Abstract
Established risk factors for thrombosis in essential thrombocythemia (ET) include age (≥60 years) and previous vascular events. Recently, also leukocytosis has been proposed in risk stratification of ET patients. We report a retrospective study on 532 ET patients followed for a median of 7.6 years. Sixty-four patients (12%) developed 95 thrombotic events during follow-up. Together with the high-risk condition, a white blood cell (WBC) value above 11 × 109/L, corresponding to the fourth percentile value, significantly correlated with a higher thrombotic risk (p = 0.033) by Cox proportional hazards. Moreover, the cumulative risk of thrombosis was significantly higher in high-risk patients with WBC >11 × 109/L. JAK2 V617F mutation did not correlate with thrombosis. Overall, 123 (23%) patients died. Three independent parameters were noted as prognostic factors for survival in multivariate analysis: age >60 years, leukocytosis >11 × 109/L, and hemoglobin level below normal values. Based on these parameters, three groups of risk were defined, with significantly different survivals. Baseline leukocytosis correlated with a higher thrombotic risk in high-risk patients and identified a cohort of patients with worse survival.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Essential thrombocythemia (ET) is a Philadelphia-negative chronic myeloproliferative neoplasm (CMPN) characterized by the abnormal proliferation of a malignant megakaryocytic clone, responsible for persistent thrombocytosis [1, 2]. The clinical course is burdened by an increased incidence of vascular complications, whose pathogenesis is multifactorial. In the last years, many retrospective studies have failed to show a clear correlation between platelet count and thrombotic events [3–5], and the role of thrombocytosis in the assessment of vascular risk has been progressively minimized. Conversely, age (≥60 years) and previous vascular events remain the main established risk factors for thrombosis, and on this basis, patients are currently stratified in low and high risk.
Recently, also the presence of JAK2V617F mutation [6–8] and baseline leukocyte (white blood cells, WBC) count have been considered as disease-related cardiovascular risk factors of ET patients. This latter has been found to play a biological role in determining thrombosis, mainly acute coronary syndromes, in CMPN and particularly in ET, although it is presently not completely defined whether elevated WBC levels actually contribute directly to causing thrombosis [9].
Consequently, several clinical studies have investigated the association between leukocytosis at diagnosis and risk of thrombosis, with not univocal results. Indeed, some authors, including our group, have reported that leukocyte count at presentation did not predict subsequent vascular events [3, 4, 10, 11], although none of these analyses investigated possible differences among low- and high-risk patients. On the contrary, some other studies concluded that leukocytosis was associated with thrombosis, particularly in low-risk patients [12–14], but no consensus was found on the numerical cutoff which should be utilized for the definition of leukocytosis. Furthermore, the implications of baseline WBC count on long-term survival remain to be determined. The aim of this article is to contribute to these issues by reporting a long-term retrospective study on 532 ET patients followed for a median of 7.6 years, with particular focus on the evaluation of the additional risk factors for thrombosis and survival.
Methods
Definitions
The diagnosis of ET was made according to the criteria proposed by the Polycythemia Vera Study Group [15] and/or, since 2002, the WHO criteria [16]. Additional risk factors for thrombosis (blood pressure >140/90 mmHg, overweight, smoking habit, mellitus diabetes, and hypercholesterolemia, defined as fasting serum total cholesterol concentration >200 mg/dL) and renal and liver function were evaluated at diagnosis and at 6 months interval thereafter.
The definition of acute leukemia and myelodysplastic syndrome was made according to the French–American–British criteria [17]; diagnosis of myelofibrosis was based on Italian Consensus Conference criteria [18]. Thrombotic episodes were objectively documented by venography or ultrasonography (deep venous thrombosis); arteriography (peripheral arterial thrombosis); and computed tomography scanning, magnetic resonance, or angiography (intracerebral vessels thrombosis). Acute myocardial infarction required acute clinical presentation, typical electrocardiograph features, and elevated creatine kinase MB fraction. Cerebral transient ischemic attack (TIA) required neurological symptoms and/or signs lasting less than 24 h.
Assessment of JAK2V617F mutational status
Total RNA was extracted from peripheral blood mononuclear cells using RNAeasy MiniKit (QIAGEN). RNA was amplified by reverse transcription-polymerase chain reaction (PCR) using 5 μM of random examers and 200 U of M-MLV Reverse Transcriptase (Invitrogen, Paisley, UK) according to the manufacturer’s recommendations. The cDNA was amplified using forward and reverse primers (5′-gatgagcaagctttctcacaagc-3′ and 5′-aaacagttggcatgggccatgc-3′); the amplified product (185 bp) was digested with Bsa XI (5 U; New England Biolabs, Germany) overnight at 37°C [19]. After digestion, 20 μL of amplified PCR products was electrophoresed on a 2% agarose gel and stained with ethidium bromide.
Statistical methods
Risk factors for thrombosis were tested using a Cox proportional hazards model. All multivariable models have been fitted after adjusting for sex, standard risk factors (age >60 years and/or previous thrombotic event), and quartile distributions of laboratory parameters measured at diagnosis (hemoglobin, hematocrit, leukocytes, and platelet count). Overall survival was calculated by the product limit method of Kaplan and Meier from the date of ET diagnosis to the date of death or last contact, whichever came first, with 95% confidence interval (95% CI). All p values were two-sided, and the significance level for all statistical tests was 0.05.
Results
Patients
From January 1978 to December 2008, 532 consecutive patients received a diagnosis of ET at the Institute of Hematology and Oncology “L. and A. Seràgnoli”, Bologna, Italy. General characteristic of the patients are summarized in Table 1. Median age at diagnosis was 64 years (range, 16–95); 307 were 60 years or older (58%). In 350 (66%) patients, one or more additional risk factors for thrombosis were present at diagnosis.
According to standard risk factors (age ≥60 years and/or history of vascular events), patients were classified as low risk (n = 186; 35%) and high risk (n = 346; 65%). However, during the years, indications for cytotoxic and antiplatelet therapy were also represented by extreme thrombocytosis (platelet count higher than 1,000 × 109/L), presence of additional cardiovascular risk factors, and/or symptoms due to microvessel disturbances. Consequently, 86% of patients received cytotoxic therapy (mainly, hydroxyurea) and 92% received an antiplatelet agent (mainly, aspirin). More specifically, during up to 32.5 years of follow-up (median, 7.6 years), 130 out of 186 low-risk patients (70%) and 330 out of 346 high-risk patients (95%) received at least one cytoreductive drug.
Overall, 64 (12%) patients (15 out of 186 low-risk patients, 8%, and 49 out of 346 high-risk patients, 14%) developed 95 thrombotic complications. Arterial events (including stroke, TIA, acute coronary syndromes, and peripheral arterial occlusions) constituted the majority (61%) of events.
Risk factors for thrombosis
Multivariable analysis of risk factors for thrombosis confirmed the value of the high-risk condition. By dividing patients in quartile distribution of leukocytes and platelet number, hemoglobin concentration, and hematocrit, also a WBC value above 11 × 109/L, corresponding to the fourth percentile value, significantly correlated with a higher thrombotic risk, together with the high-risk condition (p = 0.033 and p = 0.003, respectively; Table 2). When arterial thrombosis was analyzed separately, only high-risk condition correlated significantly with a higher risk of such events (HR = 2.2, 95% CI 1.07–4.85, p = 0.032). The presence of the JAK2V617F mutation was not found to be an independent risk factor for thrombotic events (11 thrombotic episodes in 121 patients without the mutation vs 17 thrombotic episodes in 202 mutated patients, p = 0.84).
Cumulative risk of thrombosis by baseline leukocytosis
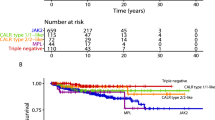
Previous reports have shown that the cumulative risk of thrombosis was significantly higher in low-risk patients with leukocyte number over a median value (8.7 × 109/L) [12] and also identified as best leukocyte cutoff for predicting thrombotic events the value of 9.4 × 109/L [13]. We performed an equivalent analysis by using both the median cutoff value of our cohort of patients (8.9 × 109/L; Fig. 1a) and the cutoff value of 9.4 × 109/L (Fig. 1b). While the latter cutoff value did not allow to recognize different incidences of thrombosis between risk categories, when the median cutoff value was utilized, the presence of leukocytosis >8.9 × 109/L identified a category of high-risk patients with a thrombotic risk significantly higher than all the other patients (52% vs 17% at 20 years, p < 0.0001), while high-risk patients with low WBC count and low-risk patients had the same cumulative thrombotic risk.
Cumulative probability of thrombosis in the follow-up according to standard risk factors and categories of WBC count at diagnosis. WBC cutoff values are as follows: 8.9 × 109/L (median WBC count; a), 9.4 × 109/L (b), 11 × 109/L (4th percentile; c). High risk: age older than 60 years and/or history of vascular events
We also tested the WBC value of 11 × 109/L (corresponding to the fourth percentile value). As shown in Fig. 1c, the cumulative risk of thrombosis was significantly higher in high-risk patients with WBC >11 × 109/L (“very” high-risk category) as compared to the group with lower leukocytes count (p = 0.002; “standard” high-risk category). The cumulative risk of thrombosis in low-risk patients with high WBC was comparable to high-risk patients with lower leukocyte count (18% vs 21.5% at 20 years from diagnosis, p = 0.24); also, low-risk patients with leukocytosis had the same thrombotic risk than low-risk patients without leukocytosis (18% vs 13% at 20 year, p = 0.92).
Overall survival
One hundred twenty-three (23%) patients died at a median age of 81 years (range 51–100); the median survival was 24 years. Causes of death were thrombotic/hemorrhagic events (eight patients, 6.5%), cardiac disease (13 patients, 10.5%), disease transformation (21 patients, 17%), second neoplasia (12 patients, 10%), senility (26 patients, 14.5%), or other causes unrelated to the hematological disease (not including cardiovascular events and development of second neoplasia) (43 patients, 27%). Thrombocytosis (>1,500 × 109/L) and leukocyte count above the median value (8.9 × 109/L) resulted associated with higher mortality but only in univariate analysis (p = 0.01 and p = 0.04, respectively). Conversely, neither a history of thrombosis nor the presence of the JAK2V617F mutation was significantly associated with poorer survival (p = 0.45 and p = 0.57, respectively).
Three independent parameters were noted as prognostic factors for survival also in multivariate analysis: age ≥60 year, leukocytosis >11 × 109/L, and hemoglobin level below normal values (<12.5 g/dL in females and <13.5 g/dL in males). Taking into account these parameters, a model for survival was constructed in order to define three groups of risk: high (two or three risk factors, 130 patients), medium (one risk factor, 265 patients), and low (no risk factors, 137 patients). Median survivals were 13 years, 28 years, and yet undefined in the three groups, respectively (p < 0.0001; Fig. 2).
Overall survival curves for ET patients stratified according to the risk including the three risk factors: age >60 year, leukocytosis >11 × 109/L, and hemoglobin level below normal values. The risk was classified as low (no risk factors, line a, 137 patients), intermediate (one risk factor, line b, 265 patients), high (two to three risk factors; line c, 130 patients, with 23 patients having all the three risk factors). Median survivals were yet undefined, 28 years, and 13 years in the three groups, respectively (p < 0.0001)
Discussion
Many recent papers have investigated the possible association between leukocytosis and thrombosis in patients with myeloproliferative disorders and particularly in ET, with controversial results [3, 4, 11–14]. In the current study, we used the database from a cohort of 532 ET patients, homogeneously followed at a single institution for a median time of 7.6 years, with the purpose to investigate the prognostic relevance of baseline leukocytosis for subsequent thrombotic events and survival. This analysis confirms the importance of the classical high-risk profile (age >60 years and/or previous thrombosis), which resulted strongly associated with the thrombotic risk. Conversely, extreme thrombocytosis was not found to correlate with thrombotic complications and rather showed a paradoxical trend toward a protection from thrombosis (HR 0.67). These findings are consistent with previous retrospective studies [12], suggesting an association between thrombocytosis and bleeding diathesis, partly explained by the occurrence of AVWS in some patients.
In addition to older age and previous thrombosis, also leukocytosis (cutoff, 11 × 109/L) had a prognostic impact on thrombosis. After patients’ stratification in high and low risk according to the standard definition, baseline leukocytosis identified a cohort of “very” high-risk group with leukocytosis (Fig. 1c). Indeed, “standard” high-risk patients without leukocytosis presented a thrombotic risk fully comparable to low-risk patients carrying leukocytosis. This result is in keeping with a recent analysis by Carobbio et al. [13], who reported that leukocytosis (median value) per se could discriminate, within the low-risk category, two subgroups of patients at different risk of future vascular complications. On the other hand, in our analysis, the thrombotic risk was not significantly different among the two subgroups of low-risk patients (p = 0.92) and the WBC cutoff was higher (4th percentile). The role of baseline leukocytosis in the low-risk category might have been masked by the fact that as many as 70% of these low-risk patients received cytotoxic therapy (plus aspirin), configuring them as high-risk from the point of view of prevention of thrombosis. The administration of cytotoxic therapy might have prevented an increase in leukocyte count over time, which has been reported to correlate with a higher thrombotic risk [20]. Moreover, the impact of leukocytosis might have been confounded by several other variables, including the co-existence or the appearance over the time of other morbidities, influencing both the thrombotic risk and long-term survival.
Our analysis also confirmed a direct correlation between survival and baseline leukocytosis and hemoglobin level below normal values, as previously reported [3, 11, 12, 21]. Patients were accordingly stratified in three categories, projected to a significantly different survival (Fig. 2). To build this model, we verified that the proportion of treated patients was the same in the three groups (p > 0.05) and that there were no myelodysplatic or fibrotic features at bone marrow examination in the 115 patients with low hemoglobin level at diagnosis. In addition, baseline leukocytosis (quartile distribution) did not correlate with disease evolution (p = 0.81), which occurred in 28 patients (acute leukemia in six cases and myelofibrosis in 22 cases). However, the possibility that some of these patients with “ET” characterized by older age, anemia, and leukocytosis may actually have an early stage primary myelofibrosis (PMF-0) [22] cannot be entirely ruled out.
In conclusion, with the limitations in the interpretation of the data due to the fact that this is a retrospective study, baseline leukocytosis seemed to be associated with higher thrombotic risk, especially in high-risk category, and worse survival. Prospective studies are needed to confirm or to rule out the accuracy of these stratifying models and to identify a WBC value which could be a widely reliable cutoff to define a significant leukocytosis.
References
Harrison CN (2005) Essential thrombocythaemia: challenges and evidence-based management. Br J Haematol 130:153–165
Barbui T, Barosi G, Grossi A et al (2004) Practice guidelines for the therapy of essential thrombocythemia. A statement from the Italian Society of Hematology, the Italian Society of Experimental Hematology and the Italian Group for Bone Marrow Transplantation. Haematologica 89(2):215–232
Palandri F, Catani L, Testoni N et al (2009) Long-term follow-up of 386 consecutive patients with essential thrombocythemia: safety of cytoreductive therapy. Am J Hematol 84(4):215–220
Passamonti F, Rumi E, Arcaini L et al (2008) Prognostic factors for thrombosis, myelofibrosis, and leukemia in essential thrombocythemia: a study of 605 patients. Haematologica 93(11):1645–1651
Tefferi A, Elliott M (2007) Thrombosis in myeloproliferative disorders: prevalence, prognostic factors, and the role of leukocytes and JAK2V617F. Semin Thromb Hemost 33(4):313–320
Vannucchi AM, Antonioli E, Guglielmelli P et al (2007) Clinical profile of homozygous JAK2 617V>F mutation in patients with polycythemia vera or essential thrombocythemia. Blood 110(3):840–846
De Stefano V, Za T, Rossi E et al (2010) Leukocytosis is a risk factor for recurrent arterial thrombosis in young patients with polycythemia vera and essential thrombocythemia. Am J Hematol 85(2):97–100
Lussana F, Caberlon S, Pagani C et al (2009) Association of V617F Jak2 mutation with the risk of thrombosis among patients with essential thrombocythaemia or idiopathic myelofibrosis: a systematic review. Thromb Res 124(4):409–417
Marchetti M, Falanga A (2008) Leukocytosis, JAK2V617F mutation, and hemostasis in myeloproliferative disorders. Pathophysiol Haemost Thromb 36(3–4):148–159
Tefferi A, Gangat N, Wolanskyj A (2007) The interaction between leukocytosis and other risk factors for thrombosis in essential thrombocythemia. Blood 109(9):4105
Girodon F, Dutrillaux F, Broséus J et al (2010) Leukocytosis is associated with poor survival but not with increased risk of thrombosis in essential thrombocythemia: a population-based study of 311 patients. Leukemia 24(4):900–903
Carobbio A, Finazzi G, Guerini V et al (2007) Leukocytosis is a risk factor for thrombosis in essential thrombocythemia: interaction with treatment, standard risk factors, and Jak2 mutation status. Blood 109(6):2310–2313
Carobbio A, Antonioli E, Guglielmelli P et al (2008) Leukocytosis and risk stratification assessment in essential thrombocythemia. J Clin Oncol 26(16):2732–2736
Wolanskyj AP, Schwager SM, McClure RF et al (2006) Essential thrombocythemia beyond the first decade: life expectancy, long-term complication rates, and prognostic factors. Mayo Clin Proc 81(2):159–166
Murphy S, Peterson P, Iland H et al (1997) Experience of the Polycythemia Vera Study Group with essential thrombocythemia: a final report on diagnostic criteria, survival, and leukemic transition by treatment. Semin Hematol 34(1):29–39
Vardiman JW, Harris NL, Brunning RD (2002) The World Health Organization (WHO) classification of the myeloid neoplasms. Blood 100(7):2292–2302
Bennett JM, Catovsky D, Daniel MT et al (1985) Proposed revised criteria for the classification of acute myeloid leukemia. A report of the French–American–British Cooperative Group. Ann Intern Med 103(4):620–625
Barosi G, Ambrosetti A, Finelli C et al (1999) The Italian consensus conference on diagnostic criteria for myelofibrosis with myeloid metaplasia. Br J Haematol 104(4):730–737
Quentmeier H, MacLeod RA, Zaborski M et al (2006) JAK2 V617F tyrosine kinase mutation in cell lines derived from myeloproliferative disorders. Leukemia 20(3):471–476
Passamonti F, Rumi E, Pascutto C, Cazzola M, Lazzarino M (2009) Increase in leukocyte count over time predicts thrombosis in patients with low-risk essential thrombocythemia. J Thromb Haemost 7(9):1587–1589
Gangat N, Wolanskyj AP, McClure RF et al (2007) Risk stratification for survival and leukemic transformation in essential thrombocythemia: a single institutional study of 605 patients. Leukemia 21:270–276
Tefferi A, Vardiman JW (2008) Classification and diagnosis of myeloproliferative neoplasms: the 2008 World Health Organization criteria and point-of-care diagnostic algorithms. Leukemia 22:14–22
Acknowledgment
The research was supported by the University of Bologna (funds for selected topics), by the EuropeanLeukemiaNet and by BolognAIL. We thank Dr. Mauro Fiacchini for his helpful assistance.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Palandri, F., Polverelli, N., Catani, L. et al. Impact of leukocytosis on thrombotic risk and survival in 532 patients with essential thrombocythemia: a retrospective study. Ann Hematol 90, 933–938 (2011). https://doi.org/10.1007/s00277-010-1154-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00277-010-1154-3