Abstract
Reversible cerebral vasoconstriction syndrome (RCVS) is a collective term used for transient noninflammatory, nonatherosclerotic segmental constriction of cerebral arteries. The angiopathies of RCVS have previously been defined by several nomenclatures. Current opinion favors the unification of these pathophysiologically related angiopathies because of their similar angiographic features and clinical course. RCVS typically presents acutely as headache, delirium, seizure, cerebral ischemia, and/or hemorrhage. The angiographic features make RCVS an important mimic of CNS vasculitides. In contrast to CNS vasculitis, RCVS is typically a transient condition with relatively good clinical outcomes. Although a complete understanding of the etiological and pathological features of RCVS has not yet been achieved, alterations in vascular tone lead to the observed arterial changes. In this review, we aim to provide a summary of RCVS and provide insight into current perspectives of the underlying pathophysiological processes, diagnosis, and treatment.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Reversible cerebral vasoconstriction syndrome (RCVS) is a collective term used for reversible angiopathies that present acutely with a sudden, severe “thunderclap” headache and multifocal arterial vasoconstriction. RCVS was initially described in 1988 by Call et al. [1] and is known by the eponym “Call–Fleming syndrome.” Recent case reports and series have identified a myriad of underlying or predisposing conditions for developing RCVS. These vasoconstrictive syndromes have previously been defined by several nomenclatures depending upon their cause and presentation. However, given their common clinical and angiographic features, a unifying diagnosis of RCVS is now favored. Although headache is a prominent feature in almost all patients with RCVS, variability in other associated symptoms and patient characteristics has previously confounded efforts to study this disease. Recent large case series have shed light on the clinicopathological features and natural history of RCVS. Despite these recent advances, several questions remain. Foremost is whether RCVS is a single disease entity or rather a common presentation of multiple disorders. Secondly, there are no accepted diagnostic criteria for establishing a diagnosis of RCVS. Lastly, questions remain regarding the appropriate treatment of patients diagnosed with RCVS. This review aims to answer some of these questions.
Patient Characteristics and Precipitating Factors
The patient and clinical characteristics of RCVS have recently been described in three relatively large case series [2–4••]. RCVS typically presents in middle-aged women. The average age of onset is between 40 and 50 years, and between 64 and 92 % of patients are female [2–4••]. However, female patients are on average a decade older than male patients with RCVS [5•]. Little is known about associated comorbid conditions, but from 20 to 40 % of patients report a prior history of headache [2–4••]. Up to half of patients may have a prior history of hypertension [2]. RCVS has also been reported in patients with diabetes; however, that alone has not been demonstrated to be a causative factor for RCVS [6].
Headache at presentation is a near ubiquitous feature of RCVS and occurs in 95–100 % of cases [2–4••]. Most patients describe the headaches as multiple, recurrent “thunderclap” headaches which are severe and sudden in onset, reaching maximal intensity in less than 1 min. RCVS patients may also present with acute elevations in blood pressure (33–39 %), focal neurological deficits (0–43 %), and/or seizures (0–17 %) depending on the clinical setting [2–4••].
Rates of recovery from RCVS are high, with up to 90 % of patients demonstrating good clinical outcomes. However, 10 % of patients may have permanent neurological disability [4••]. Complications of RCVS can include cerebral infarction (4–40 %), intracerebral hemorrhage (6–20 %), subarachnoid hemorrhage (SAH) (22–34 %), and reversible posterior leukoencephalopathy syndrome (less than 10 %) [2–4••]. Occasionally, complications of RCVS may prove fatal, with mortality rates as high as 2 % [4••, 7, 8]. Early diagnosis and initiation of therapy may prevent complications and improve outcomes.
Risk Factors/Susceptibility to RCVS
Possible causes of and reported risk factors for developing RCVS are reported in Table 1. Female gender appears to be a clear risk factor for developing RCVS. Physiological changes during pregnancy and the postpartum period may increase susceptibility to developing RCVS. Placental growth factor, soluble placental growth factor receptor (soluble Flt-1), and soluble endoglin are factors important in angiogenesis and have been implicated in the development of postpartum angiopathy, a relatively common subtype of RCVS [9–11]. Hormonal therapies also appear to be a risk factor [5•]. Migraine headaches are more frequent in women than in men and current migraine therapies include vasoactive medications which may cause RCVS [12].
In addition to gender differences, genetic variations may increase susceptibility to developing RCVS or may affect the severity and clinical course. Brain-derived neurotrophic factor (BDNF) is a neurotrophin that is important in neurogenesis, neuronal survival, and synaptic plasticity [5•]. Alterations in BDNF have been associated with disorders that affect vascular tone. Carriers of the valine allele of the BDNF polymorphism may be at particularly increased risk of developing severe vasoconstriction and subsequent complications association with RCVS [13].
Therapeutic agents and recreational drugs that affect vascular tone are commonly linked to RCVS. In a recent report, Palma et al. [14] described the case of a woman who was administered epinephrine to treat anaphylactic shock. Administration of epinephrine resulted in an episode of RCVS, typified by a thunderclap headache followed by apparent cerebral vasoconstriction. Although this is a singular case, the authors claim that administration of exogenous epinephrine was the causative factor behind the attack. Physiological changes induced by epinephrine or other vasoactive substances may shed light on the pathophysiological processes of RCVS and make it possible to develop animal models and allow future research.
Diagnostic Studies
A summary of proposed diagnostic criteria for RCVS are presented in Table 2. Most patients admitted to hospitals with thunderclap headache will have a noncontrast computed tomography (CT) scan of the brain looking for evidence of aneurysmal SAH. In contrast to blood in the basal cisterns, which is observed in patients with aneurysmal SAH, there is often evidence of cortical SAH over the convexities of the cerebral hemispheres in RCVS patients. Magnetic resonance imaging may be more sensitive than CT for detecting subtle cortical SAH. Magnetic resonance imaging is also more sensitive for detecting cerebral edema, infarction, and the changes in leukoencephalopathy [15].
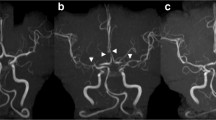
The gold standard for diagnosing RCVS is detecting the presence and subsequent resolution of arterial changes by conventional catheter-based cerebral angiography. The typical angiographic pattern consists of alternating areas of arterial constriction and dilatation, often called “beading” or “sausage on a string,” in multiple vascular beds (Fig. 1). CT angiography and magnetic resonance angiography (MRA) are newer, less invasive methods for identifying the arterial changes observed in RCVS. MRA is likely to emerge as the preferred method for cerebrovascular imaging. Chen et al. [5•] proposed that thunderclap headaches or sentinel headaches that occur prior to SAH be considered as symptoms indicating RCVS in the primary diagnosis of a patient. Since the vasoconstriction associated with thunderclap headaches persists after the resolution of pain, follow-up MRA investigations should be performed to identify temporal angiographic changes confirming the diagnosis of RCVS. Resolution should be seen 12 weeks after onset in most cases [16].
Conventional cerebral angiogram (lateral projection) showing typical angiographic features consisting of alternating areas of arterial constriction and dilatation (arrows) in multiple vascular beds. (Image provided courtesy of Brett L Cucchiara, Department of Neurology, School of Medicine, University of Pennsylvania)
There are no specific laboratory tests for RCVS. However, cerebrospinal fluid (CSF) analysis is essential to rule out SAH in thunderclap headache patients with normal brain imaging and to differentiate RCVS from infectious or inflammatory vasculopathies. The findings of CSF studies should be normal or near normal in patients with RCVS. In a series of 139 RCVS cases, 95 % of patients had CSF white blood cell counts below ten cells per microliter and protein levels below 80 mg/dL, and 80 % of patients had completely normal results [4••]. In some cases, and especially in patients who do not present with the typical thunderclap headaches or who follow a typical clinical course, a brain biopsy may be warranted to exclude alternative diagnoses.
Treatment
There is no established treatment for RCVS Clinical and angiographic resolution occurs spontaneously. However, calcium channel blockers, intravenously administered magnesium, and glucocorticoids have been used with variable success. Clinical trial data on the safety and efficacy of current therapeutic options are limited to retrospective case reports and series.
In a recent case series, the use of glucocorticoid steroids was associated with a trend toward poorer outcomes when compared with patients who received no specific therapy or calcium channel blockers alone (odds ratio 2.7, confidence interval 0.8–8.8, p = 0.08) [4••]. On the basis of these limited data, the use of steroids cannot be recommended. The calcium channel blocker nimodipine may improve headache symptoms and clinical outcomes [2, 17, 18]. Nimodipine is usually administered orally, but intravenous administration is also possible [19]. Verapamil hydrochloride is an alternative calcium channel blocker that may also be effective in treating RCVS [4••, 20]. Intravenous magnesium infusions are common for treatment of postpartum angiopathy. Although this is the standard of care for preeclampsia/eclampsia, there are variable data regarding the utility of magnesium in treating RCVS [11, 21]. Intravenous administration of prostacyclin, an eicosanoid with vasodilatory and antiplatelet effects, has been reported to improve cognitive deficits from RCVS [22]. The appropriate duration of therapy is unclear for all of these agents and requires further study. These agents may also decrease arterial blood pressure, thereby reducing cerebral perfusion pressure and exacerbating cerebral ischemia [11]. This should be considered when treating patients with RCVS and stroke.
In cases of cerebral ischemia due to severe flow limiting cerebral vasoconstriction, endovascular therapies may be warranted. Intra-arterial administration of verapamil and intracranial angioplasty have been reported to successfully treat the angiographic changes occurring in RCVS [23]. Intra-arterial administration of milrinone, a phosphodiesterase inhibitor, has also been reported to be effective in a case of RCVS refractory to nimodipine [24]. Balloon angioplasty or direct intra-arterial infusion of vasospasm-relieving agents should likely be reserved for refractory cases with clinical deterioration as these therapies carry a risk of stroke, arterial dissection or perforation, and reperfusion injury [25, 26].
Conclusion
RCVS is an increasingly recognized cause of acute, severe thunderclap headache associated with segmental cerebral arterial narrowing. There is likely significant underreporting owing to extensive subclassification schemes of RCVS, which stems in part from the diverse associated causes and symptoms. RCVS should be prominent in the differential diagnosis of any patient presenting with recurrent thunderclap headaches. Vascular imaging is paramount in detecting the typical vascular changes. Treatment options for RCVS are currently limited; however, calcium-channel blockers appear to be favored as the first-line therapy. Further elucidation of the pathophysiological processes of RCVS is needed to develop targeted therapies against this disease.
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Call GK, Fleming MC, Sealfon S, et al. Reversible cerebral segmental vasoconstriction. Stroke. 1988;19:1159–70.
Chen SP, Fuh JL, Lirng JF, et al. Recurrent primary thunderclap headache and benign CNS angiopathy: spectra of the same disorder? Neurology. 2006;67:2164–9.
Ducros A, Boukobza M, Porcher R, et al. The clinical and radiological spectrum of reversible cerebral vasoconstriction syndrome. A prospective series of 67 patients. Brain. 2007;130:3091–101.
•• Singhal AB, Hajj-Ali RA, Topcuoglu MA, et al. Reversible cerebral vasoconstriction syndromes: analysis of 139 cases. Arch Neurol. 2011;68:1005–12. Describe the features of RCVS in patients requiring hospitalization. In addition to the clinical features of 139 cases, the authors discuss RCVS treatment and outcome.
• Chen SP, Fuh JL, Wang SJ. Reversible cerebral vasoconstriction syndrome: current and future perspectives. Expert Rev Neurother. 2011;11:1265–76. Provide a comprehensive review of RCVS based on their experience with a large cohort of patients presenting to an outpatient headache center.
Marder CP, Donohue MM, Weinstein JR, Fink KR. Multimodal imaging of reversible cerebral vasoconstriction syndrome: a series of 6 cases. AJNR Am J Neuroradiol. 2012;33:1403–11.
Williams TL, Lukovits TG, Harris BT, Harker Rhodes C. A fatal case of postpartum cerebral angiopathy with literature review. Arch Gynecol Obstet. 2007;275:67–77.
Thakur JD, Chittiboina P, Khan IS, Nanda A. Unique case of postpartum cerebral angiopathy requiring surgical intervention: case report and review of literature. Neurol India. 2011;59:891–4.
Levine RJ, Maynard SE, Qian C, et al. Circulating angiogenic factors and the risk of preeclampsia. N Engl J Med. 2004;350:672–83.
Levine RJ, Lam C, Qian C, et al. Soluble endoglin and other circulating antiangiogenic factors in preeclampsia. N Engl J Med. 2006;355:992–1005.
McKinney JS, Messe SR, Pukenas BA, et al. Intracranial vertebrobasilar artery dissection associated with postpartum angiopathy. Stroke Res Treat. 2010;2010:320627.
Macgregor EA, Rosenberg JD, Kurth T. Sex-related differences in epidemiological and clinic-based headache studies. Headache. 2011;51:843–59.
Chen SP, Fuh JL, Wang SJ, et al. Brain-derived neurotrophic factor gene Val66Met polymorphism modulates reversible cerebral vasoconstriction syndromes. PLoS One. 2011;6:e18024.
Palma JA, Fontes-Villalba A, Irimia P, et al. Reversible cerebral vasoconstriction syndrome induced by adrenaline. Cephalalgia. 2012;32:500–4.
Schellinger PD, Bryan RN, Caplan LR, et al. Evidence-based guideline: the role of diffusion and perfusion MRI for the diagnosis of acute ischemic stroke: report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 2010;75:177–85.
Calabrese LH, Dodick DW, Schwedt TJ, Singhal AB. Narrative review: reversible cerebral vasoconstriction syndromes. Ann Intern Med. 2007;146:34–44.
Lu SR, Liao YC, Fuh JL, et al. Nimodipine for treatment of primary thunderclap headache. Neurology. 2004;62:1414–6.
Ducros A, Fiedler U, Porcher R, et al. Hemorrhagic manifestations of reversible cerebral vasoconstriction syndrome: frequency, features, and risk factors. Stroke. 2010;41:2505–11.
Elstner M, Linn J, Muller-Schunk S, Straube A. Reversible cerebral vasoconstriction syndrome: a complicated clinical course treated with intra-arterial application of nimodipine. Cephalalgia. 2009;29:677–82.
Theeler BJ, Krasnokutsky MV, Scott BR. Exertional reversible cerebral vasoconstriction responsive to verapamil. Neurol Sci. 2010;31:773–5.
Chik Y, Hoesch RE, Lazaridis C, et al. A case of postpartum cerebral angiopathy with subarachnoid hemorrhage. Nat Rev Neurol. 2009;5:512–6.
Grande PO, Lundgren A, Bjartmarz H, Cronqvist M. Segmental cerebral vasoconstriction: successful treatment of secondary cerebral ischaemia with intravenous prostacyclin. Cephalalgia. 2010;30:890–5.
Farid H, Tatum JK, Wong C, et al. Reversible cerebral vasoconstriction syndrome: treatment with combined intra-arterial verapamil infusion and intracranial angioplasty. AJNR Am J Neuroradiol. 2011;32:E184–187.
Bouchard M, Verreault S, Gariepy JL, Dupre N. Intra-arterial milrinone for reversible cerebral vasoconstriction syndrome. Headache. 2009;49:142–5.
Takis C, Kwan ES, Pessin MS, et al. Intracranial angioplasty: experience and complications. AJNR Am J Neuroradiol. 1997;18:1661–8.
Singhal AB, Kimberly WT, Schaefer PW, Hedley-Whyte ET. Case records of the Massachusetts General Hospital. Case 8-2009. A 36-year-old woman with headache, hypertension, and seizure 2 weeks post partum. N Engl J Med. 2009;360:1126–37.
Disclosure
No potential conflicts of interest relevant to this article were reported.
Author information
Authors and Affiliations
Corresponding author
Additional information
This article is part of the Topical Collection on Stroke
Rights and permissions
About this article
Cite this article
Velez, A., McKinney, J.S. Reversible Cerebral Vasoconstriction Syndrome: A Review of Recent Research. Curr Neurol Neurosci Rep 13, 319 (2013). https://doi.org/10.1007/s11910-012-0319-y
Published:
DOI: https://doi.org/10.1007/s11910-012-0319-y