Abstract
Logopenic progressive aphasia is the most recently described clinical variant of primary progressive aphasia (PPA), defined by impairment of lexical retrieval and sentence repetition. Unlike other PPA variants, the logopenic variant of PPA (lv-PPA) is commonly associated with Alzheimer’s disease (AD), a fact that is relevant to the selection of patients for clinical trials and disease-modifying therapies. Despite the straightforward definition and coherent pathological association, the existence of lv-PPA has been challenged, as its distinction from AD or other PPA variants can be difficult. Despite these issues, lv-PPA patients display characteristic linguistic deficits, a pattern of brain atrophy, and possibly genetic susceptibility, which warrant considering this variant as a discrete AD endophenotype. More specific clinical and anatomical markers can strengthen the consistency of this syndrome.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Logopenic progressive aphasia is the most recently described variant of primary progressive aphasia (PPA), and is characterized by deficits in lexical retrieval and impaired repetition of sentences or phrases [1, 2]. The growing interest in this condition is reflected in the increasing number of publications on the topic and in its incorporation into the international consensus criteria for the classification and diagnosis of PPA [3••]. The relevance of the syndrome is twofold. First, although up to a third of patients with PPA have underlying Alzheimer’s disease (AD) [4, 5], no specific aphasic profile had been linked to this disease before the inception of the logopenic variant of PPA (lv-PPA). Second, lv-PPA provides a natural paradigm to investigate the clinical–anatomical heterogeneity of AD given that patients with lv-PPA display a distinctive clinical profile, distribution of brain atrophy, and possibly genetic susceptibility, which differ from those patients with typical AD presentation.The existence of lv-PPA as an independent entity remains, however, somewhat controversial [6•]. First, the clinical definition of lv-PPA rests largely on the absence of deficits that define the other variants of PPA [7, 8]. Second, since language deficits are common in AD and, conversely, nonverbal cognitive involvement in lv-PPA occurs early on [9, 10•], the clinical boundary between lv-PPA and other AD presentations can be somewhat blurry. Each of these issues will be discussed in this review.
Clinical Features
Language and speech deficits in lv-PPA have been described and formalized by the international consensus criteria for diagnosis and classification of PPA [3••]. According to these criteria, impaired confrontation naming or word-finding difficulties and impaired sentence repetition are the core language deficits that typify lv-PPA. In addition to these core deficits, all patients must have any of three of the following features: phonological errors in spontaneous speech and naming, preservation of semantic knowledge, spared motor speech, and absence of frank agrammatism. Although these criteria capture the vast majority of lv-PPA cases, a proportion of patients present with ambiguous identification owing to the presence of overlapping deficits with other PPA variants [8, 11]. For instance, a patient with word-finding difficulties, impaired sentence repetition, and some level of impaired productive syntax, but otherwise preserved semantic knowledge and motor aspects of speech, can be identified as having either lv-PPA or the nonfluent/agrammatic variant of PPA (nfv-PPA). As such, several clinical series have enriched the clinical and linguistic characterization, providing a more thorough account of this syndrome, which can be useful to discriminate such borderline cases [8, 12•, 13, 14].
During spontaneous conversation, patients with lv-PPA display marked word-finding pauses with hesitations that interrupt the flow of conversation and give their speech a nonfluent quality [12•]. Their speech is interrupted with filling sounds such as “mmm,” “uhh,” and “ohh,” with words as if searching for the “right word,” or alternatively, the patient simply repeats segments of the utterance such as “my mother went, my mother went, to my home.” Some patients have false starts or hesitant speech, but in contrast to nonfluent patients with apraxia of speech, articulation is preserved without distortion or loss of prosody. Phonological paraphasias can be present during spontaneous speech or during repetition of naming tasks. Unlike phonetic errors that reflect a disruption of temporal and spatial parameters of speech, phonological paraphasias are linguistic-based errors that reflect a disorder of the selection and ordering of the target word. Thus, these errors are characterized by substitution, addition, or deletion of well-articulated phonemic segments. Despite this theoretical demarcation, in clinical practice, phonological errors are easily confused with phonetic errors that typify nfv-PPA patients with apraxia of speech [7]. The thorough evaluation of speech and language deficits, therefore, can assist in the distinction between the aforementioned variants, as shown in Table 1.
Although the current criteria explicitly state absence of “frank” agrammatism, it is well recognized that lv-PPA patients can display oversimplification of grammatical structures and syntactical errors, findings attributed to a reduced verbal short-term memory capacity and word-finding problems causing incorrect lexical usage rather than lost capacity to establish syntactical relationships [12•, 15].
Deficits in confrontation naming may be as severe as in the semantic variant, but errors are usually “don’t knows” or phonological rather than the semantic coordinate and superordinate errors seen in semantic variant cases. In addition, it is clear that the items are known by the patient (“it’s the Australian one… it hops around”), and phonological cueing is said to improve performance on this task [11, 13, 16]. Another key differentiating feature between these two variants is the sparing of single-word comprehension in logopenic patients [8, 11].
Repetition of short words is usually normal, although patients with lv-PPA can have problems with multisyllabic words (e.g., “hippopotamus”) and produce phonological errors in these cases. To complicate things further, some nfv-PPA patients have borderline performance on this task, which may limit the usefulness of a single-word repetition task in the differentiation of PPA syndromes [11, 16]. Sentence and phrase repetition is characteristically very impaired and is considered one of the clinical hallmarks of lv-PPA [2, 17]. In this task, patients often omit words or replace words with similar ones, a type of error that is thought to be caused by reduced verbal short-term memory capacity [2]. As such, instead of repeating, “The Chinese fan contained the rare emerald,” the patient may say, “The Chinese fan contained…” or “ The Chinese fan had an emerald.” The same mechanism can cause impairment in sentence comprehension, which is more influenced by the length and familiarity than by the grammatical complexity of the given sentence.
Phonological dyslexia, characterized by difficulties in reading nonwords, has been documented in patients with lv-PPA and has been interpreted as a reflection of the phonological disintegration [18]. In addition, we have observed several lv-PPA patients with dyslexia characterized by the relative facility of reading short over long words and by the use of letter-by-letter reading instead of reading the whole word as a single entity. This finding has been described in patients with posterior cortical atrophy and could account for a nosological continuum across atypical AD presentations, as logopenic deficits can also be present in patients with posterior cortical atrophy [19, 20].
Besides the evident language involvement, several clinical reports have pointed out nonverbal deficits affecting attention and visuospatial skills [13, 14, 21]. In fact, two independent longitudinal series have demonstrated that such deficits overshadow language impairments, and virtually most lv-PPA patients become demented within 1 or 2 years of follow-up [9, 10•]. The widespread cognitive involvement observed later in lv-PPA mirrors the cognitive profile of patients with advanced AD and supports the notion that the natural history of dementias is largely determined by their underlying disease. Despite this clinical continuum, lv-PPA patients exhibit clinical and anatomical peculiarities, suggesting that specific functional networks are predominantly involved in each AD phenotype [22•]. The study of the neural correlates of the core deficits in lv-PPA and the contrast of clinical and anatomical findings between this variant and other AD presentations can clarify this issue.
Neural Correlates of Logopenic Progressive Aphasia
Multiple imaging and neuropathology studies have consistently demonstrated that brain atrophy in lv-PPA is focused on the left temporoparietal junction [1, 2, 13, 17, 23–25]. Despite this well-established pattern of atrophy, the cortical regions that are critical for the emergence of the core deficits of lv-PPA are not entirely defined. In fact, the determination of these neural correlates can be hindered by the rapid cognitive deterioration of lv-PPA patients, which adds to the complexity [26] of the extensive swathe of cortical atrophy that spreads to incorporate the left temporal lobe and the medial parietal lobe [13, 17, 24].
In our recent study, we correlated proxy measures for the main cognitive deficits of lv-PPA with the whole left hemisphere cortical mantle [17]. As a proxy of lexical retrieval, confrontation-naming performance was chosen since lv-PPA patients, by definition, have spared semantic knowledge and absence of motor speech disorders. In turn, the performance on forward digit span was selected as a proxy of impaired sentence repetition since this deficit is thought to be secondary to a reduced verbal short-term memory capacity [2]. Surprisingly, each proxy correlated with separate, nonoverlapping, cortical regions localized within the distribution of cortical atrophy observed in lv-PPA: low confrontation-naming performance was associated with thinning in the inferior-posterior parietal lobe, whereas low digit span was correlated with the posterior third of the superior temporal gyrus.
These correlates raise interesting contentions. First, atrophy in the left inferior parietal lobe has also been associated with impaired naming in AD [27–30], suggesting that the impairment of naming in lv-PPA and AD hinges on defective lexical retrieval [31–34]. This clinical–anatomical convergence reinforces the concept of nosological continuity, in which components of the same neuronal network are affected in AD, irrespective of the initial clinical phenotype [22•].
The second proxy, however, seems to be characteristic of lv-PPA. Although reduced verbal short-term memory is present in patients with typical AD, the cognitive mechanisms and, thereby, anatomical basis in AD differs from that in lv-PPA. The preponderant mechanism involved in AD is thought to be an executive control deficit caused by pathological involvement of frontal regions [35, 36], whereas in lv-PPA, reduced verbal short-term memory is secondary to a breakdown of the phonological loop [2]. This component of the verbal short-term memory appears to depend on the integrity of the posterior left superior temporal gyrus, as damage to this region is associated with reduced performance in digit span and deficits in sentence-level tasks that place a high demand on short-term memory resources [37–41]. Other evidence that gives a coherent account of verbal short-term memory deficits in lv-PPA is provided by experimental neuropsychology. On the basis of data obtained from normal volunteers, Page et al. [42] demonstrated that phonological errors and reduced verbal short-term memory are underpinned by a common cognitive system. This finding suggests that the emergence of phonological errors and the breakdown of the phonological loop are closely related and, therefore, share the same anatomical substrates in lv-PPA. Accordingly, the presence of phonological errors in a mixed PPA sample was associated with atrophy in the left superior temporal gyrus [12•], a finding that is in line with cortical stimulation mapping studies that have linked this region to the presence of phonological errors [43]. In addition, impaired sentence repetition in a mixed sample of PPA has also been associated with the left superior temporal gyrus [41], emphasizing the relevance of this region in the genesis of both deficits.
This converging evidence points to the relevance of phonological integration as a key cognitive process affected in lv-PPA. In fact, patients with typical AD rarely, if ever, display phonological errors, a finding that has been evidenced by older clinical series conducted before the inception of lv-PPA [44, 45] and by a recent series that systematically sought phonological errors in a pathologically confirmed sample of AD [46]. In addition, it has been reported that lv-PPA can evolve into phonological jargon aphasia, a condition characterized by profound phonological disintegration that renders words unrecognizable (neologism) [47]. Although the precise cognitive mechanism that yields this aphasia is not entirely established, it is clear that the left temporal–parietal junction is involved and that AD is the strongly associated disease [48, 49•]. It is not yet known whether this aphasia represents an advanced stage of progression in lv-PPA or if it is a different clinical variant of lv-PPA.
Given the uniqueness of phonological errors in lv-PPA, tasks tapping phonological integrity can contribute to distinguishing this variant from other AD phenotypes or PPA variants. Accordingly, a study that contrasted performances on several verbal repetition tasks across PPA variants demonstrated that lv-PPA patients performed worse on tasks that demand integrity of the phonological loop, whereas nfv-PPA patients performed worse on tasks that tapped the subvocal rehearsal component of the verbal short-term memory (Leyton CE, Savage S, Irish M, Schubert S, Piguet O, Ballard KJ, Hodges JR. Verbal repetition in primary progressive aphasia, under revision). In accordance with this putative clinical marker, atrophy of the posterior left superior temporal gyrus can be considered as the anatomical signature of lv-PPA. Several reports, in fact, have coincidentally demonstrated that this is the main region where imaging changes of lv-PPA exceed those of other AD presentations [22•, 50].
Typical AD and lv-PPA: Two Sides of the Same Coin?
Although there are anecdotal reports of lv-PAA cases caused by non-AD such as Creutzfeldt–Jakob disease [51, 52], compelling evidence, which includes pathology studies [49•, 53, 54], β-amyloid imaging studies [11, 55], and other biomarkers [56, 57], confirms that almost all lv-PPA patients exhibit AD pathological changes. Nevertheless, lv-PPA patients exhibit some distinctive features that suggest that other, not yet identified, factors modify the clinical phenotype of AD. Consequently, besides obvious cognitive and anatomical differences, lv-PPA and AD seem to have a diverging biological behavior. A longitudinal study that contrasted cognitive and functional decline in PPA variants and AD demonstrated that the former progressed more rapidly than AD [26]. This rapid progression is concordant with clinicopathology studies that have analyzed quantitatively the pathological load of both AD phenotypes, demonstrating that lv-PPA patients not only display greater overall deposition of neurofibrillary tangles, but also that the left perisylvian language cortices have a higher proportion of neurofibrillary tangles than typical AD patients display [58•]. In addition, studies conducted before the inception of lv-PPA have posited that AD patients presenting with prominent language deficits at the onset [59] as well as those with more atrophy in the parietal–temporal region [60]—the key region involved in lv-PPA—show a more rapid decline than the amnestic AD presentation. In accordance with the contrasting clinical phenotypes, different genetic risk factors are likely to be involved in lv-PPA, since apolipoprotein ε4 polymorphism, which is linked to AD, is not associated with the logopenic presentation [61, 62].
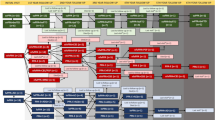
Despite these suggestive assertions, there are some reservations to consider. The core defining deficits of lv-PPA are rather unspecific; hence, the current diagnostic criteria may be lenient on forcing the inclusion of a heterogeneous group of patients labeled as having lv-PPA. Accordingly, Machulda et al. [63] suggest the existence of a least one distinctive endophenotype, characterized by more confined neuronal involvement and a slower progression. As suggested in Fig. 1, the clinical spectrum of lv-PPA may comprise AD patients displaying prominent language deficits that potentially differ from those actually called lv-PPA, characterized by prominent phonological deficits. The level of discreteness across this clinical spectrum is a matter for future research.
Spectrum of language impairment in Alzheimer’s disease (AD). Phonological disintegration and impaired lexical retrieval are the key cognitive processes involved in the logopenic variant of primary progressive aphasia (lv-PPA). A subset of lv-PPA patients can hypothetically develop jargon aphasia as expression of widespread phonological breakdown. This schema suggests that phonological errors and impaired sentence repetition result from impaired phonological processing, which is secondary to the damage to the posterior left superior temporal gyrus. Like lv-PPA, impaired lexical retrieval is present in patients with amnesic presentation of AD and is associated with the involvement of the left inferior parietal lobe. Amnesic AD patients, however, display no phonological errors, and the dissolution of semantic representations can affect naming and cause single-word comprehension deficits. The predominant involvement of one cognitive process over other cognitive processes can account for the syndromic heterogeneity in AD
Conclusions
Although lv-PPA is in essence an aphasic manifestation of AD, this PPA variant seems to have certain peculiarities that suggest a distinctive AD endophenotype. Anomia, one of the core deficits, is caused by impaired lexical retrieval which is associated with atrophy of inferior-posterior parietal lobe, a region involved consistently in AD, irrespective of its clinical presentation [64]. Impaired sentence repetition, the other core deficit, is caused by a reduced verbal short-term memory capacity. Phonological disintegration can explain both the reduced verbal short-term memory capacity and the phonological errors in lv-PPA. As such, we suggest that the thinning of the posterior left superior temporal gyrus can be the anatomical signature of this variant. A better clinical delineation of lv-PPA is necessary to understand the genetic and other unknown factors involved in this particular phenotype.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Gorno-Tempini ML, Dronkers NF, Rankin KP, Ogar JM, Phengrasamy L, Rosen HJ, et al. Cognition and anatomy in three variants of primary progressive aphasia. Ann Neurol. 2004;55:335–46.
Gorno-Tempini ML, Brambati SM, Ginex V, Ogar J, Dronkers NF, Marcone A, et al. The logopenic/phonological variant of primary progressive aphasia. Neurology. 2008;71:1227–34.
•• Gorno-Tempini ML, Hillis AE, Weintraub S, Kertesz A, Mendez M, Cappa SF, et al. Classification of primary progressive aphasia and its variants. Neurology. 2011;76:1006–14. This international consensus provides a common framework and operational definitions for each clinical variant of primary aphasia.
Knibb JA, Xuereb JH, Patterson K, Hodges JR. Clinical and pathological characterization of progressive aphasia. Ann Neurol. 2006;59:156–65.
Forman MS, Farmer J, Johnson JK, Clark CM, Arnold SE, Coslett HB, et al. Frontotemporal dementia: clinicopathological correlations. Ann Neurol. 2006;59:952–62.
• Sajjadi SA, Patterson K, Arnold RJ, Watson PC, Nestor PJ. Primary progressive aphasia: a tale of two syndromes and the rest. Neurology. 2012;78:1670–7. This report offers an alternative view to the current tripartite PPA classification and challenges the existence of lv-PPA as a discrete PPA variant.
Croot K, Ballard K, Leyton CE, Hodges JR. Apraxia of speech and phonological errors in the diagnosis of nonfluent/agrammatic and logopenic variants of primary progressive aphasia. J Speech Lang Hear Res. 2012;55:S1562–72.
Mesulam MM, Wieneke C, Thompson C, Rogalski E, Weintraub S. Quantitative classification of primary progressive aphasia at early and mild impairment stages. Brain. 2012;135:1537–53.
Etcheverry L, Seidel B, Grande M, Schulte S, Pieperhoff P, Sudmeyer M, et al. The time course of neurolinguistic and neuropsychological symptoms in three cases of logopenic primary progressive aphasia. Neuropsychologia. 2012;50:1708–18.
• Leyton CE, Hsieh S, Mioshi E, Hodges JR. Cognitive decline in logopenic aphasia: more than losing words. Neurology. 2013;80:897–903. This longitudinal study demonstrates the rapid cognitive deterioration of lv-PPA and suggests that most lv-PPA patients become demented after the first year of follow-up.
Leyton CE, Villemagne VL, Savage S, Pike KE, Ballard KJ, Piguet O, et al. Subtypes of progressive aphasia: application of the international consensus criteria and validation using beta-amyloid imaging. Brain. 2011;134:3030–43.
• Wilson SM, Henry ML, Besbris M, Ogar JM, Dronkers NF, Jarrold W, et al. Connected speech production in three variants of primary progressive aphasia. Brain. 2010;133:2069–88. This was one of the first studies that offer a comprehensive description of linguistic findings and their neural correlates across PPA variants.
Rohrer JD, Ridgway GR, Crutch SJ, Hailstone J, Goll JC, Clarkson MJ, et al. Progressive logopenic/phonological aphasia: erosion of the language network. Neuroimage. 2009;49:984–93.
Magnin E, Chopard G, Ferreira S, Sylvestre G, Dariel E, Ryff I, et al. Initial neuropsychological profile of a series of 20 patients with logopenic variant of primary progressive aphasia. J Alzheimers Dis. 2013;36:799–808.
Ash S, Evans E, O'Shea J, Powers J, Boller A, Weinberg D, et al. Differentiating primary progressive aphasias in a brief sample of connected speech. Neurology. 2013;81:329–36.
Savage S, Hsieh S, Leslie F, Foxe D, Piguet O, Hodges JR. Distinguishing subtypes in primary progressive aphasia: application of the Sydney language battery. Dement Geriatr Cogn Disord. 2013;35:208–18.
Leyton CE, Piguet O, Savage S, Burrell J, Hodges JR. The neural basis of logopenic progressive aphasia. J Alzheimers Dis. 2012;32:1051–9.
Brambati SM, Ogar J, Neuhaus J, Miller BL, Gorno-Tempini ML. Reading disorders in primary progressive aphasia: a behavioral and neuroimaging study. Neuropsychologia. 2009;47:1893–900.
Crutch SJ, Lehmann M, Warren JD, Rohrer JD. The language profile of posterior cortical atrophy. J Neurol Neurosurg Psychiatry. 2013;84:460–6.
Magnin E, Sylvestre G, Lenoir F, Dariel E, Bonnet L, Chopard G, et al. Logopenic syndrome in posterior cortical atrophy. J Neurol. 2013;260:528–33.
Foxe DG, Irish M, Hodges JR, Piguet O. Verbal and visuospatial span in logopenic progressive aphasia and Alzheimer's disease. J Int Neuropsychol Soc. 2013;19:247–53.
• Lehmann M, Ghosh PM, Madison C, Laforce Jr R, Corbetta-Rastelli C, Weiner MW, et al. Diverging patterns of amyloid deposition and hypometabolism in clinical variants of probable Alzheimer's disease. Brain. 2013;136:844–58. This study, by means of a multimodal imaging analysis, demonstrates that atypical presentations of AD are associated with degeneration of specific functional networks.
Sapolsky D, Bakkour A, Negreira A, Nalipinski P, Weintraub S, Mesulam MM, et al. Cortical neuroanatomic correlates of symptom severity in primary progressive aphasia. Neurology. 2010;75:358–66.
Mesulam M, Wieneke C, Rogalski E, Cobia D, Thompson C, Weintraub S. Quantitative template for subtyping primary progressive aphasia. Arch Neurol. 2009;66:1545–51.
Josephs KA, Dickson DW, Murray ME, Senjem ML, Parisi JE, Petersen RC, et al. Quantitative neurofibrillary tangle density and brain volumetric MRI analyses in Alzheimer's disease presenting as logopenic progressive aphasia. Brain Lang. 2013. doi:10.1016/j.bandl.2013.02.003.
Hsieh S, Hodges JR, Leyton CE, Mioshi E. Longitudinal changes in primary progressive aphasias: differences in cognitive and dementia staging measures. Dement Geriatr Cogn Disord. 2012;34:135–41.
Harasty JA, Halliday GM, Kril JJ, Code C. Specific temporoparietal gyral atrophy reflects the pattern of language dissolution in Alzheimer's disease. Brain. 1999;122(4):675–86.
Ahn HJ, Seo SW, Chin J, Suh MK, Lee BH, Kim ST, et al. The cortical neuroanatomy of neuropsychological deficits in mild cognitive impairment and Alzheimer's disease: a surface-based morphometric analysis. Neuropsychologia. 2011;49:3931–45.
Teipel SJ, Willoch F, Ishii K, Burger K, Drzezga A, Engel R, et al. Resting state glucose utilization and the CERAD cognitive battery in patients with Alzheimer's disease. Neurobiol Aging. 2006;27:681–90.
Apostolova LG, Lu P, Rogers S, Dutton RA, Hayashi KM, Toga AW, et al. 3D mapping of language networks in clinical and pre-clinical Alzheimer's disease. Brain Lang. 2008;104:33–41.
Croot K, Hodges JR, Xuereb J, Patterson K. Phonological and articulatory impairment in Alzheimer's disease: a case series. Brain Lang. 2000;75:277–309.
Becker JT, Huff FJ, Nebes RD, Holland A, Boller F. Neuropsychological function in Alzheimer's disease. Pattern of impairment and rates of progression. Arch Neurol. 1988;45:263–8.
Hodges JR, Salmon DP, Butters N. Semantic memory impairment in Alzheimer's disease: failure of access or degraded knowledge? Neuropsychologia. 1992;30:301–14.
Hodges JR, Patterson K. Is semantic memory consistently impaired early in the course of Alzheimer's disease? Neuroanatomical and diagnostic implications. Neuropsychologia. 1995;33:441–59.
Peters F, Collette F, Degueldre C, Sterpenich V, Majerus S, Salmon E. The neural correlates of verbal short-term memory in Alzheimer's disease: an fMRI study. Brain. 2009;132:1833–46.
Huntley JD, Howard RJ. Working memory in early Alzheimer's disease: a neuropsychological review. Int J Geriatr Psychiatry. 2009;25:121–32.
Leff AP, Schofield TM, Crinion JT, Seghier ML, Grogan A, Green DW, et al. The left superior temporal gyrus is a shared substrate for auditory short-term memory and speech comprehension: evidence from 210 patients with stroke. Brain. 2009;132:3401–10.
Richardson FM, Ramsden S, Ellis C, Burnett S, Megnin O, Catmur C, et al. Auditory short-term memory capacity correlates with gray matter density in the left posterior STS in cognitively normal and dyslexic adults. J Cogn Neurosci. 2011;23:3746–56.
Acheson DJ, Hamidi M, Binder JR, Postle BR. A common neural substrate for language production and verbal working memory. J Cogn Neurosci. 2011;23:1358–67.
Baldo JV, Katseff S, Dronkers NF. Brain regions underlying repetition and auditory-verbal short-term memory deficits in aphasia: evidence from voxel-based lesion symptom mapping. Aphasiology. 2012;26:338–54.
Amici S, Ogar J, Brambati SM, Miller BL, Neuhaus J, Dronkers NL, et al. Performance in specific language tasks correlates with regional volume changes in progressive aphasia. Cogn Behav Neurol. 2007;20:203–11.
Page MPA, Madge A, Cumming N, Norris DG. Speech errors and the phonological similarity effect in short-term memory: evidence suggesting a common locus. J Mem Lang. 2007;56:49–64.
Corina DP, Loudermilk BC, Detwiler L, Martin RF, Brinkley JF, Ojemann G. Analysis of naming errors during cortical stimulation mapping: implications for models of language representation. Brain Lang. 2010;115:101–12.
Appell J, Kertesz A, Fisman M. A study of language functioning in Alzheimer patients. Brain Lang. 1982;17:73–91.
Cummings JL, Benson F, Hill MA, Read S. Aphasia in dementia of the Alzheimer type. Neurology. 1985;35:394–7.
Ahmed S, de Jager CA, Haigh AM, Garrard P. Logopenic aphasia in Alzheimer's disease: clinical variant or clinical feature? J Neurol Neurosurg Psychiatry. 2012;83:1056–62.
Caffarra P, Gardini S, Cappa S, Dieci F, Concari L, Ghetti C, et al. Degenerative jargon aphasia: unusual progression of logopenic/phonological progressive aphasia? Behav Neurol. 2012;26:89–93.
Rohrer JD, Rossor MN, Warren JD. Neologistic jargon aphasia and agraphia in primary progressive aphasia. J Neurol Sci. 2009;277:155–9.
• Deramecourt V, Lebert F, Debachy B, Mackowiak-Cordoliani MA, Bombois S, Kerdraon O, et al. Prediction of pathology in primary progressive language and speech disorders. Neurology. 2010;74:42–9. This clincopathology series defines clinical subtypes of PPA associated with specific disorders.
Madhavan A, Whitwell JL, Weigand SD, Duffy JR, Strand EA, Machulda MM, et al. FDG PET and MRI in logopenic primary progressive aphasia versus dementia of the Alzheimer's type. PLoS One. 2013;8:e62471.
Johnson DY, Dunkelberger DL, Henry M, Haman A, Greicius MD, Wong K, et al. Sporadic Jakob-Creutzfeldt disease presenting as primary progressive aphasia. JAMA Neurol. 2013;70:254–7.
Martory MD, Roth S, Lovblad KO, Neumann M, Lobrinus JA, Assal F. Creutzfeldt-Jakob disease revealed by a logopenic variant of primary progressive aphasia. Eur Neurol. 2012;67:360–2.
Mesulam M, Wicklund A, Johnson N, Rogalski E, Leger GC, Rademaker A, et al. Alzheimer and frontotemporal pathology in subsets of primary progressive aphasia. Ann Neurol. 2008;63:709–19.
Rohrer JD, Rossor MN, Warren JD. Alzheimer's pathology in primary progressive aphasia. Neurobiol Aging. 2012;33:744–52.
Rabinovici GD, Jagust WJ, Furst AJ, Ogar JM, Racine CA, Mormino EC, et al. Aβ amyloid and glucose metabolism in three variants of primary progressive aphasia. Ann Neurol. 2008;64:388–401.
Gil-Navarro S, Llado A, Rami L, Castellvi M, Bosch B, Bargallo N, et al. Neuroimaging and biochemical markers in the three variants of primary progressive aphasia. Dement Geriatr Cogn Disord. 2013;35:106–17.
Kas A, Uspenskaya O, Lamari F, de Souza LC, Habert MO, Dubois B, et al. Distinct brain perfusion pattern associated with CSF biomarkers profile in primary progressive aphasia. J Neurol Neurosurg Psychiatry. 2012;83:695–8.
• Gefen T, Gasho K, Rademaker A, Lalehzari M, Weintraub S, Rogalski E, et al. Clinically concordant variations of Alzheimer pathology in aphasic versus amnestic dementia. Brain. 2012;135:1554–65. This quantitative clinicopathology study demonstrates that lv-PPA patients have a cortical distribution of neurofibrillary changes that differs from that of typical AD cases.
Atchison TB, Bradshaw M, Massman PJ. Investigation of profile difference between Alzheimer's disease patients declining at different rates: examination of baseline neuropsychological data. Arch Clin Neuropsychol. 2004;19:1007–15.
Nagahama Y, Nabatame H, Okina T, Yamauchi H, Narita M, Fujimoto N, et al. Cerebral correlates of the progression rate of the cognitive decline in probable Alzheimer's disease. Eur Neurol. 2003;50:1–9.
Rogalski EJ, Rademaker A, Harrison TM, Helenowski I, Johnson N, Bigio E, et al. ApoE E4 is a susceptibility factor in amnestic but not aphasic dementias. Alzheimer Dis Assoc Disord. 2011;25:159–63.
Wolk DA, Dickerson BC. Apolipoprotein E (APOE) genotype has dissociable effects on memory and attentional-executive network function in Alzheimer's disease. Proc Natl Acad Sci U S A. 2010;107:10256–61.
Machulda MM, Whitwell JL, Duffy JR, Strand EA, Dean PM, Senjem ML, et al. Identification of an atypical variant of logopenic progressive aphasia. Brain Lang. 2013. doi:10.1016/j.bandl.2013.02.007.
Migliaccio R, Agosta F, Rascovsky K, Karydas A, Bonasera S, Rabinovici GD, et al. Clinical syndromes associated with posterior atrophy: early age at onset AD spectrum. Neurology. 2009;73:1571–8.
Acknowledgment
John R. Hodges has received grant support from the National Health and Medical Research Council of Australia.
Compliance with Ethics Guidelines
ᅟ
Conflict of Interest
Cristian E. Leytona declares that he has no conflict of interest.
John R. Hodges is a Senior Principal Research Fellow for Neuroscience Research Australia, and an ARC Federation Fellow and Professor of Cognitive Neurology at the University of New South Wales. He receives editor royalties and speaker royalties for Henry Stewart Talks.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Behavior
Rights and permissions
About this article
Cite this article
Leyton, C.E., Hodges, J.R. Towards a Clearer Definition of Logopenic Progressive Aphasia. Curr Neurol Neurosci Rep 13, 396 (2013). https://doi.org/10.1007/s11910-013-0396-6
Published:
DOI: https://doi.org/10.1007/s11910-013-0396-6