genitalia), other socially inappropriate behaviors, and self-injurious behaviors. Examples of common simple phonic tics are grunting, squeaking, coughing, sniffing, snorting, and throat clearing. Complex phonic tics include meaningful utterances and vocalizations, such as echolalia, coprolalia, and palilalia (repeating one’s own words) [10]. Although coprolalia has been characterized as a cardinal feature of TS, it occurs in only 10–19 % of individuals [11].
It is common for tics to be exacerbated during periods of anticipation, stress, excitement, or fatigue and to be reduced when concentrating on mental or physical tasks. Several studies have documented that tics may persist during all stages of sleep [12]. Tics are considered to be involuntary, but can also be voluntarily suppressed [13]. This ability to volitionally inhibit tics is useful for differentiating TS from other hyperkinetic movement disorders. Following voluntary tic suppression, patients may experience a rebound “release” of tics, and this release has been frequently reported as worse than the baseline symptoms [14].
The onset of both motor and phonic tics frequently is preceded by premonitory urges, described as a buildup of tension, pressure, or energy localized to the tic region, or a general psychological tension, associated with a pressing need to act. Some examples of premonitory urges are muscle tension, nasal stuffiness, and dry throat—all preceding the tic. Executing the tic relieves these inner sensations and results in a feeling of relief. Tics have been described by some experts as a “voluntary response to an involuntary sensation” [8]. About 90 % of adults with TS report the occurrence of premonitory urges [15, 16], whereas 37 % in the pediatric population report similar sensations [17]. Recent studies showed that certain urges could be selectively associated with tics (e.g., physical sensations), and similarly some urges could be associated with obsessive compulsive symptoms (e.g., feelings of unease and urgency) [18].
The lifetime prevalence of any psychiatric comorbidity among individuals with TS is reported to be 66 % [19]. Seventy-two percent of TS patients meet the criteria for OCD and ADHD [20]. Fifty-eight percent of the TS population had two or more psychiatric disorders including autism spectrum disorders, depression, personality disorder, anxiety disorder, or self-injurious behavior [19, 21]. These symptoms add to the complex comorbidities associated with TS, and these comorbidities can impact quality of life (Fig. 1).
The clinical course of TS and coexisting disorders. The vertical axis represents the approximate “amount” the disorder affects a TS patient. TS symptom severity peaks around age 11 years, and ∼50 % of patients experience complete or near to complete tic remission. Thirty to 50 % experience significantly reduced symptom severity, whereas 5–10 % of patients will experience sustained or worsened symptoms
The clinical course of TS is variable, and the average age at onset of motor tic is 5.6 years [22]. Phonic tics typically follow the onset of motor tics, but sniffing, coughing, and other sounds may precede tics but are often initially wrongly attributed to “allergies.” Studies have revealed that the severity of symptoms usually peaks just before puberty [23]; in one study, the peak was at a mean of 10.6 years [23]. The majority of patients with TS achieve complete or near complete remission of tics by 21 years of age, but in 10–20 % of TS cases, the symptoms fluctuate, persist, or worsen [24]. Figure 1 summarizes the natural history of TS [23, 24] and the associated comorbid conditions. The average age at onset of ADHD has been shown to precede tic symptoms (∼3 years old), whereas the onset for OCD and the peak age of OCD severity occur 3–4 years following tic onset and peak tic severity [22]. ADHD and OCD symptoms usually persist to a variable degree through adulthood.
The estimated prevalence of TS ranges from 3 to 9 per 1000 in school-age children [23, 24]. The prevalence is higher in males compared to females, with the ratio varying from 2:1 to 4:1 [25••]. The number of diagnosed cases in the USA is lower among African Americans and Hispanic Americans, yet this may be related to differences in access to care [26].
Although TS is not a degenerative disorder, it can be socially crippling and motor tics may be painful, severe, and even life threatening. It has been estimated that 5 % of TS patients will be admitted to hospitals each year due to tic-related injuries, self-injurious behavior, uncontrollable violence, or suicidal ideation with or without suicide attempts [27]. Such “malignant” cases have been associated with greater severity of motor symptoms and the presence of two or more behavioral comorbidities.
Clinical Features: Where Is the Field Headed?
An important focus of research on clinical features has been aimed at better understanding the mysterious premonitory urges of TS. The idea that interoceptive awareness could be a strong predictor of premonitory urges has been proposed. The association of greater tic severity to higher rates of premonitory urges has recently been shown. The high levels of interoceptive awareness have been hypothesized to reflect a self-attentive capacity to perceive urges [28]. Experts have focused on this clinical feature as important to better understand TS, but also to develop improved treatment paradigms. One group has been recording from pre-motor human cortex in an effort to define and use the premonitory urge as a potential treatment approach [29].
Another clinical focus will likely be on the improved characterization of comorbidities, which cause most of the heterogeneity in clinical features across patients. Comorbidities define “types of TS” as TS only, TS + OCD, and TS + OCD + ADHD [30], which has raised questions as to whether these should be considered a continuum of the phenotype. The next decade will likely bring clarity to the issue of TS-associated comorbidities, and this improved understanding will fill an important knowledge gap as the field moves toward more effective treatments.
Moreover, early-onset longitudinal studies will be crucial to uncover clinical features that point towards remission of the disease versus those that point to a lifelong disorder.
Diagnosis
Tic disorders are identified and diagnosed through a careful history documenting childhood onset, neurological examination, recognition of the broad spectrum of motor and behavioral phenomenology, and family history. The diagnostic criteria for TS were modified in 2012 by the fifth edition of the American Psychiatric Association Diagnostic and Statistical Manual of Mental Disorders (DSM-V). This publication is the primary diagnostic reference for Mental Health professionals practicing in the USA and Canada [31]. The new diagnostic criteria for TS and other tic disorders are summarized in Table 1. A diagnosis of TS is made when both motor and vocal tics have been present at some time during the patient’s history (criterion A) and have been persistent for greater than a year (criterion C). The age at onset of tics must occur before the age of 18 years (criterion B). The presence of comorbid disorders (e.g., ADHD, OCD), though common, is not a requirement for the diagnosis of TS. Other potential causes of tics including drug-induced or associated other medical conditions such as Huntington’s disease should be eliminated prior to a formal diagnosis of TS (criterion D).
Although tics may be confused with other hyperkinetic movement disorders such as chorea, myoclonus, stereotypies, dystonia, or epileptic seizures [32], most neurologists and psychiatrists can differentiate tics based on the history, the examination, and the ability to suppress tics as well as the presence of premonitory urges. Chorea, in contrast to TS, represents continuous, non-stereotyped motor movements, which randomly involve different body parts and are not associated with premonitory urges. Motor stereotypies, such as hand waving or rotating, can be differentiated from tics based on predictability, prolonged duration (seconds to minutes), constant repetitive movements without variability, and the occurrence in the same body region. Stereotypies can be exacerbated by physical activities, lack a premonitory urge, and generally abate with distraction. Stereotypies have an earlier age at onset compared to TS (<3 years of age). Dystonia is the simultaneous sustained contracture of both agonist and antagonist muscles, and dystonia usually results in a distorted posture or movement and may be frequently triggered by voluntary movements. Myoclonus is differentiated from tics by its rapidity, difficulty in suppression, and absence of a premonitory urge. Presentations differentiating obsessive-compulsive behaviors from tics include a cognitively based and goal-directed drive, precise numbers of repetitions of the movements, and persistence until a “just right” feeling is achieved.
Diagnosis: Where Is the Field Headed?
The biggest challenge in this area has been to address the frequent multiyear delay in establishing an accurate diagnosis. Additionally, Scharf et al. [19] recently refined the population prevalence estimate of 0.3–0.9 % in children and reported that clinically referred cases had prevalence estimates that were lower than those derived from population-based samples. Study sample size, which is likely a proxy for the case assessment method, and the use of DSM-IV-TR diagnostic criteria were the major sources of heterogeneity in diagnosis. Experts generally agree that TS is common and that making an earlier diagnosis has the potential to impact outcome. Chronic multiple tic disorders have a higher prevalence, and though controversial, the field may be slowly moving toward merging these diagnoses.
Genetics
Several lines of evidence suggest that genetic factors are primary contributors to the etiology of TS. Careful family studies have found tics or a history of tics occurring in a majority of parents [25••], and in many cases TS is bilineally transmitted (both parents are affected to some degree) [33]. First-degree relatives are at significantly higher risk of developing TS when compared to controls [34]. Studies with twins have revealed concordance rates of TS to be 53–56 % in monozygotic twins, compared to a considerably lower rate of 8 % in dizygotic twins [35]. One study showed a heritability point estimate of 0.58 for TS (and 0.37 for OCD) using genome-wide complex trait analysis (GCTA) [36, 37].
Despite all the lines of evidence implicating multiple genes and chromosomal regions in the pathogenesis of TS, no causative gene mutation or common variant has been uncovered that can account for the majority of TS cases [38]. The Tourette Syndrome Association International Consortium for Genetics (TSAICG) has been conducting genome screens in large cohorts (110 individuals in 1999 [39] and 2040 individuals in 2007 [40]). Several international collaborative efforts are currently underway, including the European Society for the Study of Tourette Syndrome (ESSTS) [41], TSAICG [42] and the more recent Tourette International Collaborative Genetics (TIC Genetics) study [40, 41]. Interpretation of these results is, however, complicated partly because of heterogeneous presentation, presence of comorbidities, bilineal transmission, diversity of genotypes, and complex interaction with various environmental factors.
Genetics: Where Is the Field Headed?
It is likely that, over the next decade, genome-wide association and other genetic studies conducted across multiple centers will uncover important genetic clues that will advance our understanding of pathogenesis and treatment of TS.
Neuroinflammation
A role for environmental factors, especially infections, in the presentation and exacerbation of tics has been postulated as early as 1929 [43]. There were many early case reports showing an association between childhood sinusitis and the onset of TS [44]. More recently, post-streptococcal autoimmunity has been postulated as a potential environmental trigger [45–47]. Also, tics have been shown to be neurological manifestations of rheumatic fever associated with Sydenham’s chorea, and this has been suggested as a potential model for TS pathophysiology. Swedo and colleagues coined the term PANDAS [48, 49], for pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections, and Mell et al. [50] provided epidemiologic evidence supporting PANDAS. While the hypothesis has stimulated clinical and basic research, it has led to considerable scientific controversy [51]. Criticism of reported results has been leveled for a variety of reasons including that approximately two thirds of study participants in PANDAS clinical trials have undergone selective recruitment. There were also issues with small sample sizes and few or no controls [52–55]. The debates over PANDAS have evolved to include an expanded clinical entity named PANS (Pediatric Acute-onset Neuropsychiatric Syndrome) [54]. This term has been used to refer to a subgroup of children with abrupt onset OCD symptoms and other acute onset symptoms such as urinary frequency or enuresis, separation anxiety, etc.
Neuroinflammation: Where Is the Field Headed?
Experts have suggested a need for a broader concept of childhood acute neuropsychiatric symptoms (CANS) [56]. It is now widely recognized that a broad spectrum of movement disorders can possibly be associated with antibodies and inflammation. It is likely that TS is not one disease and that different entities may emerge as we begin to separate tics into different phenotypes based on genetics, pathogenesis, and clinical presentation. Future directions should involve large-scale epidemiologic studies and centralized registries with standardized and longitudinal data collection strategies, as well as randomized, controlled clinical trials of novel therapies. Many experts believe that most, if not all, cases of PANDAS and associated neuropsychiatric manifestations are actually tics and possibly fit the criteria for TS.
Pathophysiology
Neurophysiology
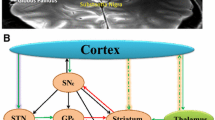
The classical models of cortico-striatal-thalamocortical circuits (CSTC) have provided frameworks for uncovering the neurophysiological basis for the manifestations of involuntary tic. The hypotheses that arise from these models suggest that the basal ganglia likely modulate behavior through a mechanism that involves changing cortical excitability through the “direct” and “indirect” basal ganglia pathways (see Fig. 3a). The direct pathway, from the striatum to the globus pallidus interna (GPi) and the substantia nigra (SNr), excites the cortex through the disinhibition of the thalamus. The indirect pathway, from the striatum to the globus pallidus externa (GPe) to the subthalamic nucleus (STN), inhibits the thalamic projections [57]. Based on these models, it has been hypothesized that tics result from competing motor patterns. According to this hypothesis, focal aberrations in the striatum cause excessive inhibition of the GPi and SNr in the direct pathway, thereby causing an involuntary motor command to be executed in the cortex due to excessive disinhibition (see Fig. 3b).
There has been a lack of human electrophysiological studies and especially a lack of studies targeting circuits. One study has found normal pre-movement (Bereitschaftspotential) in TS patients prior to execution of tics suggesting that some components of tics may be mediated via volitional pathways [58]. Further studies, however, are needed to determine whether the characteristic premonitory sensations are in any way related to this physiologic finding or whether it represents an abnormality in motor-sensory integration [59].
Deep brain stimulation (DBS) surgery offers a unique opportunity to invasively record from TS-related brain areas to study physiological abnormalities in TS. Moreover, next-generation DBS devices, such as the NeuroPace RNS and Medtronic Activa PC+S, are facilitating chronic recordings from stimulation electrodes. Most human neurophysiology studies point to low-frequency (<10 Hz) activity during tics in the ventralis oralis (VO) complex [60] or centromedian nucleus (CM) of the thalamus [61–63]. Similar activity was not present in the thalamus during voluntary mimicking of the tics [64] or during tic suppression [65•, 66].
Neurophysiology: Where Is the Field Headed?
Recent advances in technology capable of recording from multiple cortical and subcortical regions simultaneously within the brain of awake human will soon facilitate testing of the TS circuit hypotheses. Much of the current understanding of TS physiology has been based on studies in anesthetized patients or inadequate animal models.
Neuroimaging
Structural imaging studies have suggested that smaller caudate nucleus volumes correlated with severity of tics in patients with TS followed longitudinally [67]. A recent longitudinal study revealed that, in young adolescents with persistent tics, there was a decrease in left putamen volume and changes in diffusivity in right caudate nucleus, thalamus, and frontal lobe [68]. Connectivity studies have shown decreased projections between the caudate nucleus and the lateral frontal cortex [69]. These findings support a cortical disinhibition theory for TS as well as the idea that there are underlying aberrations in the striatum.
Imaging studies have also revealed changes outside of the TS striatum. These include reduced cortical thickness in motor, pre-motor, pre-frontal, and lateral orbitofrontal cortical areas [70] and structural alterations in somatosensory pathways [71] and in the corpus callosum [72]. Comorbidity studies with OCD and ADHD have revealed decreased gray matter volume in the lateral frontal cortex (inferior frontal gyrus) [73]. Abnormal cortical development in TS is supported by other imaging studies that have found abnormal structural patterns of cortical sulci which correlated with severity of clinical symptoms [74–76]. One study compared sulcal depth, length, and thickness of gray matter in 52 adult patients with TS and 52 matched controls [77•]. Patients with TS had lower depth and reduced thickness of gray matter in the pre- and post-central as well as superior, inferior, and internal frontal sulci, bringing further support for abnormal brain development.
Functional imaging studies are challenging to perform in patients with TS largely due to motion artifacts. Nevertheless, functional imaging studies of tic generation suggest increased activity in the supplementary motor association areas [78] and motor pathways [79] as well as a decreased activity in CSTC circuits responsible for motor control [80]. A resting state fMRI study revealed abnormal activity in the CSTC circuits, in pre-motor, sensorimotor, and cingulate cortices, and in the medial thalamus [81].
Neuroimaging: Where Is the Field Headed?
There are substantial difficulties in capturing motion artifact-free images in TS patients. Structural and functional neuroimaging will however be critical for elucidating the understanding of this circuit disorder. Neuroimaging may offer the intriguing possibility of parsing individual clinical manifestations into specific causative brain regions, and early-onset longitudinal studies can provide insight into the role of brain development in the manifestations and the natural history of TS. Moreover, neuroimaging can allow the study of response to various treatments. Consortia, such as the one set up by the Tourette Syndrome Association, which pool multiple scans from multiple institutions, will likely aid in filling a large knowledge gap in TS neuroimaging.
Neurochemistry
Many studies of drug trials [82], imaging [83], and human sample analysis [84, 85] have collectively led to the hypothesis of neurochemical abnormalities in TS [86, 87]. The most common neurochemical hypothesis of TS is dopaminergic dysfunction, based initially on the observation that dopamine receptor-blocking drugs (neuroleptics) were most effective in reducing tics. While some studies have found abnormalities of dopamine transporter binding capacity [88] and increases of cortical [89, 90] and striatal [91] dopamine receptors, no dopaminergic hyperinnervation has been demonstrated by PET studies [92].
Neurochemistry: Where Is the Field Headed?
Dopamine is no longer considered the exclusive neurotransmitter involved in TS. Studies have demonstrated that serotonergic pathways play an important role in the pathophysiology of TS [93]. Other hypotheses include imbalances in noradrenergic, glutamatergic, serotonergic, opioid, cholinergic, and GABA-ergic systems [84, 85, 94, 95]. A recent study by Xu et al. [96••] showed that ablation of ∼50 % of cholinergic interneurons in the striatum of mice led to TS-like stereotypies, thus implicating striatal cholinergic system in TS. The most compelling observation related to neurochemical abnormalities in TS is the finding, based on PET ligand studies, of decreased density of receptors to GABA, an inhibitory neurotransmitter, in the striatum, globus pallidus, thalamus, and amygdala and increased binding in the SNr, posterior cingulate cortex, and cerebellum. This is consistent with the hypothesis of GABA-ergically mediated disinhibition as the main neurotransmitter underpinning TS. A recent study has also brought attention to deficiencies in histamine as a rare cause of TS [97]. We will likely observe clarification of the neurochemical bases of TS over the next 5–10 years.
Treatment
A critical step in TS treatment is to provide education to the patient, parents, caregivers, and peers about the condition [98, 99]. This is important in establishing appropriate expectations and optimal treatment strategies and in creating more informed relationships [100]. A comprehensive evaluation of comorbid psychiatric conditions is also essential for treatment. A common strategy is to tailor therapy to address the symptoms (tics or comorbidities), which are likely contributing to problems of daily functioning or impacting quality of life. Treatment of TS comorbidities may diminish tic severity [22].
Several rating scales for tics have been developed to facilitate the assessment and monitoring of symptoms and to measure treatment outcomes. Two of the most commonly used scales are the Yale Global Tic Severity Scale (YGTSS) [101] and the Modified Rush Tic Rating Scale (MRTRS) [102]. Discrepancies between these outcome measures have been reported across TS studies [103, 104], and this may be due to the fluctuation of tics and the difficulty in precise measurement of the phenomenon. Additionally, measures for OCD, ADHD, self-injurious behavior, and other comorbidities are important since these symptoms may impact quality of life even more than simple motor and vocal tics.
Treatment decisions are often guided by individual needs and the experience of clinicians. It is common for clinicians to adopt a sequential approach to TS [6], in order to improve the risk-benefit ratio for any intervention. Following family education, behavioral and pharmacological approaches may be addressed. Occasional medication refractory cases may lead to discussion of surgical treatment strategies. Figure 2 provides an outline and summary of TS management. Treatment should be tailored in each case. If easily accessible, behavioral therapy such as Comprehensive Behavioral Intervention for Tic Disorders (CBIT), which may include habit reversal training (HRT), can be offered to the patients as a first line of treatment. The aim of such behavioral approach is to facilitate control of tics by disrupting the pattern of premonitory urges and the relief sensation that follows the execution of some tics. Two randomized controlled trials reported that HRT reduced tic severity with an outcome improvement of 10.5 points (out of 100) on the YGTSS at 5 months [105, 106]. Two multisite studies have demonstrated the efficacy of CBIT especially in mild to moderate TS populations [107, 108] and have suggested behavioral therapy as a safe first-line treatment for TS [6]. This treatment approach may be combined with exposure and response prevention therapy, especially if severe OCD is present [109, 110]. These trials have collectively demonstrated the superiority of CBIT over supportive psychotherapy in improving tic severity in children [107] and adults [108] as rated by the YGTSS (3.7 points mean group difference after 10 weeks of treatment). A systematic review of behavior therapy in TS highlights that there are no studies directly comparing the efficacy of behavioral therapies with pharmacotherapies for tics [111]. A meta-analysis of eight randomized controlled trials involving 438 subjects with TS concluded that CBIT produced moderate treatment effects and participants receiving CBIT were more likely to exhibit a treatment response compared to control interventions [112].
A generalized flowchart of therapeutic strategies for TS compiled from literature. Therapies should be tailored to each patient’s symptoms and needs, with priority given to the most disabling symptom (tic or comorbidity). This table is an illustration of potential medications and treatments, but is not a comprehensive list, as we recognize, for example, that many different dopamine blockers may be utilized
There is a paucity of standardized, large evidence-based drug trials in TS [113]. Pharmacologically, often the first line of TS treatment are the alpha agonists guanfacine [114] and clonidine [115]. These drugs are reasonable choices for mild to moderate tics and in general have been associated with few adverse effects (e.g., drowsiness, dry mouth). Antipsychotic drugs that act by blocking dopamine receptors (neuroleptics) are usually as second-line pharmacological treatments, although their beneficial effects in TS have been documented since the 1960s [116]. These drugs, however, can lead to adverse reactions such as drowsiness, severe weight gain, excessive sedation, parkinsonism, akathisia, and even tardive dyskinesia [83], and most experts try to reserve their use for cases when more conservative approaches fail.
Atypical antipsychotic drugs have been preferred by some experts over typical antipsychotic therapies, presumably because of a better adverse event profile and a lower risk of tardive dyskinesia [117]. Although haloperidol and pimozide are the only two drugs currently approved by the Food and Drug Administration (FDA) for the treatment of TS, fluphenazine appears to be possibly more effective and better tolerated [118].
Tetrabenazine, a dopamine-depleting agent, has been used to control motor and phonic tics [119–121], but well-designed controlled trials have not been reported. Tetrabenazine is considered by some experts to be more effective and safer than conventional neuroleptics, although it may be associated with dose-related side effects such as drowsiness, depression, parkinsonism, and restlessness.
Other medications, such as topiramate, have been less studied, but may also improve tic severity [122]. In patients with focal motor or phonic tics, injections with botulinum toxin into the affected muscles usually provides 3–4 months of relief with minimal side effects [123]. A summary of drugs and dosages used to treat TS is presented in Table 2.
In cases of “malignant” TS and for patients unresponsive to pharmacological or behavioral therapy, DBS has been increasingly used to treat not only tics but also associated OCD [124] High-frequency DBS (>100 Hz) modulates basal ganglia-thalamo-cortical circuits and may suppress motor and phonic tics (Fig. 3). In the 1970s, Rolf Hassler reported stereotactic lesioning of the thalamus and basal ganglia as a way to modulate abnormal brain circuits and to suppress tic-related behavior [125]. The most beneficial target for TS DBS has yet to be determined. According to the Tourette Syndrome Association International DBS Database and Registry Study Group since 1999, 120 patients have been reported across 13 countries to have undergone TS DBS therapy, and most have reported significant clinical improvements [126•]. The most commonly targeted structures have been the centromedian-parafascicular complex (CM-PF) of the thalamus (approximately 70 patients) and the motor and non-motor portions of the GPi (approximately 30 patients). Determination of optimal targets will require controlled studies performed in large cohorts, as well as standardization of surgical methods and outcome assessments. The Tourette Syndrome Association International Deep Brain Stimulation Database and Registry Study Group has also recently updated the recommendations for selection of candidates for TS DBS [126•]. The revised guidelines require a DSM-V diagnosis of TS by an expert clinician and have removed the previously suggested age limit of 25 years, with the specification that a multidisciplinary team approach should be employed [127]. A local Ethics Committee or Institutional Review Board consultation is recommended for patients younger than 18 years of age and for those with urgent indications. TS patients with DBS implants may be at an increased risk for hardware malfunction (such as fractures of the leads or lead extensions) due to head and neck snapping tics [128].
a The cortico-striatal-thalamocortical circuits under normal conditions. Desired motor patterns are disinhibited by the direct pathway, and competing motor patterns are suppressed by the indirect pathway. Abbreviations: GPi globus pallidus pars interna, SNr substantia nigra pars reticulata, STN subthalamic nucleus. b A cortico-striatal-thalamocortical hypothesis regarding TS (adapted from Albin and Mink [63]). The relative activity of projections is represented by line thickness. When aberrant groups of striatal neurons with inhibitory projections onto the SNr and GPi become inappropriately active, thalamocortical circuits in turn are disinhibited. This leads to undesired competing motor patterns in the cortical output, such as involuntary tics
Repetitive transcranial magnetic stimulation (rTMS) targeting the supplementary motor areas (SMA) at a rate of 1 Hz has been shown to improve symptoms in children [129, 130]. Studies of this therapy have been limited by small numbers and a lack of control conditions [131].
Treatment: Where Is the Field Heading?
CBIT therapy administered in person or through telemedicine has been evolving as an important part of the treatment armamentarium for mild to moderate tics. Many experts are also using tetrabenazine as opposed to classical neuroleptic drug treatments. DBS is a promising approach for malignant and severe medication refractory cases. The way we administer DBS will likely change over the next decade. A recent long-term study demonstrated the potential of scheduled TS DBS of less than 2 h of stimulation per day, and closed-loop responsive DBS approaches may be feasible in the TS population [132]. Closed-loop DBS strategies use neurophysiological or neurochemical feedback to deliver stimulation only when pathological activity is detected in order to reduce adverse effects of DBS and to increase battery life [133].
Conclusion
There is an abundance of future directions for research in the TS field. On the clinical side, it will be important to better understand premonitory urges, to characterize variations in TS phenotypes, and to better characterize comorbidities. Gene discovery will be applied to larger cohorts, and we will more effectively separate different clinical and genetic phenotypes of TS. More electrophysiological studies will be needed, especially in human patients performing intraoperative tasks, or through advanced DBS devices capable of recording brain signals and correlating them to awake human behavior. The manifestations of TS, which are paroxysmal, present a unique opportunity to develop responsive closed-loop neurotechnologies designed to suppress tics. All of these approaches should be conducted with the goal of developing new and improved therapies to improve the quality of life for those suffering from TS.
Looking to the future, there is a dire need for increased knowledge, awareness, and specialist care for both children and adults with TS. The First World Congress on TS and Tic Disorders was held in London in 2015, which promoted outreach and advocacy for the disorder and highlighted the important advances in all areas of TS.
Search Strategy and Selection Criteria
The authors searched PubMed for peer-reviewed articles published from 2000 to January 2015. The search terms “Tourette syndrome,” “Tourette,” “tic,” “involuntary movement,” “involuntary vocalizations,” “neurodevelopmental disorders,” “neuropsychiatric disorders,” “movement disorders,” “comorbidities,” “OCD,” “ADHD,” “genetics,” “pathophysiology,” “PANDAS,” “diagnosis,” “treatment,” “neurophysiology,” “neuroimaging,” and “deep brain stimulation” were used. Additional articles were identified by searching the reference lists of identified articles. Only papers in English were reviewed, with the exception of historical papers published in French. Articles were selected mostly from the past decade, but included older articles that were considered highly relevant to this review and to the history of TS. Review papers were included that provided insightful and comprehensive overviews on relevant aspects of Tourette syndrome.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Trivet HA, Chien HF, Munhoz RP, Barbosa ER. Charcot’s contribution to the study of Tourette syndrome. Arq Neuropsiquiatr. 2008;66:918–21.
Gilles de la Tourette G. Étude sur une affection nerveuse caractérisée par de l’incoordination motrice accompagnée d’écholalie et de coprolalie [French]. Arch Neurol. 1885;9:158–200.
Itard JM. Mémoire sur quelques functions involontaires des appareils de la locomotion, de la préhension et de la voix [French]. Arch Gen Med. 1825;8:385–407.
Trousseau A. Clinique Médicale de l’Hôtel Dieu de Paris Paris: J.-B. Bailliere; 1868.
Hughlings JJ. Clinical lectures and reports to the London Hospital. 1868;1.
McNaught KS, Mink JW. Advances in understanding and treatment of Tourette syndrome. Nat Rev Neurol. 2011;7:667–76.
Kurlan R. Clinical practice. Tourette’s syndrome. N Engl J Med. 2010;363:2332–8.
Singer HS. Tourette’s syndrome: from behaviour to biology. Lancet Neurol. 2005;4:149–59.
Jankovic J. Phenomenology and classification of tics. Neurol Clin. 1997;15:267–75.
Ganos C, Ogrzal T, Schnitzler A, Münchau A. The pathophysiology of echopraxia/echolalia: relevance to Gilles De La Tourette syndrome. Mov Disord. 2012;27:1222–9.
Freeman RD, Zinner SH, Muller-Vahl KR, et al. Coprophenomena in Tourette syndrome. Dev Med Child Neurol. 2009;51:218–27.
Hanna PA, Jankovic J. Sleep and tic disorders. Woburn: Butterworth-Heinemann; 2003.
Demirkiran M, Jankovic J. Paroxysmal dyskinesias—clinical-features and classification. Ann Neurol. 1995;38:571–9.
Jankovic J. Tourette’s syndrome. N Engl J Med. 2001;345:1184–92.
Leckman J, Walker D, Cohen D. Premonitory urges in Tourette’s syndrome. Am J Psychiatry 1993;150.
Kwak C, Vuong KD, Jankovic J. Premonitory sensory phenomenon in Tourette’s syndrome. Mov Disord. 2003;18:1530–3.
Banaschewski T, Woerner W, Rothenberger A. Premonitory sensory phenomena and suppressibility of tics in Tourette syndrome: developmental aspects in children and adolescents. Dev Med Child Neurol. 2003;45:700–3.
Rajagopal S, Cavanna AE. Premonitory urges and repetitive behaviours in adult patients with Tourette syndrome. Neurol Sci. 2014;35:969–71.
Tallur K, Minns RA. Tourette’s syndrome. Paediatr Child Health. 2010;20:88–93.
Hirschtritt ME, Lee PC, Pauls DL, et al. Lifetime prevalence, age of risk, and genetic relationships of comorbid psychiatric disorders in Tourette syndrome. JAMA Psychiatry. 2015;72:325–33.
Canitano R, Vivanti G. Tics and Tourette syndrome in autism spectrum disorders. Autism. 2007;11:19–28.
Leckman JF. Tourette’s syndrome. Lancet. 2002;360:1577–86.
Leckman JF, Zhang HP, Vitale A, et al. Course of tic severity in Tourette Syndrome: the first two decades. Pediatrics. 1998;102:14–9.
Bloch MH, Peterson BS, Scahill L, et al. Adulthood outcome of tic and obsessive-compulsive symptom severity in children with Tourette syndrome. Arch Pediatr Adolesc Med. 2006;160:65–9.
Scharf JM, Miller LL, Gauvin CA, Alabiso J, Mathews CA, Ben-Shlomo Y. Population prevalence of Tourette syndrome: a systematic review and meta-analysis. Mov Disord. 2015;30(2):221–8. This study is a systematic review of published studies on TS prevalence. The authors performed random-effects meta-analysis weighted by sample size and meta-regressions to examine covariates that were potential sources of heterogeneity. This study refined the population prevalence estimate of TS in children to be 0.3 to 0.9 %.
Knight T, Steeves T, Day L, Lowerison M, Jette N, Pringsheim T. Prevalence of tic disorders: a systematic review and meta-analysis. Pediatr Neurol. 2012;47:77–90.
Bitsko RH, Holbrook JR, Visser SN, et al. A national profile of Tourette syndrome. J Dev Behav Pediatr. 2014;35:317–22.
Cheung MY, Shahed J, Jankovic J. Malignant Tourette syndrome. Mov Disord. 2007;22(12):1743–50.
Ganos C, Garrido A, Navalpotro-Gomez I, et al. Premonitory urge to tic in Tourette’s is associated with interoceptive awareness. Mov Disord 2015.
Grados MA, Mathews CA. Clinical phenomenology and phenotype variability in Tourette syndrome. J Psychosom Res. 2009;67:491–6.
American Psychiatric Association. Diagnostic and Statistal Manual of Mental Disorders (DSM-V). Washington: American Psychiatric Association Press; 2012.
Jankovic J, Mejia N. Tics associated with other disorders. Philadelphia: Lippincott Williams & Wilkins; 2006.
Khalifa N, von Knorring A-L. Tourette syndrome and other tic disorders in a total population of children: clinical assessment and background. Acta Paediatr. 2007;94:1608–14.
Hanna P, Janjua F, Contant C, Jankovic J. Bilineal transmission in Tourette syndrome. Neurology. 1999;53:813–8.
Pauls DL, Raymond CL, Stevenson JM, Leckman JF. A family study of Gilles de la Tourette syndrome. Am J Hum Genet 1991;48.
Hyde TM, Aaronson BA, Randolph C, Rickler KC, Weinberger DR. Relationship of birth weight to the phenotypic expression of Gilles de la Tourette’s syndrome in monozygotic twins. Neurology. 1992;42:652–8.
Price RA, Kidd KK, Cohen DJ, Pauls DL, Leckman JF. A twin study of Tourette syndrome. Arch Gen Psychiatry. 1985;42:815–20.
Davis LK, Yu D, Keenan CL, Gamazon ER, Konkashbaev AI, et al. Partitioning the heritability of Tourette syndrome and obsessive compulsive disorder reveals differences in genetic architecture. PLoS Genet 2013;9.
Deng H, Gao K, Jankovic J. The genetics of Tourette syndrome. Nat Rev Neurol. 2012;8:203–13.
Genetics TTSAICf. A complete genome screen in sib pairs affected by Gilles de la Tourette syndrome. Am J Hum Genet. 1999;65:1428–36.
Genetics TTSAICf. Genome scan for Tourette disorder in affected-sibling-pair and multigenerational families. Am J Hum Genet. 2007;80:265–72.
Roessner V, Plessen KJ, Rothenberger A, et al. European clinical guidelines for Tourette syndrome and other tic disorders. Part II: pharmacological treatment. Eur Child Adolesc Psychiatry. 2011;20:173–96.
Dietrich A, Fernandez TV, King RA, et al. The Tourette International collaborative genetics (TIC Genetics) study, finding the genes causing Tourette syndrome: objectives and methods. Eur Child Adolesc Psychiatry 2014.
Selling L. The role of infection in the etiology of tics. Arch Neurol Psychiatry. 1929;22:1163–71.
Petek E, Windpassinger C, Vincent JB, et al. Disruption of a novel gene (IMMP2L) by a breakpoint in 7q31 associated with Tourette syndrome. Am J Hum Genet. 2001;68:848–58.
Patel C, Cooper-Charles L, McMullan DJ, Walker JM, Davison V, Morton J. Translocation breakpoint at 7q31 associated with tics: further evidence for IMMP2L as a candidate gene for Tourette syndrome. Eur J Hum Genet. 2011;19:634–9.
Bertelsen B, Melchior L, Jensen LR, et al. Intragenic deletions affecting two alternative transcripts of the IMMP2L gene in patients with Tourette syndrome. Eur J Hum Genet. 2014;22:1283–9.
Whatley SA, Curti D, Marchbanks RM. Mitochondrial involvement in schizophrenia and other functional psychoses. Neurochem Res. 1996;21:995–1004.
Lin H, Williams KA, Katsovich L, et al. Streptococcal upper respiratory tract infections and psychosocial stress predict future tic and obsessive-compulsive symptom severity in children and adolescents with Tourette syndrome and obsessive-compulsive disorder. Biol Psychiatry. 2010;67:684–91.
Swedo SE, Leonard HL, Garvey MA, Mittleman B, Allen JP, Perlmutter JS. Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry. 1998;155:264–71.
Mell LK, Davis RL, Owens D. Association between streptococcal infection and obsessive-compulsive disorder, Tourette’s syndrome, and tic disorder. Pediatrics. 2005;116:56–60.
Kaplan EL. PANDAS? or PAND? Or Both? Or Neither? Assessing a possible temporal or pathogenetic relationship with the group A “strep- tococcal diseases complex”. Contemp Pediatr. 2000;8:81–96.
Kurlan R. Tourette’s syndrome and ‘PANDAS’: will the relationship bear out? Neurology. 1998;50:1530–4.
Singer HS. PANDAS and immunomodulatory therapy: commentary. Lancet. 1999;354:1137–8.
Kurlan R, Kaplan EL. The pediatric autoimmune neuropsychiatric disorders associated with streptococcal infection (PANDAS) etiology for tics and obsessive-compulsive symptoms: hypothesis or entity? Practical considerations for the clinician. Pediatrics. 2004;113:883–6.
Swedo SE, Leckman JF, Rose NR. From research subgroup to clinical syndrome: modifying the PANDAS criteria to describe PANS (Pediatric Acute-onset Neuropsychiatric Syndrome). Pediatrics & Therapeutics 2012;2.
Lerner A, Bagic A, Simmons JM, et al. Widespread abnormality of the γ-aminobutyric acid-ergic system in Tourette syndrome. Brain. 2012;135:1926–36.
van der Salm S, Tijssen M, Koelman J, van Rootselaar A. The bereitschaftspotential in jerky movement disorders. J Neurol Neurosurg Psychiatry. 2012;83:1162–7.
Patel N, Jankovic J, Hallett M. Sensory aspects of movement disorders. Lancet Neurol. 2014;13:100–12.
Albin RL, Young AB, Penney JB. The functional-anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–75.
Mink JW. The basal ganglia and involuntary movements—impaired inhibition of competing motor patterns. Arch Neurol. 2003;60:1365–8.
Mink JW. Basal ganglia dysfunction in Tourette’s syndrome: a new hypothesis. Pediatr Neurol. 2001;25:190–8.
Albin RL, Mink JW. Recent advances in Tourette syndrome research. Trends Neurosci. 2006;29:175–82.
Marceglia S, Servello D, Foffani G, et al. Thalamic single-unit and local field potential activity in Tourette syndrome. Mov Disord. 2010;25:300–8.
Maling N, Hashemiyoon R, Foote KD, Okun MS, Sanchez JC. Increased thalamic gamma band activity correlates with symptom relief following deep brain stimulation in humans with Tourette’s syndrome. PLoS One. 2012;7, e44215. This study presents the first chronic electrophysiological data in humans with TS, which shows correlation between increased gamma band activity and clinical improvement.
Shute J, Maling N, Rossi PJ, et al. Neural correlates of Tourette syndrome within the centromedian thalamus, premotor and primary motor cortices. Neuroscience Annual Meeting 2014; Washington, DC.
Franzkowiak S, Pollok B, Biermann-Ruben K, et al. Altered pattern of motor cortical activation-inhibition during voluntary movements in Tourette syndrome. Mov Disord. 2010;25:1960–6.
Biermann-Ruben K, Miller A, Franzkowiak S, et al. Increased sensory feedback in Tourette syndrome. NeuroImage. 2012;63:119–25.
Peterson BS, Thomas P, Kane MJ, et al. Basal ganglia volumes in patients with Gilles de la Tourette syndrome. Arch Gen Psychiatry. 2003;60:415–24.
Bloch M, Leckman J, Zhu H, Peterson B. Caudate volumes in childhood predict symptom severity in adults with Tourette syndrome. Neurology. 2005;65:1253–8.
Makki MI, Munian Govindan R, Wilson BJ, Behen ME, Chugani HT. Altered Fronto-Striato-Thalamic connectivity in children with tourette syndrome assessed with diffusion tensor MRI and probabilistic fiber tracking. J Child Neurol. 2009;24:669–78.
Worbe Y, Gerardin E, Hartmann A, et al. Distinct structural changes underpin clinical phenotypes in patients with Gilles de la Tourette syndrome. Brain. 2010;133:3649–60.
Thomalla G, Siebner HR, Jonas M, et al. Structural changes in the somatosensory system correlate with tic severity in Gilles de la Tourette syndrome. Brain. 2009;132:765–77.
Cavanna AE, Stecco A, Rickards H, et al. Corpus callosum abnormalities in Tourette syndrome: an MRI-DTI study of monozygotic twins. J Neurol Neurosurg Psychiatry. 2010;81:533–5.
Jackson SR, Parkinson A, Jung J, et al. Compensatory neural reorganization in Tourette syndrome. Curr Biol. 2011;21:580–5.
Bäumer T, Thomalla G, Kroeger J, et al. Interhemispheric motor networks are abnormal in patients with Gilles de la Tourette syndrome. Mov Disord. 2010;25:2828–37.
Muellner J, Delmaire C, Valabrégue R, et al. Altered structure of cortical sulci in Gilles de la Tourette syndrome: further support for abnormal brain development. Mov Disord. 2015;30:655–61. This comprehensive study with 52 adult patients with TS (and 52 matched controls) shows abnormal structural patterns of cortical sulci, which correlated with severity of clinical symptoms.
Bohlhalter S. Neural correlates of tic generation in Tourette syndrome: an event-related functional MRI study. Brain. 2006;129:2029–37.
Debes N, Hansen A, Skov L, Larsson H. A functional magnetic resonance imaging study of a large clinical cohort of children with Tourette syndrome. J Child Neurol. 2011;26:560–9.
Mazzone L, Yu S, Blair C, et al. An FMRI study of frontostriatal circuits during the inhibition of eye blinking in persons with Tourette syndrome. Am J Psychiat. 2010; 341–9.
Worbe Y, Malherbe C, Hartmann A, et al. Functional immaturity of cortico-basal ganglia networks in Gilles de la Tourette syndrome. Brain. 2012;135:1937–46.
Singer HS, Gilbert DL, Wolf DS, Mink JW, Kurlan R. Moving from PANDAS to CANS. J Pediatr. 2012;160:725–31.
Roessner V, Schoenefeld K, Buse J, Bender S, Ehrlich S, Munchau A. Pharmacological treatment of tic disorders and Tourette syndrome. Neuropharmacology. 2013;68:143–9.
Saporta ASD, Chugani HT, Juhász C, et al. Multimodality neuroimaging in Tourette syndrome: alpha-[11C] methyl-L-tryptophan positron emission tomography and diffusion tensor imaging studies. J Child Neurol. 2010;25:336–42.
Wong DF, Brasic JR, Singer HS, et al. Mechanisms of dopaminergic and serotonergic neurotransmission in Tourette syndrome: clues from an in vivo neurochemistry study with PET. Neuropsychopharmacology. 2007;33(6):1239–51.
Singer HS. Neurochemical analysis of postmortem cortical and striatal brain tissue in patients with Tourette syndrome. Adv Neurol. 1992;58:135–44.
Swedo SE, Leonard HL, Kruesi MJP, et al. Cerebrospinal fluid neurochemistry in children and adolescents with obsessive-compulsive disorder. Arch Gen Psychiatry 1992;49.
Harris K, Singer HS. Tic disorders: neural circuits, neurochemistry, and neuroimmunology. J Child Neurol. 2006;21:678–89.
Cheon KA, Ryu YH, Namkoong K, Kim CH, Kim JJ, Lee JD. Dopamine transporter density of the basal ganglia assessed with [123I]IPT SPECT in drug-naive children with Tourette’s disorder. Psychiatry Res. 2004;130:85–95.
Serra-Mestres J, Ring H, Costa D. Dopamine transporter binding in Gilles de la Tourette syndrome: a [123I]FP-CIT/ SPECT study. Acta Psychiatr Scand. 2004;109:140–6.
Minzer K, Lee O, Hong JJ, Singer HS. Increased prefrontal D2 protein in Tourette syndrome: a postmortem analysis of frontal cortex and striatum. J Neurol Sci. 2004;219:55–61.
Wong DF, Singer HS, Brandt J, et al. D2-like dopamine receptor density in Tourette syndrome measured by PET. J Nucl Med. 1997;38:1243–7.
Albin RL, Koeppe RA, Wernette K, et al. Striatal [11C]dihydrotetrabenazine and [11C]methylphenidate binding in Tourette syndrome. Neurology. 2009;72:1390–6.
Müller-Vahl KR, Meyer GJ, Knapp WH, et al. Serotonin transporter binding in Tourette syndrome. Neurosci Lett. 2005;385:120–5.
Steeves TDL, Ko JH, Kideckel DM, et al. Extrastriatal dopaminergic dysfunction in Tourette syndrome. Ann Neurol. 2010;67:170–81.
Xu M, Kobets AJ, Du J-C, et al. Targeted ablation of cholinergic interneurons in the dorsolateral striatum produces behavioral manifestations of Tourette syndrome. Proc Natl Acad Sci PNAS. 2015;112:893–8. The authors developed a strategy for targeted ablation of cholinergic interneurons in the mice dorsolateral striatum, which demonstrated for the first time that these interneurons can cause behavioral changes that resemble aspects of TS.
Castellan Baldan L, Williams KA, Gallezot J-D, et al. Histidine decarboxylase deficiency causes tourette syndrome: parallel findings in humans and mice. Neuron. 2014;81:77–90.
Singer HS. Treatment of tics and tourette syndrome. Curr Treat Options Neurol. 2010;12:539–61.
Jankovic J. Treatment of hyperkinetic movement disorders. Lancet Neurol. 2009;8:844–56.
Conelea CA, Woods DW, Zinner SH, et al. Exploring the impact of chronic tic disorders on youth: results from the Tourette syndrome impact survey. Child Psychiatry Hum Dev. 2011;42:219–42.
Leckman JF, Riddle MA, Hardin MT, et al. The Yale Global Tic Severity Scale: initial testing of a clinician-rated scale of tic severity. J Am Acad Child Adolesc Psychiatry. 1989;28:566–73.
Goetz CG, Pappert EJ, Louis ED, Raman R, Leurgans S. Advantages of a modified scoring method for the Rush Video-Based Tic Rating Scale. Mov Disord. 1999;14:502–6.
Ackermans L, Duits A, van der Linden C, et al. Double-blind clinical trial of thalamic stimulation in patients with Tourette syndrome. Brain 2011; 832–44.
Maciunas RJ, Maddux BN, Riley DE, et al. Prospective randomized double-blind trial of bilateral thalamic deep brain stimulation in adults with Tourette syndrome. J Neurosurg. 2007;107:1004–14.
Wilhelm S, Deckersbach T, Coffey BJ, Bohne A, Peterson AL, Baer L. Habit reversal vs supportive psychotherapy for Tourette’s disorder: a randomized controlled trial. Am J Psychiatry. 2003;160:1175–6.
Deckersbach T, Rauch S, Buhlmann U, Wilhelm S. Habit reversal vs supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther. 2006;44:1079–90.
Piacentini J, Woods DW, Scahill L, et al. Behavior therapy for children with Tourette disorder a randomized controlled trial. JAMA J Am Med Assoc. 2010;303:1929–37.
Wilhelm S, Peterson AL, Piacentini J, et al. Randomized trial of behavior therapy for adults with Tourette syndrome. Arch Gen Psychiatry. 2012;69:795–803.
O’connor KP, Laverdure A, Taillon A, Stip E, Borgeat F, Lavoie M. Cognitive behavioral management of Tourette’s syndrome and chronic tic disorder in medicated and unmedicated samples. Behav Res Ther. 2009;47:1090–5.
Verdellen CWJ, Keijsers GPJ, Cath DC, Hoogduin CAL. Exposure with response prevention versus habit reversal in Tourettes’s syndrome: a controlled study. Behav Res Ther. 2004;42:501–11.
Wile DJ, Pringsheim TM. Behavior therapy for Tourette syndrome: a systematic review and meta-analysis. Curr Treat Options Neurol. 2013;15:385–95.
McGuire J, Piacentini J, Brennan E, et al. A meta-analysis of behavior therapy for Tourette syndrome. J Psychiatr Res. 2014;50:106–12.
Weisman H, Qureshi IA, Leckman JF, Scahill L, Bloch MH. Systematic review: pharmacological treatment of tic disorders—efficacy of antipsychotic and alpha-2 adrenergic agonist agents. Neurosci Biobehav Rev. 2013;37:1162–71.
Scahill L, Chappell PB, Kim YS, et al. A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry. 2001;158:1067–74.
Leckman JF, Cohen DJ, Detlor J, Young JG, Harcherik D, Shaywitz BA. Clonidine in the treatment of Tourette syndrome: a review of data. Adv Neurol. 1982;35:391–401.
Kushner HI. From Gilles de la Tourette’s disease to Tourette syndrome: a history. CNS Spectr. 1999;4:24–35.
Pena MS, Yaltho TC, Jankovic J. Tardive dyskinesia secondary to Aripiprazole. Ann Neurol. 2010;68:S21–S.
Wijemanne S, Wu L, Jankovic J. Long-term efficacy and safety of fluphenazine in patients with Tourette syndrome. Mov Disord. 2014;29:126–30.
Ondo WG, Jong D, Davis A. Comparison of weight gain in treatments for Tourette syndrome: tetrabenazine versus neuroleptic drugs. J Child Neurol. 2008;23:435–7.
Kenney C, Hunter C, Mejia N, Jankovic J. Tetrabenazine in the treatment of Tourette syndrome. J Pediatr Neurol. 2007;5:9–13.
Jimenez-Shahed J, Jankovic J. Tetrabenazine for treatment of chorea associated with Huntington’s disease. Expert Opin Orphan Drugs. 2013;1:423–36.
Jankovic J, Jimenez-Shahed J, Brown LW. A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurol Neurosurg Psychiatry. 2010;81:70–3.
Marras C, Andrews D, Sime E, Lang A. Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology. 2001;56:605–10.
Viswanathan A, Jimenez-Shahed J, Baizabal Carvallo J, Jankovic J. Deep brain stimulation for Tourette syndrome: target selection. Stereotact Funct Neurosurg. 2012;90:213–24.
Hassler R, Dieckmann G. Traitement stéréotaxique des tics et cris inarticulés ou coprolaliques considérés comme phenomena d’obsession motrice au cours de la maladie de Gilles de la Tourette. Rev Neurol (Paris). 1970;123:89–100.
Tourette Syndrome Association International Deep Brain Stimulation (DBS) Database and Registry Study Group. Tourette syndrome deep brain stimulation: a review and updated recommendations. Move Disord in press. This paper presents a review of all reported cases of TS DBS and provides updated recommendations for selection, assessment, and management of potential TS DBS cases based on the literature and implantation experience.
Shprecher DR, Schrock L, Himle M. Neurobehavioral aspects, pathophysiology, and management of Tourette syndrome. Curr Opin Neurol. 2014;27:484–92.
Okun MS, Fernandez HH, Foote KD, Murphy TK, Goodman WK. Avoiding deep brain stimulation failures in Tourette syndrome. J Neurol Neurosurg Psychiatry. 2008;79:111–2.
Le K, Liu L, Sun M, Hu L, Xiao N. Transcranial magnetic stimulation at 1 Hertz improves clinical symptoms in children with Tourette syndrome for at least 6 months. J Clin Neurosci. 2013;20:257–62.
Kwon HJ, Lim WS, Lim MH, et al. 1-Hz low frequency repetitive transcranial magnetic stimulation in children with Tourette’s syndrome. Neurosci Lett. 2011;492:1–4.
Ganos C, Roessner V, Münchau A. The functional anatomy of Gilles de la Tourette syndrome. Neurosci Biobehav Rev 2013; 1050–62.
Rossi PJ, Shute J, Gunduz A, Bowers D, Foote KD, Okun MS. Fewer than 2 hours of daily thalamic stimulation reduces tics in Tourette syndrome: two-year follow-up of scheduled deep brain stimulation. Brain 2014;submitted.
Almeida L, Martinez-Ramirez D, Rossi PJ, Peng Z, Gunduz A, Okun MS. Chasing tics in the Human brain: development of open, scheduled and closed loop responsive approaches to deep brain stimulation for Tourette syndrome. J Clin Neurol 2015;11.
Acknowledgments
We would like to acknowledge the support of the TSA Center of Excellence located at the University of Florida Center for Movement Disorders and Neurorestoration. We would also like to acknowledge the following grant support NIH R34MH080764 (Okun), NIH R211NS072897 (Okun), and UF CTSI NIH KL2 Scholarship (Gunduz). The authors would also like to sincerely thank Drs. Don Gilbert, Tamara Hershey, Joseph Jankovic, Carol Mathews, Jon Mink, and Doug Woods for their insightful comments on this manuscript.
Authors’ contributions
Aysegul Gunduz planned the outline of the manuscript, performed the literature search, drafted the text, designed the tables and figures, and approved the manuscript. Michael S. Okun planned the outline of the manuscript, contributed to and edited the text, contributed to the tables and figures, and approved the manuscript.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding Source
The authors were not paid to write this article by a pharmaceutical company or other agency.
Conflict of Interest
Aysegul Gunduz has received research grants from the Michael J. Fox Foundation, UF Clinical and Translational Sciences Institute, and DARPA. The institution and not Dr. Gunduz receives grants from Medtronic, and Dr. Gunduz has financial interest in these grants.
Michael S. Okun serves as a consultant for the National Parkinson Foundation and has received research grants from NIH, NPF, the Michael J. Fox Foundation, the Parkinson Alliance, Smallwood Foundation, the Bachmann-Strauss Foundation, the Tourette Syndrome Association, and the UF Foundation. Dr. Okun has previously received honoraria, but in the past >60 months has received no support from industry. Dr. Okun has received royalties for publications with Demos, Manson, Amazon, Smashwords, Books4Patients, and Cambridge (movement disorders books). Dr. Okun is an associate editor for New England Journal of Medicine Journal Watch Neurology. Dr. Okun has participated in CME and educational activities on movement disorders (in the last 36 months) sponsored by PeerView, Prime, Quantia, Henry Stewart, and by Vanderbilt University. The institution and not Dr. Okun receives grants from Medtronic, Abbvie, and ANS/St. Jude, and the PI has no financial interest in these grants. Dr. Okun has participated as a site PI and/or co-I for several NIH, foundation, and industry-sponsored trials over the years but has not received honoraria.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Movement Disorders
Rights and permissions
About this article
Cite this article
Gunduz, A., Okun, M.S. A Review and Update on Tourette Syndrome: Where Is the Field Headed?. Curr Neurol Neurosci Rep 16, 37 (2016). https://doi.org/10.1007/s11910-016-0633-x
Published:
DOI: https://doi.org/10.1007/s11910-016-0633-x