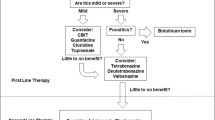
Opinion statement
Tics come in a variety of types and frequencies; have a waxing and waning course; are exacerbated by stress, anxiety, and fatigue; and often resolve or improve in the teenage or early adult years. Tourette syndrome requires the presence of chronic, fluctuating motor and phonic tics. In addition to tics, individuals with Tourette syndrome often have a variety of comorbid conditions such as attention deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder, depression and anxiety, episodic outbursts, and academic difficulties. These conditions often are a greater source of difficulty than the tics themselves. All patients with tics should be evaluated to assure proper diagnosis and to identify any associated psychopathology or academic difficulty. The treatment of tics begins with education of the patient and family, including discussions about the fundamentals of tics: their characteristics, etiology, outcomes, and available treatments. Therapy should be individualized based on the extent of impairment, available support, ability to cope, and the presence of other problems. Indications for the treatment of tics include psychosocial problems (loss of self-esteem, comments from peers, excessive worries about tics, diminished participation in activities), functional difficulties, classroom disruption, and physical discomfort. A variety of behavioral approaches can be used. Recent studies have emphasized the value of comprehensive behavioral intervention for tics (CBIT). Because habit reversal is the major component of CBIT, a cooperative patient, the presence of a premonitory urge, and a committed family are essential ingredients for success. If tic-suppressing medication is required, a two-tier approach and monotherapy are recommended. First-tier medications, notably the α-adrenergic agonists, are recommended for individuals with milder tics, especially persons with both tics and ADHD. Second-tier medications include various typical and atypical neuroleptics. Their sequence of prescription is often based on physician experience; I favor pimozide and fluphenazine. Atypical antipsychotics, such as risperidone and aripiprazole, have some advantages based on their side-effect profile and are particularly beneficial in individuals with significant co-existing behavioral issues. As will become readily apparent, however, few medications have been adequately assessed. Deep brain stimulation is an emerging therapy, but further data are required to optimize the location of electrode placement and stimulation and to determine precise indications for its implementation. Stimulant medication is effective in treating ADHD in children with tics; studies reducing concerns about its use are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tourette syndrome (TS) represents one end of a spectrum of tic disorders, which includes other entities such as transient tic disorder, chronic motor and vocal tic disorders, and tic disorders not otherwise specified [1]. Tics are common in children: epidemiologic studies have shown that about 20% of children in a classroom setting exhibit tics, and the prevalence of TS cases causing impairment is 1–10 per 1000. Milder forms of TS occur in about 0.6% of the general population. In addition to tics, individuals with TS often have a variety of concomitant psychopathologies including obsessive-compulsive disorder (OCD), attention deficit hyperactivity disorder (ADHD), learning difficulties, and sleep abnormalities. Although the presence of neurobehavioral problems is not required for the diagnosis of a tic disorder, their clinical impact on the patient may be more significant than the tics themselves. Strong support for a genetic basis is provided by twin studies, which show an 86% concordance rate for chronic tic disorder in monozygotic twins, compared with 20% in dizygotic twins. Nevertheless, neither a clear mode of inheritance nor a specific, broadly encompassing genetic linkage has been identified.
Tics
Tics are sudden, repetitive, rapid, involuntary movements or vocalizations with differing degrees of intensity and frequency and unpredictable durations. They are often subdivided into distinct categories such as motor or vocal (phonic) and simple or complex behaviors. Distinctions between motor and vocal have been questioned, recognizing that some vocal tics may be due to muscle contractions of the oropharynx or diaphragm. Simple motor tics are brief, sudden, meaningless movements such as eye blinking, shoulder shrugging, and head turning. Complex motor tics usually involve a variety of muscle groups, can appear to be purposeful (touching, jumping, body contortions, copropraxia), or involve the prolonged maintenance of a posture (dystonic tic). Simple phonic tics include throat clearing, grunting, barking, or sniffing, whereas complex vocalizations include the use of words—ie, echolalia, palilalia, or coprolalia. It is important to note, that coprolalia occurs in only about 10–19% of patients.
Common characteristics of tics include a waxing and waning course; exacerbation during periods of stress, anxiety, excitement, anger, or fatigue; appearance during inquiries about specific movements; reduction during periods of concentration or active engagement; and reported absence during sleep. A premonitory sensation (an urge, impulse, tension, pressure, itch, or tingle) often occurs before a motor or phonic tic, more typically in adults than young children. The ability to briefly suppress tics is relatively common, but suppression is often associated with a build-up of tension that resolves when the tic is permitted to occur. Although several scales are available for the ranking of tic severity, the Yale Global Tic Severity Scale (YGTSS) is most widely used in clinical trials.
Tics usually present in the first decade of life, with a peak onset at approximately 5–7 years of age. They tend to be most severe between ages 7 and 12, after which there is generally a decline in tic severity. One follow-up study of 58 teenagers and young adults (ages 15–25 years) found that by self-report, tics almost disappeared in 26%, diminished substantially in 46%, were stable in 14%, and were increased in 14%. Follow-up studies, however, suggest that about half of adults reporting the resolution of tics actually had tics confirmed on direct observation. Factors that appear to correlate with positive outcomes, regardless of tic severity, include intelligence, coping and social skills, meaningful daily activities, and good family and social support.
Tic disorders
The diagnosis of a tic disorder is based solely on historical features and a clinical examination that confirms their presence and eliminates other conditions. No diagnostic blood test, brain scan, culture, or genetic screen is currently available. The Diagnostic and Statistical Manual categorization of tics is under review [2•]. Existing categories include:
Transient tic disorder (307.21)
Transient tic disorder is defined as tics, either motor or vocal or both, occurring for less than 12 months.
Chronic motor or vocal tic disorder (307.22)
Chronic tic disorders are characterized by tics which have been present for greater than 12 months. The tics can be either solely motor, or less commonly, only vocal in nature.
Tourette syndrome/disorder (307.23)
Formal diagnostic criteria for TS, established by the Tourette Syndrome Classification Study Group, include the following:
-
Onset prior to age 21
-
Multiple motor tics and at least one vocal tic at some time, though not necessarily concurrently
-
Waxing and waning course with progressive evolution
-
Presence of tics for greater than 12 months
-
Absence of precipitating illness (eg, encephalitis, stroke or degenerative disease) or association with potential tic-inducing medication
-
Observation by a knowledgeable individual
Tic disorder, not otherwise specified (307.20)
Tic disorder-NOS includes disorders in which tics are atypical in age of onset (adult onset), are the direct physiological consequence of a general medical condition, or are due to substance intoxication or withdrawal.
Associated problems
Several studies have emphasized that most TS patients have other diagnoses or psychopathology, and the list of identified neuropsychiatric problems continues to expand [3–5]. Additionally, for most patients the clinical impact of an associated psychopathology is more significant than the tics themselves [6•].
Attention deficit hyperactivity disorder
ADHD is common in children and adolescents with TS, occurring in about 50% (range 21–90%) of referred patients with TS. This disorder generally presents at age 4–5 years and often precedes the onset of tics. In children with tics, the addition of ADHD symptoms is associated with increased psychosocial difficulties, disruptive behavior, peer rejection, emotional problems, functional impairment, family conflict, learning disabilities, and school problems.
Obsessive-compulsive disorder
Obsessive-compulsive behaviors are common in individuals with TS. In some patients it may be difficult to separate complex tics from compulsions. The incidence of obsessive-compulsive behaviors is reported to be between 20% and 89%. Symptoms generally manifest several years after the onset of tics, typically becoming more severe at a later age, and are more likely to persist than tic symptoms.
Anxiety and depression
Increased rates of both anxiety disorder and depression have been reported in patients with TS. The prevalence of depression has ranged from 13% to 76% in different studies. The etiology of depression is multifactorial. Some investigators believe depression positively correlates with earlier onset and longer duration of tics, whereas others find no correlations between depression and the number of tics.
Episodic outbursts and self-injurious behavior
Episodic outbursts or rage attacks (screaming, threatening behaviors, stomping, kicking, destroying objects, punching holes in walls, etc.) and self-injurious behaviors are relatively common in individuals with TS.
Academic difficulties
Despite typically having normal intellectual functioning, poor school performance is common in children with tics. Depending on the academic skill tested, 16–68% of children with TS function below educational expectancy and about one quarter of children receiving special education have tic disorders. Some affected individuals have executive dysfunction, discrepancies between performance and verbal IQ, impairment of visual perceptual development, and a decrease in visual-motor skills.
General principles of treatment
Important steps in the assessment and care of individuals with tic disorders are outlined in Table 1. All patients with tics should be evaluated to ensure the proper diagnosis and to eliminate the possibility that tics are secondary to another medical condition (Tic disorder-NOS) [7]. A personal interview of the patient and parent and the use of standardized parent/teacher questionnaires are helpful in identifying comorbid psychopathology and academic problems. It is also essential to identify the level of adaptive functioning, degree of impairment, and extent of distress associated with tics and with each comorbid condition. The physician must determine, based on psychosocial, classroom, and functional impairment, whether it is the patient’s tics or associated problems that require initial attention. In general, a discussion of tic symptoms and comorbid diagnoses (eg, ADHD, OCD, behavioral and learning problems) as separate entities enables the family, as well as other health care workers, to focus on individual needs.
Treatment should begin with educating the patient and family about the characteristics of tics: more specifically, that tics wax and wane; are variable in type, frequency, and intensity; have periodic fluctuations; and usually improve in the teenage or early adult years. It should be emphasized that tics are involuntary and often are exacerbated during periods of anticipation, anxiety, excitement, and fatigue. Explain that tics are caused by biologic factors (genetic with environmental influences) and, although exacerbated by stress, are not caused by emotional difficulties. The effect of environmental factors should be clarified. For example, although acute infections, such as a β-hemolytic streptococcal infection, may be associated with increased tics, the diagnosis of PANDAS (pediatric autoimmune neuropsychological disorder associated with streptococcal infections) as an etiology for TS is controversial. Finally, it is important to note that most patients with tic disorders have few difficulties secondary to their tics; parents are often more concerned about tics than their affected child.
Decisions regarding the treatment of tics need to be individualized, especially since other comorbid conditions can influence the clinical course. For many families, education regarding the diagnosis, natural history, outcome, and treatment options eliminates or at least delays the need for medical treatment. It should be emphasized that treatment is symptomatic and does not cure tics. Further, all tic-suppressing medications, even the mildest, have potential side effects. The aim of tic-suppressing pharmacotherapy is to reduce tics to a level at which they no longer cause significant psychosocial disturbance, not to completely suppress all motor and phonic tic activity. Lastly, treatment of a child with TS requires a chronic commitment and, at times, a comprehensive multidisciplinary approach.
Indications for tic-suppressing therapy |
Specific criteria for the initiation of pharmacotherapy include the following: |
• Psychosocial impairment: Psychosocial difficulties include the loss of self-esteem, comments from peers, excessive worries about tics, and failure to participate in family, social, or after-school activities. |
• Functional impairment: Tics may interfere with concentration and physical skills such as penmanship or reading. |
• Classroom disruption (usually due to vocal tics). |
• Musculoskeletal discomfort: Repetitive movements can lead to muscle strain and soreness or bone dislocation. |
• Persistence of impairing tic symptoms: Tics have a waxing and waning course and a proposed fractal pattern. Decisions to treat should be based on the presence and persistence of significantly impairing tics. |
Treatment
Nonpharmacologic treatment
Behavioral approaches
-
A variety of behavioral approaches have been used, although most have not been adequately investigated. The treating physician must recognize that these therapies, if used, do not negate ongoing educational and supportive care.
Contingency management
Contingency management maintains that tics have the potential to be modified by the contingencies that surround them, such as positive reinforcement (praise, rewards) for the reduction of tics or punishment (electric shock, time out) for tic recurrence. Cognitive behavioral therapy (discussed below) includes the management of environmental contingencies.
Relaxation training
Relaxation training (biofeedback, breathing exercises, muscle relaxation, maintaining postures, and autogenic training) has been used to promote relaxation, given that tics are exacerbated by stress and anxiety. This approach may be beneficial for some individual patients, but results failed to reach significance and were short-lived [8].
Cognitive behavioral therapy
Cognitive behavioral therapy focuses on the functional analysis and management of environmental contingencies, using approaches such as psychoeducation, awareness training, a high/low risk situational/activity profile, relaxation and muscle discrimination exercises, modification of activity planning, and development of alternative competing responses using cognitive and behavioral strategies. In a study using an individualized, manual-based, 4-month progressive protocol that included the aforementioned approaches, cognitive behavioral therapy was equally effective for tics when used with and without medication [9].
Habit reversal training
Habit reversal training (HRT), the most extensively researched technique, consists of two primary training components: tic awareness and competing response. Awareness training involves teaching the individual to become aware of his or her tics, their description and detection, the presence of premonitory urges, situation awareness, and self-monitoring. Competing response training involves performing a physically competing or incompatible response to prevent or interrupt the presence of tics. This training differs from deliberate tic suppression in that it instructs the individual to initiate a voluntary behavior to manage the premonitory urge. Randomized controlled trials of HRT have reported improved tic control [10–12].
Comprehensive behavioral intervention for tics
Comprehensive behavioral intervention for tics (CBIT) incorporates several approaches described above (psychoeducation, functional intervention, reward system, relaxation training, HRT), with the primary component being HRT. In a randomized, control trial of 126 children with impairing tics (TS or chronic tic disorder), those receiving comprehensive therapy did significantly better than those in the control group, who received only supportive and educational therapy [13••]. Attrition during the 10 weeks of behavioral therapy was low, and continued benefit was evident for 6 months following the treatment. Although this CBIT trial demonstrated efficacy, some patients did not respond, and future studies are needed to address patient selection criteria.
Alternative dietary therapies
-
Therapies in this category include the use of vitamin B6 and magnesium [14], Qufeng Zhidong Recipe, and the Clerodendrum inerme plant. Nevertheless, to date, there is no convincing scientific evidence to support the use of diets, food restrictions, or preparations of minerals or vitamins.
Acupuncture
-
Several small trials have suggested improvement following acupuncture [15].
Repetitive transcranial magnetic stimulation
-
Several studies of repetitive transcranial magnetic stimulation (rTMS), using stimulation of 1–15 Hz and targeting premotor and motor areas, showed only minimal success in suppressing tics. In five unblinded adult patients with severe tics, low-frequency stimulation of bilateral supplementary motor areas produced a 67% reduction in tics [16]. Studies in two additional patients replicated the improvement [17]. Further studies are necessary to confirm the efficacy of rTMS.
Pharmacologic treatment
-
Once the decision to use tic-suppressing medication has been reached, a two-tiered approach is recommended, recognizing that tic-suppressing medications can be broadly divided into two groups: Tier 1,“milder” non-neuroleptic medications, and Tier 2, neuroleptic and atypical neuroleptic medications. Tier 1 medications should be used first, especially in patients with milder tics. The sequence of prescription within each group is frequently based on physician experience rather than the results of direct comparison protocols (Table 2). In studies showing drug superiority to placebo, the percentage improvement of tics ranged from only 30% to 65% [18••]. The goal of treatment is not to suppress tics completely, but to eliminate the psychological or physical problems that were the specific indication for initiating treatment. In general, medications should be started at the lowest dose and gradually increased as necessary. Monotherapy should be used whenever possible. After several months of successful treatment, it is appropriate to consider a gradual taper of medication. This taper is generally scheduled during a less stressful period, such as over a student’s summer vacation.
First-tier medications
Clonidine
Clonidine is an α2-adrenergic receptor agonist that primarily activates presynaptic autoreceptors in the locus ceruleus and reduces norepinephrine release and turnover. Despite its frequent use there is a paucity of evidence for the use of clonidine in TS or other tic disorders. Several small, early studies reported conflicting data on efficacy. More recent studies have been generally supportive of its use. A blinded placebo-controlled study in children with TS and ADHD showed a significant decrease in tic severity for the clonidine group versus the placebo group [19•]. In a small, double-blind, flexible-dose, crossover trial with levetiracetam, clonidine was more effective [20]. Clonidine and risperidone showed similar efficacy in a small, single-blind, randomized trial [21]. The clonidine adhesive patch was more effective than a control adhesive patch after 4 weeks of treatment [22]. Clonidine is often used as the first agent and is particularly useful for patients with both tics and ADHD [23•].
- Standard dosage:
-
Start with 0.05 mg orally at bedtime. Increase the dose as needed every 3–7 days, by 0.05 mg per day. Use in divided doses. Because the drug has a short half-life (about 6 h), some suggest a more frequent dosage schedule for tics and definitely for ADHD. The usual maximum dose is 0.3–0.4 mg per day. Clonidine is also available as a transdermal patch that can be applied once weekly. The patch is not favored because of local skin irritation and the possibility of displacement. Clonidine should be gradually tapered to avoid rebound hypertension and tic exacerbation.
- Contraindications:
-
Documented hypersensitivity, pregnancy, breastfeeding. Use with caution if hepatic or renal function is impaired.
- Main drug interactions:
-
Clonidine may enhance the CNS-depressive effects of alcohol, barbiturates, or other sedating drugs.
- Main side effects:
-
Sedation, irritability, dizziness, dry mouth, headache, orthostatic hypotension, dysphoria, sleep disturbance. The sedating effects are usually self-limited. Sedation is the most common adverse effect.
Guanfacine
Guanfacine a longer-acting α2-adrenergic receptor agonist, is thought to be more selective for receptors in the prefrontal cortex. There have been two placebo-controlled trials of guanfacine in children with tic disorders and TS. One study showed a statistically significant improvement in tics when compared with placebo [24]. Although the second study showed a 30% decrease in the total tic score for guanfacine. compared with 11% reduction with placebo, this difference did not reach significance [25]. Similar to clonidine, guanfacine has also been used successfully in patients with tics and comorbid ADHD [23•]. Guanfacine tends to be less sedating and have less hypotensive effect than clonidine and requires less frequent dosing.
- Standard dosage:
-
The initial dose is 0.5 mg at bedtime and should be increased by 0.5 mg every 5–7 days, if necessary, to a maximum dose of 4 mg per day given in a once-a-day or twice-a-day schedule.
- Contraindications:
-
Hypersensitivity. Caution should be used in patients with cerebrovascular disease and cardiac, renal, or hepatic insufficiency.
- Main drug interactions:
-
May potentiate the CNS-depressive effects of alcohol, barbiturates, or other sedating drugs. Increases the effect of other hypotensive agents.
- Main side effects:
-
Dizziness, drowsiness, confusion, fatigue, headache, hypotension, and mental depression. Constipation and dry mouth are common.
Topiramate
Topiramate, an antiepileptic medication used for headache prophylaxis, has been reported to have some benefit for tic control. Its multiple mechanisms of action include inhibiting voltage-gated sodium channels, augmenting the inhibitory chloride ion influx mediated by gamma-aminobutyric acid (GABA), increasing endogenous GABA production, modestly inhibiting carbonic anhydrase activity, and antagonizing the AMPA/kainate subtype of the glutamate receptor.
One open-label study of two patients and a retrospective chart review have suggested a potential benefit [26, 27]. In a randomized, double-blind, placebo-controlled parallel study of 26 patients with moderately severe TS, topiramate significantly improved several YGTSS scales, as compared with the placebo group [28]. The coexistence of obesity or migraine may be an indication for the first-tier selection of topiramate.
- Standard dosage:
-
Start with a dose of 25 mg per day, gradually increasing until achieving benefit or side effects. The medication is given in divided doses. The usual effective dose is 50–300 mg per day in one study the mean effective dose was 118 mg per day [28].
- Contraindications:
-
Use lower doses with renal impairment. Monitor serum bicarbonate levels.
- Main drug interactions:
-
May decrease the efficacy of estrogen-based oral contraceptives and the levels of phenytoin. Combined use with other carbonic anhydrase inhibitors (such as acetazolamide) may increase the risk of nephrolithiasis.
- Main side effects:
-
Memory impairment, difficulty with concentration, confusion, paresthesias, altered taste, ataxia, diplopia, somnolence, dizziness, fatigue, depression, nervousness, and weight loss. Uncommon but potentially serious side effects include nephrolithiasis, metabolic acidosis, oligohidrosis, hyperthermia, and acute angle-closure glaucoma.
Levetiracetam
The precise mechanism of action of levetiracetam remains unclear; studies have suggested that it binds to SV2a, a synaptic vesicle protein found in neurons, and has an atypical GABAergic effect by enhancing chloride ion influx at the GABAA receptor complex. Several open-label clinical trials have suggested that levetiracetam may be a useful treatment for tics [29, 30] and may have a prolonged beneficial effect. Despite these reports, a double-blind, placebo-controlled trial in 20 children showed no difference between levetiracetam and placebo [31], and levetiracetam was not as beneficial as clonidine [20].
- Standard dosage:
-
Patients are usually started on 10–15 mg/kg per day, divided twice daily, and increased weekly to a total dose of 30–40 mg/kg per day.
- Contraindications:
-
Lower doses should be used in patients with impaired renal function.
- Main drug interactions:
-
No significant drug interactions have been identified.
- Main side effects:
-
Common side effects are somnolence, dizziness, headache, ataxia, fatigue, and emotional lability. Uncommon, but potentially serious, side effects include depression and/or suicidality, psychosis, pancreatitis, and pancytopenia.
Baclofen
Baclofen is a GABAB receptor agonist used in the treatment of spasticity. Its binding reduces the release of the excitatory neurotransmitters glutamine and aspartate. One open-label study evaluated baclofen in a small number of patients and demonstrated no benefit [32], whereas a second such study reported a beneficial effect in 95% of children [33]. In a small, double-blind, placebo-controlled crossover study, baclofen (20 mg three times daily) showed statistically significant improvement in an overall impairment score but no reduction in motor or vocal tics [34].
- Standard dosage:
-
In children over 7 years of age, start with 5–10 mg three times daily and titrate slowly, if necessary, to a maximum total daily dose of 60 mg.
- Contraindications:
-
Use with caution in patients with diabetes, renal insufficiency, seizure disorders, stroke, severe psychiatric disturbances, or confusional states.
- Main drug interactions:
-
Synergistic effect with other CNS depressants.
- Main side effects:
-
Somnolence, dizziness, weakness, hallucinations, confusion, headache, gastrointestinal problems, hypotonia, paresthesias, ataxia, and hyperglycemia. Sudden cessation can cause seizures and psychosis.
Clonazepam
Clonazepam, a benzodiazepine, acts as a GABAA receptor agonist and an α2-adrenergic receptor agonist; it upregulates 5-HT1 binding sites. There are no placebo-controlled trials to date, but small, open-label trials have shown some beneficial tic control. One study suggested that clonazepam is superior to clonidine [35]. Clonazepam is probably best reserved for patients with significant comorbid anxiety. Clonazepam is a controlled substance with the potential for psychological and physical dependence.
- Standard dosage:
-
Start with 0.25–0.5 mg at bedtime and titrate slowly as tolerated. The usual maintenance dose is 0.5–4 mg per day divided two to three times daily.
- Contraindications:
-
Contraindicated in patients with significant hepatic dysfunction, respiratory depression, or acute narrow-angle glaucoma.
- Main drug interactions:
-
CNS depressant action may be potentiated by other sedative hypnotic drugs (eg, alcohol, opioids, barbiturates, monoamine oxidase (MAO) inhibitors, anxiolytic, antipsychotic, anticonvulsant, or antidepressant drugs).
- Main side effects:
-
Sedation, somnolence, fatigue, confusion, dizziness, hyperactivity, and ataxia. Serious side effects include hypotension and respiratory depression. Medication may cause a paradoxic change in behavior with increased aggression, hyperexcitability, and irritability. Abrupt discontinuation of clonazepam may precipitate withdrawal symptoms, including seizures.
Second-tier medications
-
If initial therapy fails or an individual presents with severe tics, Tier 2 (classic neuroleptic or atypical neuroleptic) medications should be initiated. Medications in this category may be more beneficial than Tier 1 treatments, but side effects often limit their usefulness. Classic neuroleptics (pimozide, fluphenazine, haloperidol, trifluoperazine) are D2 dopamine receptor antagonists. These drugs act to decrease dopaminergic input from the substantia nigra to the basal ganglia and from the ventral tegmentum to the frontal cortex. They are effective at suppressing tics (70–80%) but have more severe side effects, which limit their usefulness. Atypical neuroleptics (risperidone, olanzapine, ziprasidone, quetiapine, aripiprazole) usually have a greater affinity for 5-HT2 receptors than for D2 receptors. At least some of the differences in efficacy between the different atypical neuroleptics is likely related to their relative potency of dopamine blockade.
-
Adverse effects with antipsychotics (which may occur even with low doses) include sedation, parkinsonism, acute dystonic reactions, bradykinesia, akathisia, tardive and withdrawal dyskinesias, cognitive blunting, depression, aggression, “fog states,” weight gain, prolonged cardiac conduction times (QTc), endocrine dysfunction, and poor school performance, with or without school phobia. Neurologic side effects occur with the use of atypical neuroleptics, but with possibly fewer extrapyramidal side effects than seen with some typical neuroleptics. Atypical neuroleptics are often associated with weight gain and metabolic abnormalities such as impaired glucose metabolism, dyslipidemia, obesity, and hypertension. With all medications, clinicians should prescribe the smallest dose for the shortest duration possible and monitor regularly for extrapyramidal side effects.
-
Electrocardiographic (EKG) abnormalities, serious ventricular arrhythmias, and sudden cardiac death have been associated with the use of antipsychotics. In a study of adults (mean age, 46 years), newer atypical antipsychotic medications, as a group, were associated with a risk of sudden cardiac death similar to that of typical neuroleptics [36••]. It has been suggested that obtaining an EKG before and after a patient is started on an antipsychotic drug might diminish the risk of sudden death by identifying individuals with a QT prolongation, which is a surrogate marker for drugs that cause torsade de pointes [37]. The degree of QTc prolongation varies between antipsychotics, reflecting their different capacity to block cardiac ion (potassium) channels. The QTc interval should not exceed 0.47 s in children or 0.52 s in adults, and it should not increase more than 25% from the patient’s baseline value.
Typical neuroleptics: pimozide
Pimozide, a diphenylbutylpiperidine derivative, is a D2 receptor antagonist which also blocks calcium channels. A comprehensive critical review of outcome studies has been published [38•, Class I]. Pimozide has been compared with placebo and haloperidol (two studies), placebo (one), haloperidol (one), and risperidone (two). Three studies have shown pimozide to be superior to placebo for tic suppression. Double-blind, placebo-controlled trials of pimozide versus haloperidol have shown mixed results, with one trial showing equal efficacy, one showing haloperidol to be slightly more effective than pimozide, and the third showing pimozide to be superior to haloperidol. Two trials have compared pimozide with risperidone with no significant differences identified between the two drugs in terms of efficacy or side-effect profile. In general, studies used different outcome measurement scales to assess tic severity, and flaws were noted in the analysis of crossover effects. Pimozide has been generally associated with fewer side effects, including sedation and extrapyramidal symptoms. Pimozide can prolong the QTc interval, and EKG monitoring is recommended.
- Standard dosage:
-
Start with 0.5–1 mg, preferably at bedtime the dose may be increased every 5–7 days. Use the smallest dose possible. The usual range is 2–4 mg per day in divided doses. Do not exceed 10 mg per day.
- Contraindications:
-
Documented hypersensitivity, history of cardiac arrhythmias and prolonged QT syndrome. Prior history of neuroleptic malignant syndrome, tardive dyskinesia, or Parkinson’s disease.
- Main drug interactions:
-
Use with caution in combination with QTc-prolonging agents and those with anticholinergic side effects. Increases the toxicity of MAO inhibitors and CNS depressants. Concurrent use of macrolide antibiotics or azole antifungals increases the risk of cardiotoxicity and sudden cardiac death. Avoid the consumption of grapefruit juice. Concurrent use of sertraline is contraindicated.
- Main side effects:
-
Sedation, dysphoria, cognitive blunting, school refusal, acute anxiety with somatizations, personality change, weight gain, gynecomastia or lactation, orthostatic hypotension, and blurred vision. Extrapyramidal reactions include akathisia, oculogyric crisis, drug-induced parkinsonism, and a risk of tardive dyskinesia. Rare but potentially serious adverse effects include cardiac arrhythmia (torsades de pointes), cardiac arrest, neutropenia, and seizures.
Typical neuroleptics: fluphenazine
Fluphenazine, a traditional neuroleptic, acts as both a D1 and D2 receptor antagonist. Small, open-label studies have shown fluphenazine to be effective in controlling tics, with fewer side effects than haloperidol [39, 40]. In a retrospective review, it has been shown to be an effective and well-tolerated therapy [41].
- Standard dosage:
-
Start at 0.5–1 mg at bedtime. Increase gradually every week to a daily dose of 2–5 mg per day, divided into two doses. Use the smallest dose and shortest duration possible.
- Contraindications:
-
Documented hypersensitivity, history of cardiac arrhythmias, and prolonged QT syndrome, tardive dyskinesia, or Parkinson’s disease, narrow-angle glaucoma, severe cardiac or liver disease, history of an acute extrapyramidal syndrome including acute dystonia or neuroleptic malignant syndrome (also see pimozide and haloperidol).
- Main drug interactions:
-
Caution with simultaneous use of compounds that prolonged the QT interval, cause sedation, or have an anticholinergic effect. Fluphenazine may increase serum concentrations of tricyclic antidepressants and the hypotensive action of antihypertensive agents. Concomitant lithium may cause an encephalopathy-like syndrome.
- Main side effects:
-
Extrapyramidal reactions, neuroleptic malignant syndrome, parkinsonism, tardive dyskinesia, drowsiness, restlessness, anxiety, agitation, euphoria, insomnia, confusion, weight gain, headache, seizures, tachycardia, galactorrhea, gynecomastia, hyperglycemia, hypoglycemia, sexual dysfunction, blurred vision, retinopathy, and visual disturbances.
Typical neuroleptics: haloperidol
Haloperidol, a butyrophenone, was the first traditional neuroleptic shown to be effective for tic suppression. Concerns regarding side effects have limited its use within the Tier-2 category of medications. Haloperidol was shown to be superior to both placebo and to pimozide in one double-blind, placebo-controlled trial [42, Class I]. Although other typical neuroleptics (see pimozide and fluphenazine) tend to have a slightly better patient tolerance, haloperidol remains a useful medication.
- Standard dosage:
-
Start with 0.25–0.5 mg per day in the evening. If the drug is tolerated and symptoms warrant, the dose can be increased weekly in increments of 0.25–0.5 mg, administered once or twice a day. The total daily dose ranges from 0.75 to 5 mg.
- Contraindications:
-
Hypersensitive reaction to this class of medications, prolonged QT syndrome, narrow-angle glaucoma, parkinsonism, severe cardiac or liver disease, or history of an acute extrapyramidal syndrome, including acute dystonia or neuroleptic malignant syndrome.
- Main drug interactions:
-
Caution with the simultaneous use of compounds that prolonged the QT interval, cause sedation, or have an anticholinergic effect. Haloperidol may increase serum concentrations of tricyclic antidepressants and the hypotensive action of antihypertensive agents. Concomitant use of lithium may cause an encephalopathy-like syndrome. Fluoxetine may inhibit metabolism and increase the effect of haloperidol.
- Main side effects:
-
Extrapyramidal reactions, neuroleptic malignant syndrome, parkinsonism, tardive dyskinesia, drowsiness, restlessness, anxiety, agitation, euphoria, insomnia, confusion, weight gain, headache, seizures, tachycardia, galactorrhea, gynecomastia, hyperglycemia, hypoglycemia, sexual dysfunction, blurred vision, retinopathy, and visual disturbances. Rarely causes photosensitivity reactions.
Atypical neuroleptics: risperidone
Risperidone is an atypical neuroleptic that acts as a 5-HT2 receptor antagonist at low doses and a D2 antagonist at higher doses. It also has moderate to high affinity for α1-adrenergic, D3, D4, and H1-histamine receptors. It is the most widely evaluated atypical antipsychotic for tic suppression. It has been shown to be superior to placebo in two randomized, double-blind, placebo-controlled trials for tics [43, Class I, 44]. In two randomized, double-blind, crossover studies, it was equally effective to possibly slightly more effective than pimozide [45, 46]. In children with autism, tics have been reported to appear during risperidone therapy, usually when used in combination with multiple other drugs for more than 6 months. Risperidone can prolong the QTc interval, and weight gain can be an issue.
- Standard dosage:
-
Begin with 0.25 or 0.5 mg once daily at night and gradually titrate up to 2–4 mg per day divided twice daily.
- Contraindications:
-
Hypersensitivity. Known history of QT prolongation or concomitant use of other drugs known to prolong the QT interval. Caution is recommended when there is concern regarding hyperglycemia and diabetes.
- Main drug interactions:
-
Additive effect with other CNS depressants or drugs that prolong the QT interval. Fluoxetine and paroxetine may increase the serum concentration of risperidone and decrease concentrations of 9-hydroxyrisperidone. Dopamine agonists may decrease therapeutic effects.
- Main side effects:
-
Hypotension, somnolence, weight gain, hyperglycemia, extrapyramidal reactions (akathisia, acute dystonic reactions, parkinsonism, or tardive syndromes), hyperprolactinemia, dysregulation of body temperature, neuroleptic malignant syndrome, and seizures.
Atypical neuroleptics: aripiprazole
Aripiprazole provides a high affinity at D2 receptors but also acts as a partial dopamine and 5-HT1a receptor agonist and antagonist at 5-HT2a receptors. Case reports and open-label and observational studies have supported its efficacy in treating tics [47–50]. Aripiprazole is generally well tolerated, although in one retrospective study, 22% of patients discontinued therapy, with the most common side effects being weight gain, akathisia, and sedation [47]. In contrast, in a 12-week open-label trial using a flexible dosing schedule, there was no difference in the mean body mass index measured at baseline and end point [51]. To date, no blinded, placebo-controlled trial of aripiprazole in patients with tic disorders has been published.
- Standard dosage:
-
In patients at least 10 years of age, start 2 mg every day for 3 days, then (if needed) 5 mg once a day. Titrate in increments of 2-5 mg the typical dose is 10–30 mg per day.
- Contraindication:
-
Hypersensitivity.
- Main drug interactions:
-
Carbamazepine decreases aripiprazole levels, whereas ketoconazole and quinidine increase aripiprazole levels.
- Main side effects:
-
Suicidal ideation and behavior, worsening depression, hypotension, hyperglycemia, weight gain, prolonged QT interval, gastrointestinal issues, akathisia, tardive dyskinesia, headache, insomnia, sedation, fatigue, anxiety, and restlessness
Atypical neuroleptics: ziprasidone
Ziprasidone antagonizes D2 and 5-HT2 receptors but also has widespread effects on other neurotransmitter systems, such as blocking effects on norepinephrine and serotonin transporters, α1 adrenergic receptor antagonism, and moderate affinity for histamine H1 receptors. In patients with TS, it has been shown to be more effective in suppressing tic symptoms (39% reduction) than placebo (16% reduction) [52]. Vocal tics have been reported as a tardive symptom secondary to treatment with ziprasidone [53]. Ziprasidone can prolong the QTc interval.
- Standard dosage:
-
Start with 5 mg orally every evening and titrate gradually as necessary to a dosage of 20 or 40 mg daily in divided doses.
- Contraindications:
-
Hypersensitivity. Known history of QT prolongation or concomitant use of other drugs known to cause torsades de pointes or prolong the QT interval. Caution is recommended in patients with hyperglycemia, orthostatic hypotension, and diabetes.
- Main drug interactions:
-
Concomitant use of other antipsychotic agents can have an additive effect on prolongation of the QT interval. Ziprasidone may enhance the effects of certain antihypertensive agents. Cytochrome P450 3A4 inhibitors, such as erythromycin and ketoconazole, may increase serum levels whereas CYP450 3A4 inducers such as carbamazepine and rifampin, may decrease serum levels.
- Main side effects:
-
Hypotension, somnolence, weight gain, hyperglycemia, akathisia, parkinsonism, tardive syndromes, hyperprolactinemia, insomnia, dysregulation of body temperature, neuroleptic malignant syndrome, and seizures. Ziprasidone causes greater prolongation of the QT interval than risperidone, olanzapine, or haloperidol.
Atypical neuroleptics: olanzapine
Olanzapine is an atypical neuroleptic with moderate to high affinity for D2, D4, 5-HT2A, 5-HT2C, and α-1 adrenergic receptors, as well as D1 receptors. Open-label trials have shown improvement in tic scores [54, 55]. A double-blind, crossover study of olanzapine versus pimozide found that olanzapine was superior to low-dose pimozide in producing tic reduction in four patients with severe tics [56]. The most widely reported side effects were drowsiness/sedation and weight gain. Tardive oculogyric crisis has been reported after 3 months of olanzapine treatment.
- Standard dosage:
-
Start with 2.5 mg orally every evening and increase as needed gradually to 5–10 mg per day in divided doses.
- Contraindication:
-
Hypersensitivity. Caution is recommended when there is concern regarding hyperglycemia and diabetes.
- Main drug interactions:
-
Use with caution in the presence of other CNS depressants because of a potential additive effect. The simultaneous use of olanzapine with dopamine agonists may decrease its therapeutic effects, and olanzapine use with antihypertensive medications may increase the risk of hypotension.
- Main side effects:
-
Hypotension, somnolence, weight gain, hyperglycemia, akathisia, parkinsonism or tardive syndromes, hyperprolactinemia, dysregulation of body temperature, neuroleptic malignant syndrome, seizures.
Atypical neuroleptics: quetiapine
Quetiapine is an atypical neuroleptic that antagonizes 5-HT1a, 5-HT2, D2, histamine H1, and α1 and α2 adrenergic receptors. To date, its use for tic suppression is based on case reports. One open-label trial with 12 children and adolescents with TS showed a significant reduction in tic scores with quetiapine [57], as did a retrospective review of 12 patients [58].
- Standard dosage:
-
Initially dosed at 25 mg daily it may be increased as tolerated to relatively high doses of 75–500 mg daily.
- Contraindication:
-
Use with caution in patients with congestive heart failure.
- Main drug interactions:
-
Worsens drowsiness associated with dopamine agonists, diphenhydramine hydrochloride, and sleep medications.
- Main side effects:
-
Sedation, worsening of Parkinson’s disease, induction of diabetes, stroke, and heart attacks.
Other medications
Dopamine agonists
Pergolide is a mixed dopamine (D1/D2/D3) agonist used primarily for the treatment of Parkinson’s disease. Its hypothesized mechanism of action is the reduction of dopamine release secondary to stimulation of presynaptic dopamine autoreceptors. There have been two double-blind, placebo-controlled trials of pergolide in TS, both of which showed significant improvement of total tic scores when compared with placebo. Despite reports of potential benefit, pergolide was voluntarily withdrawn from the US market in March 2007 because of ergot-induced pleural, retroperitoneal, and pericardial fibrosis, vasospasm, and concerns about cardiac valvular disease with long-term use. Nonergot dopamine agonists, such as the D2 and D3 receptor agonist pramipexole, are being investigated, given the positive results with pergolide.
Tetrabenazine
Tetrabenazine depletes presynaptic storage of catecholamines by blocking vesicular monoamine transporters, and it also is a mild postsynaptic dopamine receptor antagonist. There have been several open-label and retrospective studies of tetrabenazine for tics [59–61]. Kenney et al. [59] reviewed the medical records of 92 patients treated for tics and showed that 77% experienced either a marked or moderate reduction in tics. Porta et al. [61], in a retrospective chart review of 77 TS patients, showed that 2 years of treatment with tetrabenazine resulted in an overall improvement in 80%. Weight gain is less than that in individuals treated with neuroleptics.
- Standard dosage:
-
Dosing is started with 12.5 mg daily and increased by 12.5 mg per day every 3–5 days according to clinical response and as tolerated, divided into three daily doses. The usual effective dose is 50–150 mg per day, with a maximum recommended dose of 200 mg per day.
- Contraindications:
-
Patients with a history of depression, suicidality, or parkinsonism. Do not initiate treatment within 14 days of the use of MAO inhibitors because of a risk of hypertensive crisis.
- Main drug interactions:
-
Potentiates the systemic and neurologic effects of other amine depleters (reserpine and α-methylparatyrosine) and dopamine antagonists. When added to existing therapy with desipramine or MAO inhibitors, tetrabenazine may cause central excitation and hypertension.
- Main side effects:
-
Side effects include sedation, depression, parkinsonism, insomnia, anxiety, postural hypotension, akathisia, nausea, vomiting, insomnia, and orthostatic hypotension. Less common but potentially serious side effects include suicidality and neuroleptic malignant syndrome.
Botulinum toxin
Botulinum toxin (Botox) is a neurotoxin produced by Clostridia that exerts its effect by inhibiting the release of acetylcholine from the presynaptic terminal at the muscle-nerve junction. An evidence–based review of botulinum neurotoxin for the treatment of tics has concluded that there is evidence to support its use for motor tics, but as yet insufficient data for phonic tics [62•]. A randomized, double-blind, controlled trial of single-treatment Botox injection for motor tics in 18 patients produced a 39% reduction in the number of tics, compared with a 6% increase in the placebo group [63]. In 35 patients treated in 115 sessions, latency to onset of benefit was 3.8 days, and the mean duration of benefit was 3.4 months [64]. In a study of 15 patients with 18 individual motor tics in which each tic was treated from 2 to 50 times, short-term efficacy was reported to be good or moderate [65]. All studies report a notable reduction in premonitory sensory symptoms (urges) following Botox treatment. In a series of 30 subjects with significant vocal tics, Botox injection into the laryngeal folds decreased the frequency and interference of vocal tics, in part via a reduction of premonitory urges [66]. In general, Botox should be considered for the treatment of a specific isolated motor tic that has been present for a prolonged time, is causing significant functional impairment, and is unresponsive to pharmacotherapy.
- Standard dosage:
-
Two serotypes, botulinum toxins type A and B, are available. The intramuscular dose is highly dependent on the muscle affected by the tic. Botulinum toxin A is given in doses of 50–400 units per injection session. Botulinum toxin B is given in doses of 5000–25,000 units per injection session. Treatment is generally repeated every 3–9 months. Patients may develop antibodies to botulinum toxin, resulting in reduced efficacy of future injections.
- Contraindications:
-
Myasthenia gravis or other neuromuscular conditions and infection at the injection sites.
- Main drug interactions:
-
Botox may be potentiated by aminoglycoside antibiotics or other drugs that interfere with neuromuscular transmission.
- Main side effects:
-
Pain, erythema, ecchymoses, and rash at the injection site. Weakness of the injected muscle, and dysphasia or hypophonia following laryngeal injections.
Sulpiride and tiapride
Tiapride and sulpiride are substituted benzamides and selective D2 antagonists that lack effects on norepinephrine, acetylcholine, serotonin, histamine, or GABA receptors. Neither is available in the United States, but studies in Europe have shown improvement in tics. One retrospective study of 63 patients with TS treated with sulpiride showed beneficial effects in 59% [67]. A prospective, open-label, 6-week study using a low dose of sulpiride in 189 patients with mild to moderate tics, showed improvement in motor and vocal tic scores and the total YGTSS scale. Sedation occurred in 16% of patients and was the most common side effect [68]. Tiapride is also commonly used in Europe and its use is supported by a positive therapeutic effect in a double-blind, placebo-controlled trial in 17 children [69].
Marijuana
Δ-9-tetrahydrocannabinol (THC) is the major psychoactive substrate in Cannabis sativa. Initial interest in THC was based on interview results of 17 patients with TS, 14 of whom reported a reduction in tics with marijuana use. A randomized, double-blind, placebo-controlled study of THC showed a significant difference (P < 0.05) or a trend toward a significant difference (P < 0.10) in tics during the 6-week treatment period [70]. THC is not currently available for clinical use in the United States.
Other agents
Based upon pathophysiologic hypotheses involving various neurotransmitter systems within cortico-striato-thalamo-cortical pathways, a variety of therapeutic options have been proposed, some of which are currently undergoing preliminary trials. Recognizing that glutamate is the major excitatory transmitter in the CNS and that glutamate has significant interactions with dopaminergic systems [71], preliminary trials with glutamatergic modulating agents (agonists/antagonists) are in progress. Other drugs claimed to have a beneficial response (but lacking rigorous investigation) include donepezil (an anticholinesterase inhibitor) [72], finasteride (a 5-α-reductase inhibitor) [73], flutamide (an androgen blocker) [74], ondansetron (a selective 5-HT3 antagonist) [75], ketanserin (a 5-HT2 blocker with additional α1 agonist and mild D2 antagonist action) [76], and nicotine patches.
Immunomodulatory therapy
-
It has been proposed that tic symptoms in a subset of children are caused by a preceding group A β-hemolytic streptococcal (GABHS) infection. Labeled as “pediatric autoimmune neuropsychiatric disorder associated with streptococcal infection” (PANDAS), it has been hypothesized that the underlying pathology involves an immune-mediated mechanism with molecular mimicry. This disorder remains extremely controversial. Intravenous immunoglobulin (IVIG) has not been effective in suppressing tics in patients with PANDAS: tics were reduced by 19% after IVIG versus 12% after placebo [77]. Similarly, in 30 unselected tic-disorder patients randomly assigned to IVIG or placebo, no significant changes were observed between treatment groups in measures of tic severity [78].
Surgical approaches
Deep brain stimulation
For patients with severe tics refractory to medical management, surgical interventions focused on the proposed cortico-striato-thalamo-cortical circuits involved in TS have been attempted [79]. Deep brain stimulation, a stereotactic treatment, has had preliminary success in treating tics [80•, 81, 82]. Target sites for high-frequency stimulation have included the centromedian-parafascicular complex of the thalamus, the globus pallidus interna, and the anterior limb of the internal capsule [83]. Although the number of cases is small, there has been a persistent reduction in tic frequency by 70–90%. Although this technique has several advantages over other neurosurgical approaches, a cautious approach is recommended pending determination of patient selection criteria and the outcome of carefully controlled clinical trials.
Other neurosurgical approaches
Surgical lesioning of a variety of target sites, including the frontal lobe (bimedial frontal leucotomy and prefrontal lobotomy), limbic system (anterior cingulotomy and limbic leucotomy), cerebellum, and thalamus, have been tried in attempts to reduce severe tics [84].
Treatment of comorbidities
Attention deficit hyperactivity disorder
-
The efficacy of stimulants for the treatment of ADHD symptoms in children with tics has been well documented. Their use in this group, however, has been controversial because early reports suggested that stimulant medications had the potential to provoke or intensify tics, an adverse effect that might persist despite medication withdrawal. More recent studies, however, have blunted these initial concerns. Several investigators have shown that the potential impact of stimulants on the development of tics is minimal or of short duration, and that a definite causal effect is present in very few children [19•, 85, 86]. The Tourette Syndrome Study Group performed a multicenter, randomized, double-blind, controlled clinical trial comparing clonidine, methylphenidate, a combination of clonidine and methylphenidate, and placebo in children with tics and ADHD [19•]. The results showed that both clonidine and methylphenidate were associated with significant improvement in ADHD symptoms, with the greatest benefit when using combined clonidine and methylphenidate therapy. When treatment groups were compared for “worsening of tics,” the rates (20–26%) did not differ among the groups, including placebo, demonstrating that stimulants did not exacerbate tics.
-
Given the significant adverse impact of ADHD on quality of life and social/school functioning in children, several recommendations are proposed: appropriate accommodations in the classroom (eg, extra time to complete assignments, preferential seating, one-to-one tutoring, and predicable routines); instruction in organizational and work-study skills; and, when appropriate, pharmacotherapy.
-
Stimulants, especially methylphenidate, can have an immediate and significant beneficial effect on ADHD symptoms without worsening tic symptoms [23•].
-
Alternative medications for the treatment of ADHD in children with tic disorders include the α2-adrenergic agonists clonidine and guanfacine, discussed above, as well as atomoxetine, desipramine, and nortriptyline.
-
Atomoxetine, a selective norepinephrine reuptake inhibitor, has documented efficacy for ADHD and has been associated with no change or a minimal reduction in tics [87]. This medication is used in doses of 1.0–1.5 mg/kg per day, given in two divided doses. Common adverse effects of atomoxetine include nausea, emesis, diminished appetite, and insomnia.
-
Several placebo-controlled studies have documented the efficacy of the tricyclic antidepressant desipramine in children and adolescents with ADHD alone and with TS plus ADHD [88, 89].
Obsessive-compulsive disorder
-
Cognitive-behavioral therapy can be an effective nonpharmacologic treatment for OCD [90]. Selective serotonin reuptake inhibitors (SSRIs) are first-line therapy for OCD and are often used for disabling symptoms [91]. Empirical support for treatment in children is best with fluoxetine, sertraline, fluvoxamine, and the tricyclic antidepressant clomipramine. Combined therapy with typical or atypical neuroleptics may be beneficial in cases resistant to SSRIs alone. A black box warning has been issued by the US Food and Drug Administration regarding increased suicidal ideation and suicidality in children and adolescents receiving SSRIs.
Academic difficulties
-
A variety of factors, including severe tics, medication side effects, comorbid behavioral/psychiatric disorders (ADHD, obsessive-compulsive behaviors, and depression), psychosocial problems, learning disabilities, and executive dysfunction, can contribute to poor school performance. A comprehensive neuropsychological assessment is often beneficial in defining underlying issues. Specific therapy should be directed at the identified pathology.
References and Recommended Reading
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Singer HS: Tourette’s syndrome: from behaviour to biology. Lancet Neurol 2005, 4:149–159.
Walkup JT, Ferrao Y, Leckman JF, et al.: Tic disorders: some key issues for DSM-V. Depress Anxiety 2010, 27:600–610.
Khalifa N, von Knorring AL: Tourette syndrome and other tic disorders in a total population of children: clinical assessment and background. Acta Paediatr 2005, 94:1608–1614.
Kurlan R, Como PG, Miller B, et al.: The behavioral spectrum of tic disorders: a community-based study. Neurology 2002, 59:414–420.
Cavanna AE, Eddy C, Rickards HE: Cognitive functioning in Tourette syndrome. Discov Med 2009, 8:191–195.
Pringsheim T, Lang A, Kurlan R, et al.: Understanding disability in Tourette syndrome. Dev Med Child Neurol 2009, 51:468–472.
Mejia NI, Jankovic J: Secondary tics and tourettism. Rev Bras Psiquiatr 2005, 27:11–17.
Bergin A, Waranch HR, Brown J, et al.: Relaxation therapy in Tourette syndrome: a pilot study. Pediatr Neurol 1998, 18:136–142.
O’Connor KP, Laverdure A, Taillon A, et al.: Cognitive behavioral management of Tourette’s syndrome and chronic tic disorder in medicated and unmedicated samples. Behav Res Ther 2009, 47:1090–1095.
Woods DW, Piacentini JC, Chang SW, et al.: Managing Tourette Syndrome: A Behavioral Intervention for Children and Adults. New York: Oxford University Press; 2008. Woods DW, Piacentini JC, Chang SW, et al.: Managing Tourette Syndrome: A Behavioral Intervention for Children and Adults. New York: Oxford University Press; 2008.
Himle MB, Woods DW, Piacentini JC, Walkup JT: Brief review of habit reversal training for Tourette syndrome. J Child Neurol 2006, 21:719–725.
Deckersbach T, Rauch S, Buhlmann U, Wilhelm S: Habit reversal versus supportive psychotherapy in Tourette’s disorder: a randomized controlled trial and predictors of treatment response. Behav Res Ther 2006, 44:1079–1090.
Piacentini J, Woods DW, Scahill L, et al.: Behavior therapy for children with Tourette disorder: a randomized controlled trial. JAMA 2010, 303:1929–1937.
Garcia-Lopez R, Romero-Gonzalez J, Perea-Milla E, et al.: [An open study evaluating the efficacy and security of magnesium and vitamin B(6) as a treatment of Tourette syndrome in children.]. Med Clin (Barc) 2008, 131:689–692.
Ma S, Liu XY, Yu RL, Chen LJ: [Clinical observation on acupuncture for treatment of Tourette’s syndrome]. Zhongguo Zhen Jiu 2006, 26:392–394.
Mantovani A, Lisanby SH, Pieraccini F, et al.: Repetitive transcranial magnetic stimulation (rTMS) in the treatment of obsessive-compulsive disorder (OCD) and Tourette’s syndrome (TS). Int J Neuropsychopharmacol 2006, 9:95–100.
Mantovani A, Leckman JF, Grantz H, et al.: Repetitive transcranial magnetic stimulation of the supplementary motor area in the treatment of Tourette syndrome: report of two cases. Clin Neurophysiol 2007, 118:2314–2315.
Scahill L, Erenberg G, Berlin CM Jr, et al.: Contemporary assessment and pharmacotherapy of Tourette syndrome. NeuroRx 2006, 3:192–206.
Tourette's Syndrome Study Group: Treatment of ADHD in children with tics: a randomized controlled trial. Neurology 2002, 58:527–536.
Hedderick EF, Morris CM, Singer HS: Double-blind, crossover study of clonidine and levetiracetam in Tourette syndrome. Pediatr Neurol 2009, 40:420–425.
Gaffney GR, Perry PJ, Lund BC, et al.: Risperidone versus clonidine in the treatment of children and adolescents with Tourette’s syndrome. J Am Acad Child Adolesc Psychiatry 2002, 41:330–336.
Du YS, Li HF, Vance A, et al.: Randomized double-blind multicentre placebo-controlled clinical trial of the clonidine adhesive patch for the treatment of tic disorders. Aust NZ J Psychiatry 2008, 42:807–813.
Bloch MH, Panza KE, Landeros-Weisenberger A, Leckman JF: Meta-analysis: treatment of attention-deficit/hyperactivity disorder in children with comorbid tic disorders. J Am Acad Child Adolesc Psychiatry 2009, 48:884–893.
Scahill L, Chappell PB, Kim YS, et al.: A placebo-controlled study of guanfacine in the treatment of children with tic disorders and attention deficit hyperactivity disorder. Am J Psychiatry 2001, 158:1067–1074.
Cummings DD, Singer HS, Krieger M, et al.: Neuropsychiatric effects of guanfacine in children with mild Tourette syndrome: a pilot study. Clin Neuropharmacol 2002, 25:325–332.
Abuzzahab FS, Brown VL: Control of Tourette’s syndrome with topiramate. Am J Psychiatry 2001, 158:968.
Kuo SH, Jimenez-Shahed J: Topiramate in treatment of Tourette syndrome. Clin Neuropharmacol 2010, 33:32–34.
Jankovic J, Jimenez-Shahed J, Brown LW: A randomised, double-blind, placebo-controlled study of topiramate in the treatment of Tourette syndrome. J Neurol Neurosurg Psychiatry 2009, 81:70–73.
Awaad Y, Michon AM, Minarik S: Use of levetiracetam to treat tics in children and adolescents with Tourette syndrome. Mov Disord 2005, 20:714–718.
Fernandez-Jaen A, Fernandez-Mayoralas DM, Munoz-Jareno N, Calleja-Perez B: An open-label, prospective study of levetiracetam in children and adolescents with Tourette syndrome. Eur J Paediatr Neurol 2009, 13:541–545.
Smith-Hicks CL, Bridges DD, Paynter NP, Singer HS: A double blind randomized placebo control trial of levetiracetam in Tourette syndrome. Mov Disord 2007, 22:1764–1770.
Shapiro AK: Gilles de la Tourette Syndrome. New York: Raven; 1988.
Awaad Y: Tics in Tourette syndrome: new treatment options. J Child Neurol 1999, 14:316–319.
Singer HS, Wendlandt J, Krieger M, Giuliano J: Baclofen treatment in Tourette syndrome: a double-blind, placebo-controlled, crossover trial. Neurology 2001, 56:599–604.
Drtilkova I, Balaotkova B, Lemanova H, Zak J: Therapeutical effects of clonidine and clonazepam in children with tic syndrome. Homeost Health Dis 1994, 35:296.
Ray WA, Chung CP, Murray KT, et al.: Atypical antipsychotic drugs and the risk of sudden cardiac death. N Engl J Med 2009, 360:225–235.
Schneeweiss S, Avorn J: Antipsychotic agents and sudden cardiac death—How should we manage the risk? N Engl J Med 2009, 360:294–296.
Pringsheim T, Marras C: Pimozide for tics in Tourette’s syndrome. Cochrane Database Syst Rev 2009, CD006996. This comprehensive review of prior studies assessed the benefits and risks of pimozide in comparison to placebo or other medications in treating tics in Tourette syndrome.
Singer HS, Gammon K, Quaskey S: Haloperidol, fluphenazine and clonidine in Tourette syndrome: controversies in treatment. Pediatr Neurosci 1985, 12:71–74.
Goetz CG, Tanner CM, Klawans HL: Fluphenazine and multifocal tic disorders. Arch Neurol 1984, 41:271–272.
Jankovic J: Treatment of hyperkinetic movement disorders. Lancet Neurol 2009, 8:844–856.
Shapiro E, Shapiro AK, Fulop G, et al.: Controlled study of haloperidol, pimozide and placebo for the treatment of Gilles de la Tourette’s syndrome. Arch Gen Psychiatry 1989, 46:722–730.
Scahill L, Leckman JF, Schultz RT, et al.: A placebo-controlled trial of risperidone in Tourette syndrome. Neurology 2003, 60:1130–1135.
Dion Y, Annable L, Sandor P, Chouinard G: Risperidone in the treatment of Tourette syndrome: a double-blind, placebo-controlled trial. J Clin Psychopharmacol 2002, 22:31–39.
Bruggeman R, van der Linden C, Buitelaar JK, et al.: Risperidone versus pimozide in Tourette’s disorder: a comparative double-blind parallel-group study. J Clin Psychiatry 2001, 62:50–56.
Gilbert DL, Batterson JR, Sethuraman G, Sallee FR: Tic reduction with risperidone versus pimozide in a randomized, double-blind, crossover trial. J Am Acad Child Adolesc Psychiatry 2004, 43:206–214.
Budman C, Coffey BJ, Shechter R, et al.: Aripiprazole in children and adolescents with Tourette disorder with and without explosive outbursts. J Child Adolesc Psychopharmacol 2008, 18:509–515.
Kawohl W, Schneider F, Vernaleken I, Neuner I: Chronic motor tic disorder and aripiprazole. J Neuropsychiatry Clin Neurosci 2009, 21:224.
Murphy TK, Mutch PJ, Reid JM, et al.: Open label aripiprazole in the treatment of youth with tic disorders. J Child Adolesc Psychopharmacol 2009, 19:441–447.
Davies L, Stern JS, Agrawal N, Robertson MM: A case series of patients with Tourette’s syndrome in the United Kingdom treated with aripiprazole. Hum Psychopharmacol 2006, 21:447–453.
Seo WS, Sung HM, Sea HS, Bai DS: Aripiprazole treatment of children and adolescents with Tourette disorder or chronic tic disorder. J Child Adolesc Psychopharmacol 2008, 18:197–205.
Sallee FR, Kurlan R, Goetz CG, et al.: Ziprasidone treatment of children and adolescents with Tourette’s syndrome: a pilot study. J Am Acad Child Adolesc Psychiatry 2000, 39:292–299.
Willmund G, Lee AH, Wertenauer F, et al.: Vocal tics associated with ziprasidone. J Clin Psychopharmacol 2009, 29:611–612.
Budman CL, Gayer A, Lesser M, et al.: An open-label study of the treatment efficacy of olanzapine for Tourette’s disorder. J Clin Psychiatry 2001, 62:290–294.
McCracken JT, Suddath R, Chang S, et al.: Effectiveness and tolerability of open label olanzapine in children and adolescents with Tourette syndrome. J Child Adolesc Psychopharmacol 2008, 18:501–508.
Onofrj M, Paci C, D’Andreamatteo G, Toma L: Olanzapine in severe Gilles de la Tourette syndrome: a 52-week double-blind cross-over study vs. low-dose pimozide. J Neurol 2000, 247:443–446.
Mukaddes NM, Abali O: Quetiapine treatment of children and adolescents with Tourette’s disorder. J Child Adolesc Psychopharmacol 2003, 13:295–299.
Copur M, Arpaci B, Demir T, Narin H: Clinical effectiveness of quetiapine in children and adolescents with Tourette’s syndrome: a retrospective case-note survey. Clin Drug Investig 2007, 27:123–130.
Kenney C, Hunter C, Jankovic J: Long-term tolerability of tetrabenazine in the treatment of hyperkinetic movement disorders. Mov Disord 2007, 22:193–197.
Kenney C, Hunter C, Davidson A, Jankovic J: Short-term effects of tetrabenazine on chorea associated with Huntington’s disease. Mov Disord 2007, 22:10–13.
Porta M, Sassi M, Cavallazzi M, et al.: Tourette’s syndrome and role of tetrabenazine: review and personal experience. Clin Drug Investig 2008, 28:443–459.
Simpson DM, Blitzer A, Brashear A, et al.: Assessment: botulinum neurotoxin for the treatment of movement disorders (an evidence-based review): report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology 2008, 70:1699–1706.
Marras C, Andrews D, Sime E, Lang AE: Botulinum toxin for simple motor tics: a randomized, double-blind, controlled clinical trial. Neurology 2001, 56:605–610.
Kwak CH, Hanna PA, Jankovic J: Botulinum toxin in the treatment of tics. Arch Neurol 2000, 57:1190–1193.
Rath JJ, Tavy DL, Wertenbroek AA, et al.: Botulinum toxin type A in simple motor tics: short-term and long-term treatment-effects. Parkinsonism Relat Disord 2010, 16:478–481.
Porta M, Maggioni G, Ottaviani F, Schindler A: Treatment of phonic tics in patients with Tourette’s syndrome using botulinum toxin type A. Neurol Sci 2004, 24:420–423.
Robertson MM, Schnieden V, Lees AJ: Management of Gilles de la Tourette syndrome using sulpiride. Clin Neuropharmacol 1990, 13:229–235.
Ho CS, Chen HJ, Chiu NC, et al.: Short-term sulpiride treatment of children and adolescents with Tourette syndrome or chronic tic disorder. J Formos Med Assoc 2009, 108:788–793.
Eggers C, Rothenberger A, Berghaus U: Clinical and neurobiological findings in children suffering from tic disease following treatment with tiapride. Eur Arch Psychiatry Neurol Sci 1988, 237:223–229.
Muller-Vahl KR, Schneider U, Prevedel H, et al.: Delta 9-tetrahydrocannabinol (THC) is effective in the treatment of tics in Tourette syndrome: a 6-week randomized trial. J Clin Psychiatry 2003, 64:459–465.
Singer HS, Morris C, Grados M: Glutamatergic modulatory therapy for Tourette syndrome. Med Hypotheses 2010, 74:862–867.
Cubo E, Fernandez Jaen A, Moreno C, et al.: Donepezil use in children and adolescents with tics and attention-deficit/hyperactivity disorder: an 18-week, single-center, dose-escalating, prospective, open-label study. Clin Ther 2008, 30:182–189.
Bortolato M, Muroni A, Marrosu F: Treatment of Tourette’s syndrome with finasteride. Am J Psychiatry 2007, 164:1914–1915.
Peterson BS, Zhang H, Anderson GM, Leckman JF: A double-blind, placebo-controlled, crossover trial of an antiandrogen in the treatment of Tourette’s syndrome. J Clin Psychopharmacol 1998, 18:324–331.
Toren P, Weizman A, Ratner S, et al.: Ondansetron treatment in Tourette’s disorder: a 3-week, randomized, double-blind, placebo-controlled study. J Clin Psychiatry 2005, 66:499–503.
Bonnier C, Nassogne MC, Evrard P: Ketanserin treatment of Tourette’s syndrome in children. Am J Psychiatry 1999, 156:1122–1123.
Perlmutter SJ, Leitman SF, Garvey MA, et al.: Therapeutic plasma exchange and intravenous immunoglobulin for obsessive-compulsive disorder and tic disorders in childhood. Lancet 1999, 354:1153–1158.
Hoekstra PJ, Minderaa RB, Kallenberg CG: Lack of effect of intravenous immunoglobulins on tics: a double-blind placebo-controlled study. J Clin Psychiatry 2004, 65:537–542.
Neimat JS, Patil PG, Lozano AM: Novel surgical therapies for Tourette syndrome. J Child Neurol 2006, 21:715–718.
Porta M, Sassi M, Ali F, et al.: Neurosurgical treatment for Gilles de la Tourette syndrome: the Italian perspective. J Psychosom Res 2009, 67:585–590.
Mink JW: Clinical review of DBS for Tourette syndrome. Front Biosci (Elite Ed) 2009, 1:72–76.
Mink JW: Clinical review of DBS for Tourette syndrome. Front Biosci (Elite Ed) 2009, 1:72–76.
Shields DC, Cheng ML, Flaherty AW, et al.: Microelectrode-guided deep brain stimulation for Tourette syndrome: within-subject comparison of different stimulation sites. Stereotact Funct Neurosurg 2008, 86:87–91.
Temel Y, Visser-Vandewalle V: Surgery in Tourette syndrome. Mov Disord 2004, 19:3–14.
Roessner V, Robatzek M, Knapp G, et al.: First-onset tics in patients with attention-deficit-hyperactivity disorder: impact of stimulants. Dev Med Child Neurol 2006, 48:616–621.
Erenberg G: The relationship between Tourette syndrome, attention deficit hyperactivity disorder, and stimulant medication: a critical review. Semin Pediatr Neurol 2005, 12:217–221.
Allen AJ, Kurlan RM, Gilbert DL, et al.: Atomoxetine treatment in children and adolescents with ADHD and comorbid tic disorders. Neurology 2005, 65:1941–1949.
Singer HS, Brown J, Quaskey S, et al.: The treatment of attention-deficit hyperactivity disorder in Tourette’s syndrome: a double-blind placebo-controlled study with clonidine and desipramine. Pediatrics 1995, 95:74–81.
Spencer T, Biederman J, Coffey B, et al.: A double-blind comparison of desipramine and placebo in children and adolescents with chronic tic disorder and comorbid attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2002, 59:649–656.
Storch EA, Bjorgvinsson T, Riemann B, et al.: Factors associated with poor response in cognitive-behavioral therapy for pediatric obsessive-compulsive disorder. Bull Menninger Clin 2010, 74:167–185.
Decloedt EH, Stein DJ: Current trends in drug treatment of obsessive-compulsive disorder. Neuropsychiatr Dis Treat 2010, 6:233–242.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Singer, H.S. Treatment of Tics and Tourette Syndrome. Curr Treat Options Neurol 12, 539–561 (2010). https://doi.org/10.1007/s11940-010-0095-4
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11940-010-0095-4