Abstract
Myeloproliferative neoplasms (MPN) are acquired clonal disorders characterized by the proliferation of bone marrow myeloid cells. Different somatic mutations have been recently associated with MPN, the most common being JAK-2 V617F. Among MPN, polycythemia vera and essential thrombocythemia are particularly associated with an increased risk to develop thrombotic complications, either arterial or venous. Cerebrovascular events (stroke and transient ischemic attacks) are prevalent, accounting for approximately two-thirds of all events. Also cerebral vein thrombosis can complicate MPN and can be the first manifestation of the disease. Risk factors for thrombosis in patients with MPN are related or unrelated to the disease. Among the former there are cellular risk factors, such as increased white blood cell counts, vascular cell activation, endothelial dysfunction, and plasmatic risk factors, such as increased plasma viscosity, reduced levels of protein S, increased thrombin generation. The latter include increased age and previous thrombotic events. In addition, common cardiovascular risk factors (smoking, hypertension, diabetes, dyslipidemia, obesity) contribute to the pathogenesis of arterial events, whereas circumstantial risk factors (particularly oral contraceptive use and pregnancy/puerperium) to that of venous events. Primary prevention of arterial thrombosis with antiplatelet therapy is warranted in the majority of patients with MPN, whereas primary prevention of venous thrombosis is limited to anticoagulant prophylaxis during high-risk situations. Secondary prevention includes long-term antiplatelet therapy for arterial and short- or long-term anticoagulant therapy for venous thrombosis, depending on the risk factors present at the first event.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Chronic myeloproliferative neoplasms (MPN) are a group of diseases characterized by a chronic clonal proliferation of myeloid cells. The annual incidence of MPN is 6–10 cases every 100,000 individuals [1], with a peak age at diagnosis between the 5th and the 6th decade of life. MPN had been historically divided in 4 subtypes: chronic myeloid leukemia (CML), polycythemia vera (PV), essential thrombocythemia (ET) and primary myelofibrosis (PMF). The diagnostic criteria of MPN have been recently revised by a WHO panel of experts [2].
CML is characterized by the abnormal proliferation of the white cell lineage. It is defined by the presence of the t(9;22) chromosomal translocation (Philadelphia chromosome), giving rise to the BCR-ABL1 aberrant fusion protein, an always active hybrid oncogene, that gives a proliferative advantage to myeloid cells carrying the translocation. Untreated CML is a progressive disorder, with a prognosis of 5–6 years since diagnosis, characterized by a chronic phase followed by an accelerated phase progressing to acute myeloid leukemia. The development of a rational engineered class of drugs, the inhibitors of BCR-ABL1, revolutionized the therapy and the life expectancy of affected patients.
PV, ET, and PMF share different clinical and biological features, making possible to group them together as Philadelphia-negative MPN. Overlapping among different subtypes is frequent. As an example, approximately 35 % of patients with PV can evolve into PMF within 15 years from diagnosis [1]. In recent years several progresses have been made in the understanding of the molecular biology of Philadelphia-negative MPN, especially with the discovery that many patients affected by PV, ET, and PMF carry a common V617F somatic mutation in the Janus Kinase 2 (JAK-2) gene [3]. JAK-2 is a tyrosine kinase that plays a pivotal role in myelopoiesis, being in the transduction pathway activated either by erythropoietin, thrombopoietin or granulocyte colony stimulating factor. The mutant form is constitutively active. JAK-2 V617F mutation is present in almost all patients with PV and in approximately 50 % of patients with ET and PMF. Recently, to reinforce the hypothesis that Philadelphia-negative MPN are clonal overlapping disorders, a substitution involving the thrombopoietin receptor gene (MPL W515L/K), shared by individuals with ET and PMF, has been described in patients without JAK-2 V617F mutation [4]. Another activating JAK2 mutation, JAK2-R564Q [5] has been described in familial thrombocytosis. Furthermore, in patients with diagnostic criteria for ET without JAK-2 and MPL mutations, Klampf et al [6•] described the presence of insertions and deletions in the calreticulin (CALR) gene. No patient with PV had this gene mutated, and no patient with JAK-2 mutations had also CALR mutations.
Thrombotic complications represent a common feature in the natural history of Philadelphia-negative MPN, being the most common cause of death in affected patients and greatly contributing to the disease burden. Particularly for PV and ET, a thrombotic episode is often the first manifestation of an underlying MPN. In patients with PV a thrombotic event is the presenting symptom in one-fifth of cases. Since few data are available on the association between thrombosis and CML, we will exclude this MPN from our review, and deal with Philadelphia-negative MPN only.
This review will focus on vascular neurologic complications associated with MPN. More specifically, we aim to discuss the pathophysiology of MPN-associated thrombosis, to describe the epidemiology of arterial and venous cerebrovascular events in MPN patients, and to give some hints on prevention and treatment of thrombosis in this setting.
Pathophysiology of Thrombosis in Patients with MPN
Several risk factors for thrombosis are recognized in patients with MPN; either general or disease-specific (Table 1). MPN patients are in an age group where cerebrovascular events become prevalent, and underlying conventional risk factors for atherosclerosis are often present. Various prospective studies followed large cohorts of patients for several years in order to identify conditions associated with an increased risk of thrombosis. In the large observational European Collaboration on Low-dose Aspirin in Polycythemia vera study (ECLAP) [7], 1638 patients with PV were followed for 2.7 ± 1.3 years and thrombotic events were recorded. Median age at recruitment was 60 ± 13 years and time since PV diagnosis was 5 ± 5 years. Risk factors significantly associated with stroke/transient ischemic attack (TIA) were age above 65 years [hazard ratio, HR, 2.47 (95%CI 1.31–4.65)] and previous arterial thrombosis [HR 2.86 (95%CI 1.57–5.23)]. In particular, a previous stroke increased the likelihood to have another cerebrovascular event during follow-up of approximately 3-folds [HR 2.85, (95 % CI 1.57–5.23)]. Since the results of this study were confirmed by many others (reviewed in [8]), patients are defined at high risk for thrombosis if they are above 65 years and had had a previous vascular event.
Recently, a thrombotic risk score [International Prognostic Score of thrombosis in Essential Thrombocythemia (IPSET-thrombosis)] was developed and validated in a large group of patients with ET, who were divided into 3 risk groups of low (1.03 % patient-years), intermediate (2.35 % patient-years) and high (3.56 % patient-years) thrombosis risk on the basis of age above 60 years, thrombosis history, cardiovascular risk factors, and the presence of JAK-2 V617F mutation [9••]. In the ECLAP study, a leukocyte count above 15 × 109/L was positively correlated with the thrombotic risk, but did not reach the statistically significant threshold for neurologic events. The impact of leukocyte count on the risk of thrombosis was assessed also by Gangat et al [10], who retrospectively reviewed 407 patients with diagnosis of PV or ET followed since 1956, finding no association between leukocytosis and the risk of developing thrombosis both in patients with PV (P = 0.26) and ET (P = 0.54). Advanced age and high hematocrit, one of the diagnostic criteria of PV, were associated with an increased risk of arterial and venous vascular events, particularly in the cerebral circulation [9••]. Proposed mechanisms include the increased blood viscosity and the marginalization of circulating platelets toward the endothelium surface, where they can be more easily activated. Several neurologic symptoms, such as tinnitus, paresthesia, and headache, commonly reported by patients with PV, had been attributed to increased blood viscosity. Platelet count apparently does not correlate with the risk of thrombosis. Two large trials [ECLAP and Polycythemia Vera Study Group (PVSG)] [7, 11] failed to demonstrate any influence of the number of circulating platelets on thrombotic risk. It is also noteworthy that a particularly high platelet count in ET patients can be associated with bleeding diathesis, caused by intrinsic platelet dysfunction (acquired alpha or delta storage pool deficiency) or acquired Von Willebrand disease because of the consumption of high-molecular-weight Von Willebrand factor multimers. Campbell et al [12] followed a multicenter cohort of 776 patients with ET, finding a direct and significant correlation between platelet count above 450 × 109/L and the risk of bleeding [HR 3.7 (95 % CI 1.7–8.2)]. As a direct consequence of this, a very high platelet count has been associated with a low risk of thrombosis. Indeed, Carobbio et al [11] showed that in patients with ET the lowest risk of thrombosis (incidence rate 1.59 % patient-years) was recorded in the group with both platelet count above 1000 × 109/L and leukocytes lower than 11 × 109/L. Conversely, the highest risk category (thrombosis rate 2.95 % patient-years) was formed by patients with both platelet count below 1000 × 109/L and leukocytes higher than 11 × 109/L, carrying also the JAK-2 V617F mutation.
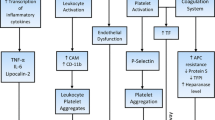
There is evidence that the thrombotic risk is not only correlated to the increased number of clonal cells, but also to several acquired qualitative alterations of these cells contributing to the prothrombotic phenotype usually seen in patients with MPN. Red blood cell membrane is altered and aggregates of red blood cells are frequently detected. One may speculate that these aggregates contribute to the neurologic symptoms causing occlusion of the small cerebral vessels. Fujita et al [13] demonstrated that JAK-2 V617F positive red blood cells express high levels of the procoagulant phosphatidylserine on their membrane, as detected by flow cytometry. Various platelet abnormalities have been described in patients with MPN. Platelets circulate in an active form (as demonstrated by increased P-selectin expression), are more apt to sustain hemostasis on their surface because of a high phosphatidylserine exposure on the outer membrane (as shown by increased annexin V binding), and generate more procoagulant microparticles [14]. Even the platelet turnover appears to be particularly accelerated in these patients. Arellano-Rodrigo et al [15] studied 53 patients with ET and found that the 26 with a history of thrombosis had a significantly higher number of reticulated platelets, which were younger and more hemostatically active than the older ones. The burden of the JAK-2 V617F positive clone appears to be directly correlated with the percentage of reticulated platelets. Panova-Noeva et al [16] investigated 46 patients with ET, 38 with PV, and 42 healthy subjects, finding that MPN JAK-2 V617F positive patients had a statistically higher percentage of reticulated platelets (2.8 % vs 2.2 %, P < 0.05) than JAK-2 negative patients. Interestingly, in this case series cytoreductive therapy with hydroxyurea appeared to decrease reticulated platelets in JAK-2 positive patients. Also leukocytes, besides contributing to blood viscosity, can increase the prothrombotic tendency through such several other mechanisms as activation of platelets, worsening inflammation in the atherosclerotic plaque, generation of tissue factor-bearing microparticles, and formation of neutrophils/platelets aggregates [17, 18].
The endothelium itself can be impaired in patients with MPN. Indeed, recent studies suggested that angiogenesis is impaired, as demonstrated by high circulating levels of vascular endothelial growth factor (VEGF) [19]. Moreover, pathologic myeloid cells express and secrete several mediators able to interfere with the homeostasis of the endothelial layer. Activated endothelial cells are strong contributors to the thrombotic process, through the release of the highly adhesive high-molecular-weight Von Willebrand factor, the expression of E-selectin and the alteration of the metabolism of free radical mediators [20].
Other than the cellular part of hemostasis, also blood clotting is impaired in patients with MPN and this could partially explain the findings that even the prevalence of venous thrombotic events is increased. Particularly, in patients with ET a reduction of circulating levels of the naturally occurring anticoagulant protein S has been described, mimicking the resistance to activated protein C. This pattern was first described by Conlan et al [21] in 2 patients with ET and subsequently confirmed by other authors [22–24]. Protein S appears to be cleaved and inactivated on the membrane of clonal platelets by a specific protease. A cytoreductive therapy is able to restore normal levels of protein S by reducing platelet count [25]. In ET patients described in the study of Arellano-Rodrigo et al [15], resistance to activated protein C was associated with an increased risk of thrombosis, being present in 8 of 26 with, and 1 of 27 without thrombosis (P = 0.001). Inherited thrombophilia might increase the thrombotic risk in patients with MPN. In a cohort of 191 patients with PV or ET the presence of the gain-of-function mutation factor V Leiden was associated with a 4-fold increased risk of thrombosis [HR = 4.3 (95 % CI 1.2–15.9)] [26]. In this study no association was found with the presence of the other common prothrombin G20210A gain-of-function mutation. All the aforementioned hemostatic changes contribute to the imbalance of hemostasis toward a procoagulant status, as demonstrated by an increase in thrombin generation, a global coagulation test that measures the amount of thrombin produced by the activated plasma. In 59 patients with ET and 30 with PV, Marchetti et al [24] showed that, despite thrombin generation was lower in patients than in healthy controls, adding activated protein C to the test caused a reduction in thrombin generation in both groups, but patients were less sensitive than controls to the anticoagulant effect of activated protein C. Duchemin et al [27] directly correlated resistance to activated protein C to circulating microparticles. Forty-four patients with PV or ET were investigated, showing that removing microparticles by ultrafiltration resulted in a normalization of the resistance to activated protein C. These data were confirmed by Tripodi et al [28], who performed thrombin generation on platelet-rich and platelet-poor plasma and thromboelastometry on whole blood in 111 patients with PV, ET, or PMF and 89 healthy controls. They found evidence of a procoagulant imbalance in MPN patients: the endogenous thrombin potential ratio was higher, and time to clot formation shorter in MPN patients than in controls.
Different studies showed that the JAK-2 V617F mutation is an independent risk factor for thrombosis. Recently published systematic reviews and meta-analyses on patients with PV, ET, or PMF showed that carriers of this mutation have a 2–3-fold increased risk of thrombosis compared with noncarriers [29–31]. Whether the underlying mechanism is due to the effect of the mutation per se on cellular proliferation or to the effect of other factors present in JAK2 V617F carriers remains to be clarified.
Epidemiology of Cerebral Arterial Thrombosis in Patients with MPN
Arterial thrombosis accounts for approximately two-thirds of all thrombotic events in MPN patients and is the main cause of death. A few studies estimating the incidence of cerebrovascular events in patients with MPN are available so far. In the multicenter ECLAP study [7], 143 episodes of arterial thrombosis were recorded during a mean follow-up of 2.7 years in the 1638 patients with PV, for an incidence rate of 32 events per 1000 patient-years. Among these arterial events 35 TIA and 36 strokes were observed, all objectively confirmed by neuroimaging or autopsy (overall incidence rate 16 per 1000 patient-years). The remaining 72 events were myocardial infarctions and peripheral arterial thromboses. A similar figure was observed in patients with ET. Two studies of 306 [32] and 532 [33] patients followed for a median period of 96 months and 7.6 years, reported an incidence of cerebrovascular events of 8.3 per 1000 patient-years [13 TIA (4 %) and 7 strokes (2 %)] and of 7.7 per 1000 patient-years [31 TIA or strokes (6 %)], respectively. The latter study confirmed that age above 60 years, leukocytosis and anemia were major risk factors for stroke.
Epidemiology of Cerebral Vein Thrombosis in Patients with MPN
Cerebral vein thrombosis (CVT) is a rare and life-threatening condition with an annual incidence of 3–4 cases per 1,000,000 in the general adult population. At diagnosis, such neurologic symptoms as headache, papilledema, and diplopia are common, whereas focal signs and symptoms are less frequent if compared with what is observed in arterial stroke. In adults, common risk factors include thrombophilia abnormalities, oral contraceptive intake, pregnancy, and puerperium. The gender imbalance among risk factors explains the observed female:male ratio of 2:1 [34]. The association between JAK-2 mutation and CVT has been confirmed in several studies. Passamonti et al [35] tested the JAK-2 V617F mutation in 152 consecutive patients with CVT referred to a single thrombosis center for thrombophilia screening within 1 year from the episode and without a diagnosis of MPN. Ten patients (6.6 %) carried the JAK-2 V617F mutation, 5 of whom had other thrombophilia abnormalities, and 3 other had such prothrombotic circumstantial conditions at the time of CVT as oral contraceptive use or inflammatory bowel diseases. Of the 10 JAK-2 V617F positive patients, 6 had a concomitant still undiagnosed MPN at the time of CVT and 3 developed MPN in the subsequent 3 years of follow-up. Also De Stefano et al [36] reported a prevalence of JAK-2 V617F mutation of 4.8 % (95 % CI 1.3–16.1) in 45 patients with CVT and no overt MPN. Recently, the clinical characteristics of a group of 48 patients with MPN and CVT were compared with those of 87 MPN patients with other venous thromboses and 178 MPN patients without thrombosis [37]. A significantly higher prevalence of thrombophilia abnormalities was found in patients with MPN and CVT compared with patients with MPN but no thrombosis (40 % vs 21 %, P = 0.015), as well as a higher prevalence of JAK-2 V617F mutation (78 % vs 55 %, P = 0.059). Moreover, patients with MPN and CVT showed a higher tendency to develop recurrent thrombosis, compared with patients with MPN and other venous thromboses.
Prevention and Treatment of Cerebral Thrombosis
MPN represent an acquired thrombophilic state, so it is very important to educate and encourage patients to control all the modifiable common cardiovascular risk factors, as smoking, hypertension, diabetes, dyslipidemia, or obesity. In PV patients, in order to avoid thrombotic complications, it is mandatory to maintain the hematocrit level below 45 %, with phlebotomy as first line therapy (Fig. 1) [38, 39••]. Primary prevention of thromboembolic events with 100 mg of aspirin once a day is indicated in all patients, unless in the presence of major contraindications such as a high risk of bleeding [40]. In high risk patients (age >65 years and/or previous cerebrovascular events), cytoreductive therapy (hydroxyurea or interferon alpha as first line) must be started. Also in patients with ET, prophylaxis with 100 mg of aspirin once a day is generally recommended, whereas cytoreduction with hydroxyurea is indicated in high risk patients (same criteria as PV). The effect of antiplatelet therapy was assessed in a study by Alvarez-Larran et al [41], that included 300 low-risk patients with ET treated with antiplatelet agents (n = 198) or just followed without treatment. Antiplatelet therapy did not influence the occurrence of arterial thrombotic events in the whole ET population (incidence rate: 9.4 per 1000 patient-years in nontreated vs 16.2 per 1000 patient-years in treated subjects; P = 0.60), but in patients with associated cardiovascular risk factors (smoking, hypertension, serum cholesterol level above 200 mg/dL, and diabetes). In these patients the incidence of arterial thrombosis was higher than in those without cardiovascular risk factors (incidence rate ratio 2.50, 95 % CI 1.02–6.10), but if they were treated with antiplatelet therapy the incidence rate ratio was reduced (1.20, 95 % CI 0.40–3.20). Special attention should be paid for ET patients with a very high platelet count (>1500 × 109/L), as antiplatelet therapy may enhance their bleeding tendency, particularly if a platelet dysfunction or acquired Von Willebrand disease are present. Cytoreductive therapy in patients with ET is required only in the high-risk group (>60 years, previous thrombosis, cardiovascular risk factors, JAK2 V617F mutation) based on the newly validated IPSET-thrombosis score (9). Following such risk stratification, only in high-risk patients the reduction of thrombotic complications counterbalance the potential damages of a cytoreductive therapy [reviewed in 42].
In order to prevent venous thromboembolic complications in high-risk situations, such as general or orthopedic surgery, prolonged immobilization, pregnancy, and puerperium, prophylaxis with appropriate doses of unfractionated or low-molecular-weight heparin is advised. A single retrospective study investigated the risk of thrombosis associated with estrogen-based hormone therapies in women with ET, showing a higher prevalence of venous thromboembolism in oral contraceptive users than in nonusers (23 % vs 7 %, P = 0.03) but a similar prevalence in hormone replacement therapy users than in nonusers (2 % vs 7 %, P = 0.86) [43]. No association was found between hormone therapies and the risk of stroke.
Thrombotic events are treated with standard therapy, ie, antiplatelet agents for arterial thrombosis and anticoagulant therapy with low-molecular-weight heparin followed by a vitamin K-antagonist for venous thrombosis. After an arterial event, long-term antiplatelet therapy and control of cardiovascular risk factors (if present) are advised in patients with MPN. In patients with a first episode of CVT the optimal duration of anticoagulant therapy is still not established. A short term (3–12 months) anticoagulant therapy is generally accepted in patients with a transient risk factor at the time of the event that is no longer present, but since MPN remains active, life-long oral anticoagulant therapy should be considered, especially in patients with no removable risk factors at the time of thrombosis [44]. Multicenter, international studies on the optimal duration of anticoagulant therapy in patients without MPN are ongoing (www.excoa-cvt.com). A careful evaluation of the individual risk of bleeding and a periodic follow-up of patients is advisable, always keeping in mind that MPN are progressive disorders and the general status of affected patients may change over time.
Conclusions
MPN cause significant alterations of both cellular and plasmatic compartments of hemostasis, which, in the final result, is an acquired prothrombotic state. Indeed, thrombosis is the leading cause of morbidity and mortality in patients affected by MPN. Following MPN diagnosis, patients require careful monitoring, education and surveillance in order to avoid thrombotic complications, and many of them are candidate to receive primary antiplatelet prophylaxis. Although abdominal vein thrombosis often complicates MPN, the absolute incidence of thrombosis remains low, and cerebrovascular accidents are the most prevalent thrombotic events. Whereas MPN should be looked for in patients with abdominal vein thrombosis, whether or not the same should be done in patients with cerebral venous thrombosis is still uncertain, and may be considered if thrombosis was idiopathic.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Muxí PJ, Oliver AC. Jak-2 positive myeloproliferative neoplasms. Curr Treat Options Oncol. 2014. doi:10.1007/s11864-014-0279-3.
Tefferi A, Vardiman JW. Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia. 2008;2:14–22.
Kiladjian JJ. The spectrum of JAK2-positive myeloproliferative neoplasms. Hematol Am Soc Hematol Educ Program. 2012;2012:561–6.
Ruan GR, Jiang B, Li LD, Niu JH, Li JL, Xie M, et al. MPL W515L/K mutations in 343 Chinese adults with JAK2V617F mutation-negative chronic myeloproliferative disorders detected by a newly developed RQ-PCR based on TaqMan MGB probes. Hematol Oncol. 2010;28:33–9.
Etheridge SL, Cosgrove ME, Sangkhae V, Corbo LM, Roh ME, Seeliger MA, et al. A novel activating, germline JAK2 mutation, JAK2R564Q, causes familial essential thrombocytosis. Blood. 2014;123:1059–68.
Klampfl T, Gisslinger H, Harutyunyan AS, Nivarthi H, Rumi E, Milosevic JD, et al. Somatic mutations of calreticulin in myeloproliferative neoplasms. N Engl J Med. 2013;369:2379–90. Identification of a novel gene mutation involved in the pathogenesis of MPN other than JAK-2 V167F mutation.
Landolfi R, Di Gennaro L, Barbui T, De Stefano V, Finazzi G, Marfisi R, et al. European Collaboration on Low-Dose Aspirin in Polycythemia Vera (ECLAP). Leukocytosis as a major thrombotic risk factor in patients with polycythemia vera. Blood. 2007;109:2446–52.
Finazzi G, Barbui T. Evidence and expertise in the management of polycythemia vera and essential thrombocythemia. Leukemia. 2008;22:1494–502.
Barbui T, Finazzi G, Carobbio A, Thiele J, Passamonti F, Rumi E, et al. Development and validation of an International Prognostic Score of thrombosis in World Health Organization—essential thrombocythemia (IPSET-thrombosis). Blood. 2012;120:5128–33. The Authors develop a prognostic score able to predict thrombotic risk in ET patients. Patients are considered at low-, intermediate- or high-risk according to the presence of cardiovascular risk factors and JAK-2 mutation status.
Gangat N, Wolanskyj AP, Schwager SM, Hanson CA, Tefferi A. Leukocytosis at diagnosis and the risk of subsequent thrombosis in patients with low-risk essential thrombocythemia and polycythemia vera. Cancer. 2009;115:5740–5.
Carobbio A, Finazzi G, Antonioli E, Guglielmelli P, Vannucchi AM, Delaini F, et al. Thrombocytosis and leukocytosis interaction in vascular complications of essential thrombocythemia. Blood. 2008;112:3135–7.
Campbell PJ, MacLean C, Beer PA, Buck G, Wheatley K, Kiladjian JJ, et al. Correlation of blood counts with vascular complications in essential thrombocythemia: analysis of the prospective PT1 cohort. Blood. 2012;120:1409–11.
Fujita H, Sakuma R, Tomiyama J, Hamaki T, Ohwada A, Nishimura S. Relationship between clotting activity and phosphatidylserine expression on erythrocyte membranes in polycythemia vera patients with the JAK2 V617F mutation. Arch Physiol Biochem. 2011;117:231–5.
Falanga A, Marchetti M. Thrombotic disease in the myeloproliferative neoplasms. Hematol Am Soc Hematol Educ Program. 2012;2012:571–81.
Arellano-Rodrigo E, Alvarez-Larrán A, Reverter JC, Colomer D, Villamor N, Bellosillo B, et al. Platelet turnover, coagulation factors, and soluble markers of platelet and endothelial activation in essential thrombocythemia: relationship with thrombosis occurrence and JAK2 V617F allele burden. Am J Hematol. 2009;84:102–8.
Panova-Noeva M, Marchetti M, Buoro S, Russo L, Leuzzi A, Finazzi G, et al. JAK2V617F mutation and hydroxyurea treatment as determinants of immature platelet parameters in essential thrombocythemia and polycythemia vera patients. Blood. 2011;118:2599–601.
Carobbio A, Finazzi G, Guerini V, Spinelli O, Delaini F, Marchioli R, et al. Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood. 2007;109:2310–3.
Falanga A, Marchetti M, Vignoli A, Balducci D, Russo L, Guerini V, et al. V617F JAK-2 mutation in patients with essential thrombocythemia: relation to platelet, granulocyte, and plasma hemostatic and inflammatory molecules. Exp Hematol. 2007;35:702–11.
Medinger M, Skoda R, Gratwohl A, Theocharides A, Buser A, Heim D, et al. Angiogenesis and vascular endothelial growth factor-/receptor expression in myeloproliferative neoplasms: correlation with clinical parameters and JAK2-V617F mutational status. Br J Haematol. 2009;146:150–7.
Karakantza M, Giannakoulas NC, Zikos P, et al. Markers of endothelial and in vivo platelet activation in patients with essential thrombocythemia and polycythemia vera. Int J Hematol. 2004;79:253–9.
Conlan MG, Haire WD. Low protein S in essential thrombocythemia with thrombosis. Am J Hematol. 1989;32:88–93.
Wieczorek I, MacGregor IR, Ludlam CA. Low proteins C and S and activation of fibrinolysis in treated essential thrombocythemia. Am J Hematol. 1995;49:277–81.
Bucalossi A, Marotta G, Bigazzi C, Galieni P, Dispensa E. Reduction of antithrombin III, protein C, and protein S levels and activated protein C resistance in polycythemia vera and essential thrombocythemia patients with thrombosis. Am J Hematol. 1996;52:14–20.
Marchetti M, Castoldi E, Spronk HM, van Oerle R, Balducci D, Barbui T, et al. Thrombin generation and activated protein C resistance in patients with essential thrombocythemia and polycythemia vera. Blood. 2008;112:4061–8.
Dienava-Verdoold I, Marchetti MR, te Boome LC, Russo L, Falanga A, Koene HR, et al. Platelet-mediated proteolytic down regulation of the anticoagulant activity of protein S in individuals with haematological malignancies. Thromb Haemost. 2012;107:468–76.
Trifa AP, Cucuianu A, Popp RA, Coadă CA, Costache RM, Militaru MS, et al. The relationship between factor V Leiden, prothrombin G20210A, and MTHFR mutations and the first major thrombotic episode in polycythemia vera and essential thrombocythemia. Ann Hematol. 2014;93:203–9.
Duchemin J, Ugo V, Ianotto JC, Lecucq L, Mercier B, Abgrall JF. Increased circulating procoagulant activity and thrombin generation in patients with myeloproliferative neoplasms. Thromb Res. 2010;126:238–42.
Tripodi A, Chantarangkul V, Gianniello F, Clerici M, Lemma L, Padovan L, et al. Global coagulation in myeloproliferative neoplasms. Ann Hematol. 2013;92:1633–9.
Ziakas PD. Effect of JAK2 V617F on thrombotic risk in patients with essential thrombocythemia: measuring the uncertain. Haematologica. 2008;93:1412–4.
Dahabreh IJ, Zoi K, Giannouli S, Zoi C, Loukopoulos D, Voulgarelis M. Is JAK2 V617F mutation more than a diagnostic index? A meta-analysis of clinical outcomes in essential thrombocythemia. Leuk Res. 2009;33:67–73.
Lussana F, Caberlon S, Pagani C, Kamphuisen PW, Büller HR, Cattaneo M. Association of V617F Jak2 mutation with the risk of thrombosis among patients with essential thrombocythaemia or idiopathic myelofibrosis: a systematic review. Thromb Res. 2009;124:409–17.
Radaelli F, Colombi M, Calori R, Zilioli VR, Bramanti S, Iurlo A, et al. Analysis of risk factors predicting thrombotic and/or haemorrhagic complications in 306 patients with essential thrombocythemia. Hematol Oncol. 2007;25:115–20.
Palandri F, Polverelli N, Catani L, Ottaviani E, Baccarani M, Vianelli N. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol. 2011;90:933–8.
Martinelli I, De Stefano V. Extra-abdominal venous thromboses at unusual sites. Best Pract Res Clin Haematol. 2012;25:265–74.
Passamonti SM, Biguzzi E, Cazzola M, Franchi F, Gianniello F, Bucciarelli P, et al. The JAK2 V617F mutation in patients with cerebral venous thrombosis. J Thromb Haemost. 2012;10:998–1003.
De Stefano V, Fiorini A, Rossi E, Za T, Farina G, Chiusolo P, et al. Incidence of the JAK2 V617F mutation among patients with splanchnic or cerebral venous thrombosis and without overt chronic myeloproliferative disorders. J Thromb Haemost. 2007;5:708–14.
Martinelli I, De Stefano V, Carobbio A, Randi ML, Santarossa C, Rambaldi A, et al. Cerebral vein thrombosis in patients with Philadelphia-negative myeloproliferative neoplasms. A European Leukemia net Study. Am J Hematol. 2014. doi:10.1002/ajh.23809.
Barbui T. How to manage thrombosis in myeloproliferative neoplasms. Curr Opin Oncol. 2011;23:654–8.
Marchioli R, Finazzi G, Specchia G, Cacciola R, Cavazzina R, Cilloni D, et al. Cardiovascular events and intensity of treatment in polycythemia vera; CYTO-PV Collaborative Group. N Engl J Med. 2013;368:22–33. Randomized prospective trial demonstrating the importance to maintain hematocrit below 45% to significantly reduce the rate of cardiovascular events and deaths in PV patients.
Landolfi R, Marchioli R, Kutti J, Gisslinger H, Tognoni G, Patrono C, et al. European Collaboration on Low-Dose Aspirin in Polycythemia Vera investigators. Efficacy and safety of low-dose aspirin in polycythemia vera. N Engl J Med. 2004;350:114–24.
Alvarez-Larrán A, Cervantes F, Pereira A, Arellano-Rodrigo E, Pérez-Andreu V, Hernández-Boluda JC, et al. Observation versus antiplatelet therapy as primary prophylaxis for thrombosis in low-risk essential thrombocythemia. Blood. 2010;116:1205–10.
Barbui T. Myeloproliferative neoplasms and thrombosis. Blood. 2013;122:2176–84.
Gangat N, Wolanskyj AP, Schwager SM, Mesa RA, Tefferi A. Estrogen-based hormone therapy and thrombosis risk in women with essential thrombocythemia. Cancer. 2006;106:2406–11.
Martinelli I, Passamonti SM, Rossi E, De Stefano V. Cerebral sinus-venous thrombosis. Intern Emerg Med. 2012; Suppl 3:221–5.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Andrea Artoni, Paolo Bucciarelli, and Ida Martinelli declare that they have no conflicts of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Neurology of Systemic Disease
Rights and permissions
About this article
Cite this article
Artoni, A., Bucciarelli, P. & Martinelli, I. Cerebral Thrombosis and Myeloproliferative Neoplasms. Curr Neurol Neurosci Rep 14, 496 (2014). https://doi.org/10.1007/s11910-014-0496-y
Published:
DOI: https://doi.org/10.1007/s11910-014-0496-y