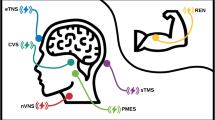
Abstract
Migraine and other chronic headache disorders are common and if inadequately treated, can lead to significant disability. The effectiveness of medications can be limited by side effects, drug interactions, and comorbid diseases necessitating alternative methods. Technological developments in the past 5 years have made it possible to use non-invasive methods of neuromodulation to treat primary headache disorders. This field includes technologies such as supraorbital transcutaneous stimulation (STS), transcranial magnetic stimulation (TMS), and non-invasive vagal nerve stimulation (nVNS). Existing trials show these modalities are safe and well tolerated and can be combined with standard pharmacotherapy. We review the technologies, biological rationales, and trials involving non-invasive neuromodulation for the treatment of primary headache disorders.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Migraine is a chronic disease in the USA affecting 18 % of women and 6 % of men, particularly in early middle age (35–45 years) leading to significant morbidity and disruption of productivity [1]. Although treatment options for migraine have drastically expanded in the past 30 years with the introduction of triptans and better clinical application of preventive treatment, the effectiveness of pharmacologic therapies can be limited by adverse events (AEs), drug-drug interactions, systemic comorbidities (e.g., cardiovascular, renal, or liver disease), pregnancy, or inadequate clinical response. Less than one fourth of patients with chronic migraine continue to use oral preventive medication more than 12 months after treatment initiation usually due to AEs or ineffectiveness [2]. Due to these limitations, a significant portion of migraineurs, amounting to 2 % of the general population, suffer from chronic migraine [3] with one in five becoming occupationally disabled [4].
Neuromodulation is a key concept in many brain disorders including migraine. The pharmacologic neuromodulation of major neurotransmitter systems such as serotonin, dopamine, and noradrenaline is widely utilized in the treatment of pain. Glutaminergic neurotransmission and cortical excitability are linked to migraine [5] and cortical spreading depression. The suppression of cortical spreading depression may be a common mechanism of action for migraine preventive treatment [6].
The prevalence and burden of migraine have led to new research in non-pharmacologically based treatments using various forms of neuromodulation. Common forms of neuromodulation in clinical practice include electrical or magnetic stimulation, which may target peripheral nerves, the spinal cord, or intracranial sites. Chronic peripheral nerve stimulation, such as the occipital nerve, appears to be effective for many patients with refractory migraine [7, 8] but only a minority experiences an excellent response. AEs such as lead migration, infection, and surgical pain are common. Intracranial stimulation, including deep brain stimulation and motor cortex stimulation, may be effective for the treatment of pain [9]. A few studies have explored the use of deep brain stimulation for intractable chronic headache disorders such as cluster [10]. Unfortunately, these procedures carry significant risks including death [11]. Non-invasive neurostimulation avoids surgical risks and their cost can be more accessible to patients. These treatments generally allow patients to self administer treatments, potentially changing the patient locus of control and improving long-term outcomes [12].
One form of neuromodulation, transcutaneous magnetic stimulation, has traditionally been used in the treatment of psychiatric conditions [13] and diagnostically used for functional brain mapping [14]. Vagal nerve stimulation previously required implanted leads and was used for the treatment of refractory epilepsy. However, with the adaptation of external vagal nerve stimulators, its use has expanded to the treatment of different chronic pain disorders, including migraine. Transcutaneous electrical stimulation is an old technology more commonly used in other pain disorders that has been adapted for use in headaches after previous reports showed effectiveness with high frequency stimulation [15, 16].
In the article, we review the non-invasive neuromodulation technologies for the treatment of primary headache disorders and the key clinical investigations into their use for headache.
Supraorbital Transcutaneous Stimulation
The Cefaly™ device (CEFALY-Technology, Belgium) is available by prescription in the USA and Europe and is the first device to use transcutaneous stimulation for targeted treatment of migraine. It was adapted from transcutaneous electrical nerve stimulation that has been safely and widely used to supplement medications and physical therapy for other chronic pain syndromes, such as in failed back syndrome [17].
The device consists of an electrode with skin adhesive placed on the forehead covering the sites of the supraorbital and supratrochlear nerves, both of which are branches of the ophthalmic nerve or the first branch of the trigeminal nerve. Biphasic rectangular impulses with an electrical mean of zero, impulse width of 250 μs, frequency of 60 Hz, and maximum intensity of 16 mA are generated with device activation. The relatively high frequency and low intensity is aimed to avoid crossing the pain threshold while still being able to activate Aβ afferents and leading to paresthesia in the distribution of the nerve and preventing the activation of Aδ and C fibers important in nociception and reducing hyperalgesia [18].
Supraorbital Transcutaneous Stimulation Studies
Piquet et al [19••]: In a double-blind, sham-controlled study on 30 healthy volunteers, high frequency stimulation (120 Hz) for 10 min with the Cefaly™ device produced a sedative effect with significantly decreased vigilance and attention compared to low frequency (2.5 Hz) and sham stimulation (low intensity stimulation below the threshold of perception). There were no significant AEs in any groups. The biological rationale for a sedative effect from STS remains speculative but other research shows possible involvement of monoaminergic brain stem nuclei [20].
PREMICE Study [21••]: The PREMICE study (PREvention of MIgraine using the STS Cefaly) showed that STS reduced the number of headache days in patients with episodic migraines (with and without aura) by the third month of use via a double-blind, randomized, sham-controlled trial. A total of 67 patients were enrolled and randomized to either verum or sham stimulation (machine-produced identical noise but no stimulation) for 20 min a day for 90 days. Patients were not on pharmacologic preventives and had an average of four migraine attacks and seven migraine days per month.
Primary outcomes studied were the number of migraine days per month at the end of 90 days and responder rate (defined as patients with ≥50 % decrease in migraine days). There were no significant differences in the number of migraine days compared to sham in the first month of use; however, a significant difference emerged by the third month of use with the treatment group having 2.06 less days of migraine and the sham group having 0.32 less days of migraine (p = 0.054). The treatment group had a significant decrease in the number of days/month requiring acute medication (11.45 to 7.25 days/month, p = 0.0057) with no changes seen in the sham group (9.24 to 9.28 days/month, p = 0.822). The 3-month responder rate (≥50 % reduction in headache frequency) in the treatment group was 38 and 12 % in the sham group (p = 0.023). No treatment effects were seen in average headache severity and there were no significant AEs in any group.
Magis et al. [22]: Further safety data was gathered via a mail-based survey involving 2312 Belgian patients showing an overall satisfaction rate of 54 % with only minor and reversible side effects in 4.3 %. This study did not specifically exclude patients with chronic migraine, but instead, allowed any patients using migraine-specific medications (triptans) to participate in the survey. The authors contacted subjects by telephone at the end of the rental period to determine satisfaction and AEs. A few patients reported some effectiveness for the treatment of acute headache using the device.
Transcranial Magnetic Stimulation
Transcranial magnetic stimulation (TMS) has been used since the 1990s as a non-invasive technology for both diagnostic and therapeutic purposes in functional brain mapping and treatment of depression [23].
TMS pulses can be delivered repetitively in rapid succession (rTMS) or singly (sTMS). Potential applications with clinical effect using rTMS include depression, schizophrenia, obsessive-compulsive disorder, and addiction among others [13, 24]. RTMS in general has a stimulatory effect and has been associated with the risk of seizure. The use of sTMS has inhibitory effects and has not been associated with seizure or other significant neurological, cardiac, or other systemic AEs [23, 25, 26].
The magnetic field generated by the TMS device at the occiput induces a secondary current in the adjacent brain tissue leading to depolarization both orthodromic and antidromic to the stimulus. By manipulating the location of the stimulus and other delivery parameters, TMS can lead to functional activation or suppression of cortical areas [14, 23, 24]. As a therapeutic modality, TMS-induced depolarization is thought to disrupt the wave of spreading cortical depression associated with migraine and, therefore, prevent migraine propagation [27••].
Transcranial Magnetic Stimulation Studies
Lipton et al. [27••]: Lipton et al. conducted the first double-blinded, randomized, sham-controlled study on the use of sTMS as abortive therapy for migraine preceded by aura. Two hundred and one subjects with aura preceding migraine >30 % of the attacks and moderate to severe pain >90 % of the attacks were randomized (sham n = 99, verum n = 102) and allowed to treat up to three auras within 60 min of onset. The treatment consisted of two pulses delivered approximately 30 s apart. Patients were on stable doses of migraine preventive drugs not allowed to use abortive therapy until 2 h after the use of the device. The sham machines were physically identical to the verum machines in addition to producing similar sounds and light display with use.
The primary outcome was pain freedom at 2 h for the first treated attack and secondary assessments included the proportion with photophobia, nausea, and phonophobia. Significantly, more patients were pain free at 2 h in the treatment group (39 %) compared to the sham group (22 % p = 0.0179). Those in the treatment group at 2 h were also significantly less likely to have associated symptoms such as photophobia and phonophobia and nausea for those with moderate to severe pain (this benefit in the reduction of migrainous symptoms was not seen in those with mild headache severity).
Additionally, those in the treatment group were more likely to meet secondary outcomes by being pain free at 24 h (29 vs 16 %, p = 0.0405) and 48 h (27 vs 13 %, p = 0.0327). Interestingly, patients on a baseline preventive medication appeared to be less likely to be pain free in the treatment group (65 %) compared to the sham group (97 %) though additional post hoc subgroup analysis [28] suggested this relationship was a spurious statistical result and no additional effects were seen between TMS and sham in those using preventive medications.
Although the above differences in the sham and verum groups are quite striking, the study did not reveal differences in the use of abortive medications or differences in perceived global assessment of relief. Adequate blinding was maintained through the study as 71 % of those in the treatment group and 67 % of those in the sham group believed they received active treatment.
Bhola et al. [29]: A post-marketing, open-label study conducted via telephone survey of patients with episodic and chronic migraine (both with and without aura) showed the device to be well tolerated without serious AEs and with 62 % of users reporting benefit at 12 weeks. The majority of users (131 of 190) had chronic migraine. The study included three women who used the device during pregnancy.
Non-Invasive Vagal Nerve Stimulation
Stimulation of the cervical vagal nerve was developed as an adjunctive treatment for severe refractory epilepsy or depression. Until recently, nVNS required surgically implanted leads. Interest in vagal nerve modulation in the treatment of headache disorders arose out of case reports and observations that epilepsy patients with comorbid migraines reported striking reduction in migraines with VNS use [30–33]. An uncontrolled experiment showed that left vagal nerve stimulation via implanted device in patients with epilepsy reduced pain threshold as tested by mechanical impact to the skin [34]. Further studies via rat models showed that VNS reduced nociceptive behaviors in mice in addition to reducing sign neuronal activation via fos-immunoreactivity with formalin-induced trigeminal activation [35].
The exact mechanism of pain relief with VNS is not well understood. VNS inhibition of glutamate release in the trigeminal nucleus caudalis has been implicated as a possible mechanism of action [36]. Additionally, it is known that the afferent nucleus of the vagus nerve (solitary tract nucleus) has a complex network of outputs to the brainstem autonomic centers as well as hypothalamus, thalamic nuclei, and cortical areas involved in pain perception [37], making it possible to modulate multiple pathways that contribute to pain and pain response. Despite the multiple potential systemic effects of VNS, the stimulation settings for the treatment of epilepsy or headache do not produce serious AEs. In fact, VNS may be useful for the treatment of a wide variety of medical conditions such as heart failure [38] and dementia [39].
A portable, battery-powered nVNS was developed in the past 5 years allowing for the stimulation of the cervical vagus nerve without the barriers or complications of surgically implanted devices.
There are two commercially available nVNS devices with the gammaCore device (electroCore LLC, Basking Ridge, NJ, USA) being the only device studied for headache treatment. The gammaCore device selective stimulates low threshold myelinated affect A fibers via 90-s pulses.
Non-Invasive Vagal Nerve Stimulation Studies
EVENT [40, 41••, 42]: The Prevention of Chronic Migraine Study was a double-blind, randomized, sham-controlled trial of patients with chronic migraine (with or without aura) who were not on other migraine prophylactics.
Vagal nerve stimulations were carried out three times a day 6–8 h apart between each session. Each session consisted of two 90-s stimulations delivered to the right vagus nerve at 5–10-min intervals. After 2 months, patients in the treatment group (n = 26) had a nearly 2-day reduction in the number of headache days per 28 days whereas the sham group (n = 23) did not show any change in headache days (p = 0.1249). A minority (11.5 %) of treated patients reported >50 % reduction in headache days whereas 0 % reported such an improvement in the sham group. Additionally, the treatment group also reported improvement in quality of life as assessed by the SF-12.
After 2 months of blinded treatment, the study was converted to an open-label extension (OLE) phase and carried out for an additional 6 months. There was significant drop out of the patients during this phase with 45.8 % of patients completing the OLE phase. There appeared to be an additive effect with sustained treatment as those who had 8 months of true nVNS showed a 42.1 % (8.8 day) reduction in the number of headache days per 28 days compared to 23.6 % (5.5 day) reduction in the number of headache days in those with 6 months of true nVNS treatment.
The rates of AEs were similar in the sham and verum groups during the randomized phase and were non-serious; bradycardia and vocal cord paralysis were not seen. During the open-label phase, one patient developed appendicitis (deemed not to be device related), and one developed worsening headaches.
PREVA [43••, 44]: The Prevention and Acute Treatment of Chronic Cluster Headache was an open-label study with patients randomized to either standard of care (n = 49) or standard of care plus nVNS (n = 48), after 4 weeks of comparative study; the study was extended for 4 weeks an open-label with all patients receiving standard of care plus nVNS. Treatment with nVNS consisted of three 90-s stimulations performed twice daily with additional stimulations performed as necessary based on the level of pain or other symptoms.
After 2 weeks of comparative treatment, patients receiving nVNS experienced a subsequent 43 % reduction in the number of cluster attacks per week compared to 12 % reduction in the standard of care alone group (p = 0.0025). Those receiving nVNS also required less rescue medications of sumatriptan and oxygen (>50 % reduction in use). AEs were generally mild to moderate in severity with the use of nVNS (e.g., neck pain and dizziness). During the open-label extension, the improvements seen during the comparative phase were maintained and the rates of AEs remained similar.
ACT1 [45]: The Efficacy and Safety Outcomes of Non-invasive Vagus Nerve Stimulation for the Acute Treatment (ACT1) of the Cluster Headache Study was a double-blind, randomized trial comparing nVNS to sham as a rescue treatment for cluster attacks in both chronic and episodic cluster patients (n = 49 and 101, respectively). Treatment consisted of three 120-s stimulations to the right cervical vagus nerve at the onset of pain or premonitory symptoms carried out over 1 month. The primary outcome of responder rate (defined as 0 to 1 level of pain on a 4-point scale with 0 as no pain and 4 as very severe pain) showed a trend of improvement, particularly for those with episodic cluster (34.2 % nVNS responder rate vs 17 % sham), though was not statistically significant (p = 0.07). The secondary outcome of sustained response (0 to 1 pain at both 15 and 60 min) was significant for those with episodic cluster (34.2 % nVNS vs 10.6 % sham, p = 0.008) but not for those with chronic cluster (13.6 % nVNS vs 15.4 % sham, p = 1.0). There were no serious device-related AEs in either group.
Nesbitt [46] published a single-arm, open-label study of cluster headache patients (11 chronic, 8 episodic) who used the gammaCore device over the course of 1 year. The device was used preventatively (two to three sessions of stimulation twice a day) and abortively (additional three consecutive sessions) on the cervical vagus nerve ipsilateral to the headache. The study showed a reduction in attack frequency from 4.5 to 2.6 every 24 h and reduced need for rescue medications.
Grazzi [47] published a single-arm, open-label study of patients (n = 30) with 5–9 migraine days per month (with and without aura) using gammaCore as rescue treatment. Treatment consisted of one 90-s pulse delivered to the right vagus nerve. Of 112 treated migraine attacks, 39.2 % (44) were aborted completely within 30 min. Most of the other attacks (44.6 %, n = 50) did not improve and were treated with rescue medication at 2 h. The remaining 16.2 % (18) attacks had unclear benefit from treatment but were not treated with subsequent rescue medication.
Goadsby [48] published a single-arm, open-label study of patients (n = 30) with episodic migraine (with and without aura) using gammaCore as rescue treatment (two 90-s stimulations at 15-min intervals performed on the right cervical vagus nerve). The 2-hour pain-free rate was 21 % for the first treated attack and was well tolerated with no serious AEs.
Rainero [49] published an open-label study of 15 patients with chronic migraine and comorbid medication overuse headache. Patients were treated with a 5-day detoxification regiment and followed for 6 months using nVNS as acute treatment. Of the 362 attacks that were treated, 2-h pain-free rate was 33.4 % with overall mild side effects.
Discussion
Recent advances in neuromodulation technology have made it possible to use portable and non-invasive devices for treatment of headache disorders. These advances are particularly exciting as they can be safely combined with existing pharmacotherapy and can be offered to patients with pharmacologic contraindications due to comorbid diseases or pregnancy.
The three non-invasive devices studied for use in headache disorders include the Cefaly (supraorbital transcutaneous stimulation), SpringTMS (transcranial magnetic stimulation), and gammaCore (external vagal nerve stimulator). For each device, there exists one to two double-blind, sham-controlled RCTs all with favorable outcomes and no severe or dangerous AEs. Table 1 provides an overview of the key studies.
The Cefaly device has been studied as a preventive in episodic migraine via a double-blind, sham-controlled RCT showing reduced headache days and less abortive medication in the treatment group after 3 months of use [21••]. As with all device-related studies, adequate blinding can be difficult to achieve; in this study, the sham treatment consisted of a device that produced identical noise but no sham stimulation. As the verum stimulation is known to have prominent sensory effects, patients’ spontaneous reports of their sensory perceptions may lead to unblinding of the investigator, if not the patient as well. Cefaly has not been studied in a clinical trial for chronic migraine.
There are no large studies of neuromodulation devices in pregnancy. Cefaly is marketed by the manufacturer as safe for use in pregnancy based on existing experience with the safety of transcutaneous electrical nerve stimulation for back pain in pregnancy [50]. Cefaly is also compatible with implanted pacemakers and most other medical devices. The SpringTMS post market survey included three women who used the device during pregnancy without reported complications. VNS may also be safe for use in pregnancy, and a few case reports describe its use with good outcomes [51]. SpringTMS is contraindicated in the presence of metallic implants or leads in the head and neck area and is likely safe for most cardiac devices (though specifics may require discussion with a cardiac device manufacturer). GammaCore is not recommended for use in the presence of a pacemaker or other electronic-implanted device although implanted VNS has been safely used in those with implanted cardiac defibrillators [38].
The SpringTMS device has been investigated by Lipton et al. [27••] as an abortive in episodic migraine via a double-blind, sham-controlled RCT which showed significantly better 2 and 24-h pain-free rates. Adequate blinding appeared to have been maintained as similar percentages of those in the verum (71 %) and sham groups (67 %) believed they received active treatment.
The gammaCore nVNS device has been investigated via double-blind, sham-controlled RCT for both cluster (ACT1 [45], used as rescue treatment) and migraine (EVENT [40, 41••, 42], used as preventive). As an abortive treatment for cluster headache, ACT1 showed nVNS is more effective for episodic cluster than chronic cluster patients. The PREVA [43••, 44] study did show significant improvements with nVNS therapy for chronic cluster patients though was not a blinded or sham-controlled trial. Additional open-label studies [46–49] were conducted with various cluster and migraine patients all with favorable results. It is of interest to note that although stimulation at the right cervical vagus nerve is the standard used in the EVENT, PREVA, and ACT1 trials (regardless of the side of the pain), this protocol was adjusted by Nesbitt [46] to be stimulation at the vagus nerve ipsilateral to the side of cluster headache. There have not been studies comparing standard right-sided approach versus ipsilateral approach for patients with cluster. Implanted VNS stimulation of the vagus nerve has been associated with events such as bradycardia which was not seen in the nVNS trials. There were no serious or dangerous device-related AEs for any of the devices, and larger post marketing surveys showed good safety and tolerability [22, 29].
Although non-invasive neuromodulation is a promising new method to treat patients with chronic headache disorders, there are significant cost and accessibility limitations due to the lack of insurance coverage for the devices and a relative dearth of physicians with experience in using these devices. The Cefaly device is available for purchase for approximately $400 with a prescription though long-term use can incur significant costs due to the cost of the specialized leads ($25 for three). SpringTMS can be rented for $2700 with a 1-year prescription. The gammaCore device is not yet available for use outside of clinical studies and there are currently no price guidelines.
Conclusion
Based on the existing RCTs and cohort studies, non-invasive neuromodulation is a safe and potentially effective treatment for multiple headache disorders, including migraine and cluster (both chronic and episodic). At this time, studies have been limited to migraine and cluster but may be expanded to include other trigeminal autonomic cephalgias and post-traumatic headache. Cost and accessibility may be improved with increased practitioner knowledge and more widespread device use and distribution.
References
Papers of particular interest, published recently, have been highlighted as: •• Of major importance
Lipton R, Stewart W, Diamond S, Diamond M, Reed M. Prevalence and burden of migraine in the United States: data from the American migraine study II. Headache. 2001;41:646–57.
Hepp Z, Dodick D, Varon S, Gillard P, Hansen R, Devine E. Adherence to oral migraine-preventive medications among patients with chronic migraine. Cephalalgia. 2014;35:478–88.
The international classification of headache disorders, 3rd edition (beta version). Cephalagia 2013;33:629-808.
Manack A, Buse D, Lipton R. Chronic migraine: epidemiology and disease burden. Curr Pain Headache Rep. 2011;15:70–8.
Ramadan N. The link between glutamate and migraine. CNS Spectr. 2003;8:446–9.
Ayata C, Jin H, Kudo C, Dalkara T, Moskowitz M. Suppression of cortical spreading depression in migraine prophylaxis. Ann Neurol. 2006;59:652–61.
Schwedt T, Dodick D, Hentz J, Trentman T, Zimmerman R. Occipital nerve stimulation for chronic headache—long-term safety and efficacy. Cephalalgia. 2007;27:153–7.
Saper J, Dodick D, Silberstein S, McCarville S, Sun M, Goadsby P. Occipital nerve stimulation for the treatment of intractable chronic migraine headache: ONSTIM feasibility study. Cephalagia. 2010;31:271–85.
Tsubokawa T, Katayama Y, Yamamoto T, Hirayama T, Koyama S. Chronic motor cortex stimulation in patients with thalamic pain. J Neurosurg. 1993;78:393–401.
Fontaine D, Lazorthes Y, Mertens P, et al. Safety and efficacy of deep brain stimulation in refractory cluster headache: a randomized placebo-controlled double-blind trial followed by a 1-year open extension. J Headache Pain. 2010;11:23–31.
Schoenen J, Clemente LD, vanderheede M, et al. Hypothalamic stimulation in chronic cluster headache: a pilot study of efficacy and mode of action. Brain. 2005;128:940–7.
Coughlin A, Badura A, Fleisher T, Guck T. Multidisciplinary treatment of chronic pain patients: its efficacy in changing patient locus of control. Arch Phys Med Rehabil. 2000;81:739–40.
Lefaucheur J, Andre-Obadia N, Antal A, Ayache S, Baeken C, Benninger D. Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation. Clin Neurophys. 2014;125:2150–206.
Narayana S, Papanicolaou A, McGregor A, Boop F, Wheless J. Clinical applications of transcranial magnetic stimulation in pediatric neurology. J Child Neurol. 2014;30:1111–24.
Solomon S, Guglielmo K. Treatment of headache by transcutaneous electrical stimulation. Headache. 1985;25:12–5.
Tayeb Z, Hafeez F, Shafiq Q. Successful treatment of nummular headache with TENS. Cephalalgia. 2008;28:897–8.
Cruccu G, Aziz T, Garcia-Larresa L, et al. EFNS guidelines on neurostimulation therapy for neuropathic pain. Eur J Neurol. 2007;14:952–70.
Sluka K, Judge M, McColley M, Reveiz P, Taylor B. Low frequency TENS is less effective than high frequency TENS at reducing inflammtion-induced hyperalgesia in morphone-tolerant rats. Eur J Pain. 2000;4:185–93.
Piquet M, Balestra C, Sava S, Schoenen J. Supraorbital transcutaneous neurostimulation has sedative effects in healthy subjects. BMC Neurol. 2011;11:135. This study showed the device to be safe for use in healthy volunteers with verum stimulation leading to mild sedative effects.
Craig A. Spinal and trigeminal lamina I input to the locus coeruleus anterogradely labeled with phaseolus vulgaris leucoagglutinin (PHA-L) in the cat and the monkey. Brain Res. 1992;584:325–8.
Schoenen J, Vandersmissen B, Jeangette S, et al. Migraine prevention with a supraorbital transcutaneous stimulator. Neurology. 2013;80:697–704. Double-blind, RTC, sham-controlled study for STS as a migraine preventive. Significantly reduced headache days at three months of use though did not reduce headache severity.
Magis D, Sava S, D'Elia T, Schoenen J. Safety and patients’ satisfaction of transcutaneous supraorbital neuroStimulation (tSNS) with the Cefaly® device in headache treatment: a survey of 2,313 headache sufferers in the general population. J Headache Pain. 2013;14:95.
Anand S, Hotson J. Transcranial magnetic stimulation: neurophysiological applications and safety. Brain and Cogn. 2002;50:366–86.
Rossini P, Rossi S. Transcranial magnetic stimulation: diagnostic, therapeutic and research potential. Neurology. 2007;68:484–8.
Wasserman E. Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol. 1997;108:1–16.
Dodick D, Schembri C, Helmuth M, Aurora S. Transcranial magnetic stimulation for migraine: a safety review. Headache. 2010;50:1153–63.
Lipton R, Dodick D, Silberstein S, et al. Single-pulse transcranial magnetic stimulation for acute treatment of migraine with aura: a randomised, double-blind, parallel-group, sham-controlled trial. Lancet Neurology. 2010;9:373–80. Double-blind, RTC, sham-controlled study for TMS as abortive migraine treatment. Treatment group more likely to be pain free at 372 h but no differences seen in abortive medication use.
Almaraz A, Dilli E, Dodick D. The effect of prophylactic medications on TMS for migraine aura. Headache Curr. 2010;50:1630–3.
Bhola R, Kinsella E, Giffin N, et al. Single-pulse transcranial magnetic stimulation (sTMS) for the acute treatment of migraine: evaluation of outcome data for the UK post market pilot program. J Headache Pain. 2015;16:51.
Sadler R, Purdy R, Rahey S. Vagal nerve stimulation aborts migraine in patient with intractable epilepsy. Cephalagia. 2002;22:482–4.
Hord E, Evans M, Mueed S, Adamolekun B, Naritoku D. The effect of vagus nerve stimulation on migraines. J Pain. 2003;4:530–4.
Mauskop A. Vagus nerve stimulation relieves chronic refractory migraine and cluster headaches. Cephalagia. 2005;25:82–6.
Cecchini A, Mea E, Tullo V, et al. Vagus nerve stimulation in drug-resistant daily chronic migraine with depression: preliminary data. Neurol Sci 2009;30.
Kirchner A, Birklein F, Stefan H, Handwerker H. Left vagus nerve stimulation suppresses experimentally induced pain. Neurology. 2000;55:1167–71.
Bohotin C, Scholsem M, Multon S, Martin D, Bohotin V, Schoenen J. Vagus nerve stimulation in awake rats reduces formalin-induced nociceptive behaviour and fos-immunoreactivity in trigeminal nucleus caudalis. Pain. 2003;101:3–12.
Oshinsky M, Murphy A, Hekierski H, Cooper M, Simon B. Non-invasive vagus nerve stimulation as treatment for trigeminal allodynia. Pain. 2014;155:1037–42.
Schachter S, Saper C. Progress in epilepsy research: vagus nerve stimulation. Epilepsia. 1998;39:677–86.
Ferrari GD, Crijns H, Borggrefe M, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:857–5.
Sjogren M, Hellstrom P, Jonsson M, Runngerstam M, Silander H, Ben-Menachem E. Cognition-enhancing effect of vagus nerve stimulation in patients with Alzheimer’s disease: a pilot study. J Clin Psych. 2002;63:972–80.
Silberstein S. Non-invasive vagus nerve stimulation in the treatment of migraine headache. Presented at the 4th European Headache and Migraine Trust International Congress; September 20, 2014; Copenhagen, Denmark.
Silberstein S, Silva AD, Calhoun A, Grosberg B, Lipton R, Cady R. Non-invasive vagus nerve stimulation for chronic migraine prevention in a prospective, randomized, sham-controlled pilot study (the EVENT Study): report from the double-blind phase. Presented at the 56th Annual Scientific Meeting of the American Headache Society; June 2014; Los Angeles, California. Double-blind, RTC, sham-controlled study for nVNS as migraine preventive. Reduced number of migraine days with more benefits seen after multiple months of use.
Silberstein S, Silva AD, Calhoun A, Grosberg B, Lipton R, Cady R. Non-invasive vagus nerve stimulation for chronic migraine prevention in a prospective, randomized, sham-controlled pilot Study (the EVENT Study): report from the open-label phase. Presented at the 56th Annual Scientific Meeting of the American Headache Society; June 2014; Los Angeles, California.
Gaul C, Diener H, Solbach K, et al. Non-invasive vagus nerve stimulation using gammaCore® for the prevention and acute treatment of chronic cluster headache: report from the randomised phase of the PREVA study. Presented at the 4th European Headache and Migraine Trust International Congress; September 18–21, 2014; Copenhagen, Denmark. RCT, open-label study for nVNS as abortive and preventive in chronic cluster. Cluster preventive and abortive. Reduced number of headache days after 2 weeks.
Gaul C, Diener H, Solbach K, et al. Non-invasive vagus nerve stimulation using gammaCore for the prevention and acute treatment of chronic cluster headache: report from the open-label phase of the PREVA study. Presented at the 4th European Headache and Migraine Trust International Congress; September 18–21, 2014; Copenhagen, Denmark.
Silberstein S, Mechtler L, Kudrow D, et al. Efficacy and safety outcomes of non-invasive vagus nerve stimulation for the acute treatment (ACT1) of cluster headache study. Presented at the 57th Annual Scientific Meeting of the American Headache Society; Jun 18–21, 2015; Washington DC.
Nesbitt A, Martin J, Tompkins E, Ruttledge M, Goadsby P. Initial use of a novel noninvasive vagus nerve stimulator for cluster headache treatment. Neurology. 2015;84:1249–53.
Grazzi L, Padovan A, Barbanti P. Role of neurostimulation in migraine. Neurol Sci. 2015;36 Suppl 1:S121–3.
Goadsby P, Grosberg B, Mauskop A, Cady R, Simmons K. Effect of noninvasive vagus nerve stimulation on acute migraine: an open label pilot study. Cephalagia. 2014;34:986–93.
Rainero I, Martino PD, Rubino E, Vaula G, Gentile S, Pinessi L. Non-invasive vagal nerve stimulation for the treatment of headache attacks in patients with chronic migraine and medication-overuse headache. Neurology 2014;82:P1.262.
Keskin E, Onur O, Keskin H, Gamus I, Kafali H, Turhan N. Transcutaneous electrical nerve stimulation improves low back pain during pregnancy. Gynecol Obstet Investig. 2012;74:76–83.
Husain M, Stegman D, Trevino K. Pregnancy and delivery while receiving vagus nerve stimulation for the treatment of major depression: a case report. Ann Gen Psych. 2005;4:1–7.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Shuhan Zhu declares no potential conflicts of interest.
Michael J. Marmura reports grants from eNeura and Teva.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Headache
Rights and permissions
About this article
Cite this article
Zhu, S., Marmura, M.J. Non-Invasive Neuromodulation for Headache Disorders. Curr Neurol Neurosci Rep 16, 11 (2016). https://doi.org/10.1007/s11910-015-0620-7
Published:
DOI: https://doi.org/10.1007/s11910-015-0620-7