Abstract
Introduction
Postpartum cerebral angiopathy (PCA) is a rare and pathophysiologically ill-characterized cerebral vasoconstriction syndrome, occurring within 30 days of a usually uncomplicated pregnancy and delivery. Its onset has been associated with the use of vasoactive medications, particularly ergot alkaloids. Other cases have occurred in the absence of these medications, prompting conjecture into possible overlap between PCA and other conditions known to cause cerebral vasoconstriction, including primary angiitis of the central nervous system and postpartum eclampsia. The vast majority of cases follow a relatively benign course; however, a fatal case has been reported. Histopathologic findings in PCA, so far limited to the fatal case and two more recent biopsies, have been nonspecific.
Objective
Here we present a second fatal case of PCA, including pre- and post-mortem histopathologic analysis. We also include a review of all PCA cases reported in the English literature.
Methods
Criteria for the clinical diagnosis of PCA are proposed and used to select case reports from the medical literature. Data pertaining to patient characteristics, clinical symptomatology, cerebral imaging findings, and clinical outcomes are compared between cases associated with the postpartum use of vasoactive medications and spontaneous cases.
Conclusions
We conclude that histopathologic findings in PCA are nonspecific and secondary to ischemic brain injury. Functional vasoconstriction is the most likely primary pathophysiologic process in PCA. The etiology in cases associated with medications may be due to idiosyncratic reactions to these agents. Significant overlap in symtomatology and clinical features exists between spontaneous cases and late postpartum eclampsia.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Postpartum cerebral angiopathy (PCA) is a rare and pathophysiologically ill-characterized cerebral vasoconstriction syndrome occurring within 30 days of a usually uncomplicated pregnancy and delivery. The handful of reported cases share a primary clinical manifestation of refractory headache accompanied by focal neurological deficits and sometimes seizures.
The onset of PCA has been associated with the postpartum use of vasoactive medications, especially ergot alkaloids, with bromocriptine being particularly implicated (Comabella et al. 1996; Iffy et al. 1996; Janssens et al. 1995). Numerous other cases have been reported in the absence of such medication use. Various authors have conjectured that these latter cases may be a manifestation of postpartum eclampsia (PPE) (Chang 2000; Donaldson 2000; Raps et al. 1993), a benign variant of primary angiitis of the central nervous system (PACNS) (Calabrese et al. 1993; Sugiyamaet al. 1997; Ursell et al. 1995;Yasuda et al. 1993), unusual reactivity to the postpartum hormonal milieu (Al-Sous et al. 1998; Calado et al. 2004; Comabella et al. 1996; Geocadin et al. 2002), or postpartum migraine (Comabella et al. 1996; Modi and Modi 2000).
The outcome is usually good, with complete resolution of cerebral vasoconstriction within days to weeks, and complete to near-complete resolution of clinical symptoms within weeks to months. However, cases resulting in permanent neurological deficits, as well as a single fatal case (Geraghty et al. 1991), have been reported.
When performed, cerebral angiograms and transcranial doppler ultrasonography (TCD) of patients with PCA almost uniformly show multi-segmental cerebral vasoconstriction and corresponding increased blood flow velocities, respectively (Bogousslavsky et al. 1989; Ursell et al. 1995; Zunker et al. 2002). Typically, these findings reverse as clinical symptoms resolve. The close correlation between symptom resolution and reversal of vascular findings suggests transient vasoconstriction as the primary pathophysiologic process in PCA (Ursell and Marras et al. 1995). However, because vasoconstriction and increased blood flow velocity are also consistent with vasculitis, the potential role of this latter process remains an open question (Calabrese et al. 1992; Calabrese and Mallek 1988; Calado et al. 2004; Yasuda et al. 1993; Zunker et al. 2002).
Histopathologic examination of tissue from PCA cases has been limited to the previously mentioned fatal case (autopsy), and biopsies from two more recent cases (Calado et al. 2004; Song et al. 2004) from which the patients variably recovered.
Here, we present a second fatal case of PCA, including findings from pre- and post-mortem histopathologic tissue evaluation. We also include a review of all PCA cases reported in the English literature.
Case presentation
A previously healthy 40-year-old woman, gravida 4, para 2, abortus 2, presented to a community hospital with a five-day history of severe pounding headache associated with nausea. Seven days earlier she had undergone successful, induced vaginal delivery of a healthy infant under epidural anesthesia, performed five weeks prematurely due to nonreassuring fetal heart decelerations. Apart from mild, diet-controlled gestational diabetes, the pregnancy was uneventful. History for recent illicit drug use and peripartum use of ergot-derivied or sympathomemetic medication was negative. The patient had a remote history of migraine-type headaches as a teenager, which resolved without recurrence after vision correction with eyeglasses. She had a long-standing one pack per day smoking history, and a history of alcohol abuse with 2 years of sobriety preceding this presentation.
On presentation, her blood pressure was 165/88 mmHg (later, dropping to 150/79) with a pulse rate of 78 beats per min and temperature of 37.1°C. Physical examination was otherwise unremarkable. Apart from marginally decreased hemoglobin (11 mg/dl) and hematocrit (34.4%), all laboratory values, including cerebrospinal fluid evaluation, were within normal limits. Computed tomography (CT) scan of the brain showed mild subcortical white matter enhancement in the left frontotemporal and parietal cortices, which were interpreted as possible indicators of meningitis or cerebritis. The patient was started on intravenous ceftriaxone and acyclovir, as well as ketorolac and morphine for pain control. On the following morning the patient developed a sudden-onset left hemiparesis with declining mental status and seizures. At that point she was transferred to our university hospital for further evaluation and management.
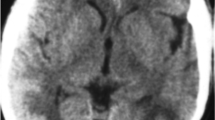
Repeat CT and magnetic resonance (MR) imaging of the brain showed a five-centimeter intraparenchymal hematoma in the right frontal lobe, bilateral occipital lobe hypodensities consistent with infarction, and no indications of venous thrombosis (Fig. 1a). The patient was started on high-dose methylprednisalone, phenytoin, and calcium channel blockers, with initial resolution of seizure activity and improvement in mental status. On hospital day-two (8 days after symptom onset) a cerebral angiogram revealed diffuse vasoconstriction involving mainly bilateral anterior and middle cerebral and basilar arteries (Fig. 1b, c). The following day, resumed decline in mental status with seizure activity and posturing consistent with impending herniation prompted neurosurgical evacuation of the right frontal hematoma. At surgery, the brain was noted to be severely edematous and herniating through the craniotomy site. Histologic evaluation of cerebral tissue included with the evacuation specimen showed hypoxic-ischemic changes with acute and subacute infarction, focal fibrin thrombi, and no evidence of vasculitis or infection (Fig. 2).
The patient’s postoperative neurological status failed to improve. The following day, transcranial duplex Doppler ultrasonography showed high-velocity blood flow in the left anterior and bilateral middle cerebral and basilar arteries, consistent with continued vasoconstriction. Cyclophosphamide and magnesium were added to the patient’s medications, and mannitol infusion was started, to mitigate increasing intracranial pressure. However, repeated CT and MR imaging showed increasing cerebral edema and multiple new hypodensities consistent with infarction in supra- and infra-tentorial brain regions (Fig. 3a–d). By this time the patient’s pupils were fixed and dilated and brainstem reflexes were severely truncated. An apnea test was performed, and the patient was subsequently declared brain dead, fourteen days postpartum, 12 days after the initial onset of symptoms.
A brain-only postmortem examination showed markedly increased brain weight at 1,720 grams (normal 1,200) and diffuse gyral flattening, consistent with massive cerebral edema. The premortem craniotomy site with herniating hematoma bed was prominently evident. Also present were the bilateral uncal and cerebellar tonsilar notching, consistent with herniations at those sites. On cut section, cerebral gray matter was generally pale with diffuse obscuring of the gray-white junction; and the lateral and third ventricles were compressed and obliterated.
Microscopic findings (Fig. 4a–c) confirmed multiple acute and subacute infarctions primarily involving bilateral frontoparietal and occipital cortices and the cerebellum. Focal necrosis of the anterior pituitary was also noted. Stains for microorganisms were negative. Apart from copious perivascular margination and diapedesis of inflammatory cells in vessels associated with areas of infarction, no vasculitis or other vascular abnormalities in large or small vessels were present.
Photomicrographs from brain-only autopsy. Large menningeal vessels (a, hematoxylin and eosin, low power) were uniformly intact and without evidence of vasculitis, thrombosis, or other vasculopathy. Intraparenchymal arterioles and capillaries (b, hematoxylin and eosin, high power; c, hematoxylin and eosin, medium power) near areas of infarction showed marked inflammatory cell infiltration and margination, and were otherwise normal. Numerous areas of acute and subacute infarction—here (c) examplified by cerebellum with inflammation and pyknotic Purkinje cells—as well as tonsilar and uncal herniation, were present
Review of literature
Methods
A PubMed (http://www.pubmed.gov) literature search was conducted, using permutations of the search term “postpartum cerebral angiopathy”, augmented by search results from the “Related Articles” tool and manual review of selected articles referenced in case reports included in the final review. As there are no established diagnostic criteria for PCA, the diagnostic criteria proposed by Calabrese and Furlan et al. (1992); Calabrese and Mallek (1988), for the diagnosis of primary angiitis of the central nervous system were modified to identify cases for the present review. For inclusion cases required: (1) Acute onset of neurological symptoms within 0–30 days postpartum; (2) cerebral vasoconstriction within that time period documented by angiography or TCD; and (3) exclusion of other pathology to which the clinical symptoms and cerebral imaging could be secondary, including cases which fully met conventional criteria for intrapartum or postpartum preeclampsia or eclampsia. Cases were categorized as “medication-related” if vasoactive medications had been used during the postpartum period. If no record of such medication use was present, cases were categorized as “spontaneous.”
Results
Using the above criteria, review of the English literature revealed 33 reports published since 1984 describing 45 cases of PCA. The data, including the present case, are presented in Tables 1, 2 and 3.
Twenty-seven (58%) PCA cases were spontaneous (S) (Akins et al. 1996; Ellison et al. 1988; Geocadin et al. 2002; Geraghty et al. 1991; Kubo et al. 2002; Langlois et al. 1989; Lee et al. 1999; Raps et al. 1993; Singhal 2004b; Song et al. 2004; Sugiyama et al. 1997; Ursell et al. 1998; Yasuda et al. 1993; Zunker et al. 2002], and 19 (42%) were medication-related (MA) (Al-Sous et al. 1998; Barinagarrementeria et al. 1992; Bogousslavsky et al. 1989; Comabella et al. 1996; Crippa et al. 2000; Garner et al. 1990; Granier et al. 1999; Henry et al. 1984; Iffy et al. 1996a; Ihara,et al. 2000; Janssens et al. 1995; Konstantinopoulos et al. 2004; Kulig et al. 1991; Modi and Modi 2000; Raroque et al. 1993; Roh and Park 1998; Sekimoto et al. 2002]. Among MA cases, 17 (89%) were associated with the use of ergot alkaloids: 12 (63%) with a single agent, including 3 associated with bromocriptine, 3 with ergometrine, 2 with methylergonovine, and 1 each with dihydroergotamine, ergonovine, lisuride, and methylergoline. Five cases (26%) were associated with the use of two agents, including two cases combining an ergot derivative with a sympathomemetic (bromocriptine and isometheptene, and bromocriptine and phenylpropanolamine), two cases combining two ergot alkaloids (methylergonovine and bromocriptine, and methylergonovine and dihydroergotoxine), and one case combining an ergot derivative with a serotonin agonist (dihydroergotamine and sumatripan). The remaining two MA cases (11%) involved the use of isometheptene alone. The mean age of all patients was 29.2 years (± 6.6). Five (11%) patients had a history of migraine. Twelve (26%) were nulliparous at the time of delivery, and an equal number had Cesarean sections. A greater proportion of MA cases had epidural anesthesia during delivery. Twenty-nine reports included blood pressure measurements at presentation. Individual values were categorized as “mild” or “severe” according to established convention (Gary Cunningham et al. 2005). When only qualitative characterizations of presenting blood pressure were provided (“normotensive,” four S cases and one MA case; and “hypertensive,” three S cases and one MA case), they were categorized as “none” and “hypertensive,” respectively. Medication-related cases had a mean earlier onset of symptoms, with a much smaller range and degree of variability. For MA cases, the mean interval of drug commencement to symptom onset (not shown in Table 1) was 2.1 days (± 2.6 days), with a minimum interval of minutes (ergonovine and methylergometrine), and maximum of 9.5 days (bromocriptine). Headache and seizure were the two most common presenting symptoms in both groups. Visual symptoms ranging from blurred vision to cortical blindness, mental status changes, and speech disturbances were more common in MA cases. Paresis and hyperreflexia were more common in S cases. Forty-three (93%) patients presented with two or more symptoms. During the course of illness, three S cases and one MA case progressed to coma; four S cases and one MA case progressed to seizures; and three MA cases and one S case progressed to paresis. Information about the duration of symptoms (not shown in tables) was reported for approximately half of the cases in each group. The mean for MA cases was 12.9 days (± 9.3, range 4–35), and for S cases 15.5 days (± 11.2, range 5–32).
The results of cerebral imaging are presented in Table 2. All patients who underwent cerebral angiography (either conventional or MR angiography) or TCD demonstrated cerebral vasoconstriction. Where the arteries involved were specified, the main branches of the Circle of Willis were involved in a majority of S cases, with the posterior cerebral arteries most frequently involved. The middle cerebral artery was most commonly involved among MA cases, with one half also involving the vertebrobasilar system (compared to a third of S cases). Approximately two-thirds of patients in each group were studied with MRI. A majority of S cases and half of MA cases showed abnormalities, most frequently involving the occipital or parieto-occiptial lobes. An equal proportion (approximately 25%) of S and MA cases had intracerebral hemorrhages by CT or MRI, while a greater proportion of S cases had cerebral infarction. Two-thirds of infarctions in both groups occurred in the occipital or parieto-occiptial cortex, while other cortical areas, including watershed zones, were involved equally as often in S cases.
The vast majority of patients in both groups had favorable outcomes (presented in Table 3), with complete to near-complete resolution of symptoms. One S case recurred in a subsequent pregnancy, with resolution of symptoms in the interim. Two fatal cases, including our case, were among the S group.
Discussion
The present case represents the second reported fatal case of PCA [Geraghty et al. 1991], and one of only four reported cases which have included histologic evaluation of cerebral tissue (Table 4) . The setting, initial clinical course, and cerebral imaging findings in both fatal cases were typical of other reported cases of PCA. However, whereas most cases followed a relatively benign course, with complete to near-complete resolution of symptoms within weeks of presentation, these two cases were atypically severe and progressed unremittingly to brain death despite aggressive therapeutic measures. Postmortem histopathologic findings in both cases were nonspecific. The marginal vascular abnormalities found by Geraghty et al.—slight intimal thickening and focal disruption of the elastic lamina—were not found in the current case. A more recent case (Calado et al. 2004) including histologic evaluation of letomeningeal and cerebral biopsies reported small-vessel vasculitis, which was thought likely secondary to prolonged arterial constriction. Another case (Song et al. 2004) describes biopsy findings of focal perivascular chronic inflammation in the absence of vasculitis or other vascular pathology. While copious inflammatory infiltrates in cerebral vessels associated with infarction were evident in the present case, vasculitis was not identified. Fibrin thrombi in vessels in cerebral tissue included with a premortem hematoma evacuation likely occurred secondary to adjacent tissue injury.
Taken together, no anatomic abnormalities to account for sustained cerebral vasoconstriction were identified in any of the four cases that have included direct evaluation of brain tissue. The histologic changes that were identified likely occurred secondary to cerebral ischemia and injury. The absence of anatomic vascular pathology coupled with the findings of cerebrovascular studies strongly suggests the primary pathophysiolgic process in PCA is functional vasoconstriction. This process seems to be reversible in the vast majority of cases.
The etiology of vasoconstriction in PCA is unclear. It has been suggested that idiosyncratic heightened sensitivity of the neurovasculature to the vasoactive effects of some medications may be causative in cases associated with the postpartum use of these agents (Comabella et al. 1996; Crippa et al. 2000; Granier et al. 1999; Janssens et al. 1995; Raroque et al. 1993; Roh and Park 1998). Bromocriptine in particular has been implicated in a wide range of adverse events in the peuperium, seemingly mediated by vasoconstriction (Iffy et al. 1996a; Iffy et al. 1996b; Iffy et al. 1998; Janssens et al. 1995). These events include postpartum hypertension, seizures, cerebrovascular accidents, myocardial infarction (Iffy et al. 1998), and acute renal failure (Makdassi et al. 1996). According to one source (de Jong-van den BergL. and Mintzes 1995), between 1980, when the use of bromocriptine for postpartum lactation suppression was introduced, and 1994, 531 adverse events, including 32 deaths, were reported among US women of child-bearing age, following bromocriptine use. Indeed, during our literature review we encountered numerous case reports and case series describing such events. These cases generally followed a clinical course very similar to PCA and displayed parallel cerebral imaging findings. However, the vast majority of cases did not document cerebral vasoconstriction, and thus, were not included in the present review. Numerous other reports were identified in the non-English literature. Largely due to concern over adverse events, in 1995 the FDA withdrew approval for the use of bromocriptine for postpartum lactation suppression. However, it is worth noting that significant controversy exists regarding this implicated causation, with other authors contending that adverse events are more likely related to pre-existing risk factors or the concomitant use of bromocriptine and other medications (Herings and Stricker 1995; Morgans 1995). While the present review excluded cases with significant preexisting morbidity, we did find that only a minority of MA cases were associated with bromocriptine, and those that were involved a second agent equally as often as bromocriptine alone. This result is similar to an FDA bulletin in 1984 (No authors listed 1984), initially raising the concern of possible adverse events, which cited 6 of 17 patients who suffered adverse events while taking bromocriptine were also receiving other drugs which could have contributed. It is also noteworthy that, in the present review, cases involving bromocriptine alone had a mean onset of 6.8 days (± 2.4; range 5–9.5) after commencing the drug, while those involving bromocriptine and another agent had a mean onset of 0.33 days (± 0.57; range 0–1), and cases involving non-bromocriptine ergot alkaloids had a mean onset of 2.4 days (± 2.2; range 0–7). This raises the question of whether bromocriptine, while perhaps alone capable of stimulating vasoconstriction in some patients with prolonged exposure, may also sensitize the cerebral vasculature to the vasoconstrictive effects of other agents. This has been suggested as a possible vasospastic mechanism for other ergot derivatives (Sekimoto and Kunishige et al. 2002). The postpartum state may also somehow potentiate sensitization in susceptible patients (Comabella et al. 1996; Granier et al. 1999; Ihara et al. 2000). Alternatively, cerebral vasoconstriction in these patients may be incidental to the postpartum setting, a possibility further suggested by the observation of cerebral vasoconstriction associated with similar medications in nonpeuperal settings (Call et al. 1988; Singhal 2004a).
As suggested by some authors (Akins and Levyet al. 1996; Donaldson 2000), cerebral vasoconstriction in spontaneous PCA cases may fall within the spectrum of postpartum eclampsia. While a review (Leitch et al. 1997) of eclampsia occurring at a large UK hospital over a 60-year period showed a significant overall decrease in incidence, there was a relative increase in the incidence of eclampsia in the postpartum period, comprising around half of all the cases. Approximately, over the past decade, increasing attention has been drawn to the occurrence of late postpartum eclampsia (PPE), with onset between 2–30 days postpartum (Chames et al. 2002; Dziewas et al. 2002; Felz et al. 2000; Lubarsky et al. 1994; Martin and Sidman 2003; Mathew et al. 2003; Matthys et al. 2004; Newbould 2002). A recent case series (Chames et al. 2002) found that, of 89 total cases of eclampsia over a 5-year period (1996–2001), fully a quarter (23/89) developed > 48 h postpartum. Similarly, an earlier study (Lubarsky et al. 1994) found that from 1977 to 1992, 16% (54/334) of eclamptic patients in the study population presented two or more days after delivery. Some authors (Leitch et al. 1997) conjecture that the occurrence of eclampsia in the late peuperium may be due to a difference in the factors associated with ante/intrapartum and postpartum eclampsia. Perhaps supporting this view is the observation, that only 22 and 56%, respectively, of women in the above studies had been diagnosed as preeclamptic (Chames et al. 2002; Lubarsky et al. 1994). Other investigators (Chang 2000; Dziewas et al. 2002; Martin and Sidman 2003; Mathew et al. 2003; Veltkamp et al. 2000) have noted that classic manifestations of eclampsia, including hypertension and proteinuria, are not always present in cases of late postpartum onset. This of course begs the nosological question of whether these patients’ diagnoses have been properly classified, which fuels some controversy over the existence of late PPE. Nonetheless, numerous similarities in the clinical course, response to treatment, and outcomes between classic eclampsia and late PPE strongly suggest related etiologies. Likewise, striking similarities involving the clinical setting, signs and symptoms, responses to treatments, and outcomes exist between PPE and PCA. Indeed, in the present review, 73% (16/22) of patients with spontaneous PCA were hypertensive at presentation, with over half in the severe range; of the 21 reports including urinalysis results, 4 (19%) noted proteinuria; and as already mentioned, seizures were initially present in 12/27 (44%) cases, and an additional four cases progressed to seizures during their course of illness. Further implicating a connection between PCA, PPE and classic eclampsia, numerous investigators (Bartynski and Sanghvi 2003; Farine et al. 1984; Qureshi et al. 1996; Ringer et al. 2001; Sengar et al. 1997; Trommer et al. 1988) have found cerebral vasoconstriction, particularly involving the posterior circulation, to commonly accompany eclampsia, both in classic and late PPE settings. These and other studies (Tank et al. 2004; Thomas 1998) have also shown similarities in MR and CT imaging. Conspicuously absent, however, among the symptomatology of PCA, are, generalized edema, liver function test abnormalities, and abdominal pain—all hallmarks of classic eclampsia. This suggests that if there is an overlap between classic eclampsia, PPE, and spontaneous PCA, the pathophysiologic process in PCA is primarily limited to the cerebral vasculature, while that of classic eclampsia and PPE involves both cerebral and systemic vascular systems.
References
Akins PT, Levy KJ, Cross AH, Goldberg MP, Schieber MH (1996) Postpartum cerebral vasospasm treated with hypervolemic therapy. Am J Obstet Gynecol 175:1386–1388
Al-Sous W, Bohlega S, Al-Kawi Z, McLean D, Shuiri K (1998) Post-partum cerebral angiopathy. A rare cerebrovascular complication. Eur J Neurol 5:411–416
Barinagarrementeria F, Cantu C, Balderrama J (1992) Postpartum cerebral angiopathy with cerebral infarction due to ergonovine use. Stroke 23:1364–1366
Bartynski WS, Sanghvi A (2003) Neuroimaging of delayed eclampsia. Report of 3 cases and review of the literature. J Comput Assist Tomogr 27:699–713
Bogousslavsky J, Despland PA, Regli F, Dubuis PY (1989) Postpartum cerebral angiopathy: reversible vasoconstriction assessed by transcranial Doppler ultrasounds. Eur Neurol 29:102–105
Calabrese LH, Gragg LA, Furlan AJ (1993) Benign angiopathy: a distinct subset of angiographically defined primary angiitis of the central nervous system. J Rheumatol 20:2046–2050
Calabrese LH, Furlan AJ, Gragg LA, Ropos TJ (1992) Primary angiitis of the central nervous system: diagnostic criteria and clinical approach. Cleve Clin J Med 59:293–306
Calabrese LH, Mallek JA (1988) Primary angiitis of the central nervous system. Report of 8 new cases, review of the literature, and proposal for diagnostic criteria. Medicine (Baltimore) 67:20–39
Calado S, Vale-Santos J, Lima C, Viana-Baptista M (2004) Postpartum cerebral angiopathy: vasospasm, vasculitis or both? Cerebrovasc Dis 18:340–341
Call GK, Fleming MC, Sealfon S, Levine H, Kistler JP, Fisher CM (1988) Reversible cerebral segmental vasoconstriction. Stroke 19:1159–1170
Chames MC, Livingston JC, Ivester TS, Barton JR, Sibai BM (2002) Late postpartum eclampsia: a preventable disease? Am Journal Obstet Gynecol 186:1174–1177
Chang GY (2000) Basilar artery vasospasm in postpartum cerebral angiopathy. Neurology 55:1596
Comabella M, Alvarez-Sabin J, Rovira A, Codina A (1996) Bromocriptine and postpartum cerebral angiopathy: a causal relationship? Neurology 46:1754–1756
Crippa G, Sverzellati E, Pancotti D, Carrara GC (2000) Severe postpartum hypertension and reversible cerebral angiopathy associated with ergot derivative (methergoline) administration. Ann Ital Med Int 15:303–305
de Jong-van den Berg L, Mintzes B (1995) Bromocriptine and lactation suppression: are the risks acceptable? Pharm World Sci 17:93–95
Donaldson JO (2000) Eclampsia and postpartum cerebral angiopathy. J Neurol Sci 178:1
Dziewas R, Stogbauer F, Freund M, Ludemann P, Imai T, Holzapfel C, Ringelstein PB (2002) Late onset postpartum eclampsia: a rare and difficult diagnosis. J Neurol 249:1287–1291
Ellison C, Martens R, Belkin R, Bourdette D (1988) Postpartum cerebral angiopathy: A benign variant of isolated CNS vasculitis? Neurolo 38:110–110
Farine D, Andreyko J, Lysikiewicz A, Simha S, Addison A (1984) Isolated angiitis of brain in pregnancy and puerperium. Obstet Gynecol 63:586–588
Felz MW, Barnes DB, Figueroa RE (2000) Late postpartum eclampsia 16 days after delivery: case report with clinical, radiologic, and pathophysiologic correlations. J Am Board Fam Pract 13:39–46
Garner BF, Burns P, Bunning RD, Laureno R (1990) Acute blood pressure elevation can mimic arteriographic appearance of cerebral vasculitis—(a postpartum case with relative hypertension). J Rheumatol 17:93–97
Gary Cunningham, Kenneth J. Leveno, Steven L. Bloom, John C. Hauth, Larry C. Gilstrap, Katharine D. Wenstrom. 2005. Williams Obstetrics. 1600
Geocadin RG, Razumovsky AY, Wityk RJ, Bhardwaj A, Ulatowski JA (2002) Intracerebral hemorrhage and postpartum cerebral vasculopathy. J Neurol Sci 205:29–34
Geraghty JJ, Hoch DB, Robert ME, Vinters HV (1991) Fatal puerperal cerebral vasospasm and stroke in a young woman. Neurology 41:1145–1147
Granier I, Garcia E, Geissler A, Boespflug MD, Durand-Gasselin J (1999) Postpartum cerebral angiopathy associated with the administration of sumatriptan and dihydroergotamine—a case report. Intensive Care Med 25:532–534
Henry PY, Larre P, Aupy M, Lafforgue JL, Orgogozo JM (1984) Reversible cerebral arteriopathy associated with the administration of ergot derivatives. Cephalalgia 4:171–178
Herings RM, Stricker BH (1995) Bromocriptine and suppression of postpartum lactation. The incidence of adverse cardiovascular effects in women of child-bearing age. Pharm World Sci 17:133–137
Iffy L, O’Donnell J, Correia J, Hopp L (1998) Severe cardiac dysrhythmia in patients using bromocriptine postpartum. Am J Ther 5:111–115
Iffy L, Lindenthal J, McArdle JJ, Ganesh V (1996a) Severe cerebral accidents postpartum in patients taking bromocriptine for milk suppression. Isr J Med Sci 32:309–312
Iffy L, McArdle JJ, Ganesh V (1996b) Intracerebral hemorrhage in normotensive mothers using bromocriptine postpartum. ZentralblGynakol 118:392–395
Ihara M, Yanagihara C, Nishimura Y (2000) Serial transcranial color-coded sonography in postpartum cerebral angiopathy. J Neuroimaging 10:230–233
Janssens E, Hommel M, Mounier-Vehier F, Leclerc X, Guerin du Masgenet B, Leys D (1995) Postpartum cerebral angiopathy possibly due to bromocriptine therapy. Stroke 26:128–130
Konstantinopoulos PA, Mousa S, Khairallah R, Mtanos G (2004) Postpartum cerebral angiopathy: an important diagnostic consideration in the postpartum period. Am J Obstet Gynecol 191:375–377
Kubo S, Nakata H, Tatsumi T, Yoshimine T (2002) Headache associated with postpartum cerebral angiopathy: monitoring with transcranial color-coded sonography. Headache 42:297–300
Kulig K, Moore LL, Kirk M, Smith D, Stallworth J, Rumack B (1991) Bromocriptine-associated headache: possible life-threatening sympathomimetic interaction. Obstet Gynecol 78:941–943
Langlois PF, Sharon GE, Gawryl MS (1989) Plasma concentrations of complement-activation complexes correlate with disease activity in patients diagnosed with isolated central nervous system vasculitis. J Allergy Clin Immunol 83:11–16
Lee C, Hsu TY, Ou CY, Chang SY, Soong YK (1999) Retinal detachment in postpartum preeclampsia and eclampsia: report of two cases. Changgeng Yi Xue Za Zhi 22:520–524
Leitch CR, Cameron AD, Walker JJ (1997b) The changing pattern of eclampsia over a 60-year period. Br J Obstet Gynaecol 104:917–922
Lubarsky SL, Barton JR, Friedman SA, Nasreddine S, Ramadan MK, Sibai BM (1994) Late postpartum eclampsia revisited. Obstet Gynecol 83:502–505
Makdassi R, de Cagny B, Lobjoie E, Andrejak M, Fournier A (1996) Convulsions, hypertension crisis and acute renal failure in postpartum: role of bromocriptine? Nephron 72:732–733
Martin J, Sidman R (2003) Late postpartum eclampsia: a common presentation of an uncommon diagnosis. J Emerg Med 25:387–390
Mathew R, Raj RS, Sudha P (2003) Late postpartum eclampsia without prodroma. Neurol India 51:539–540
Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM (2004) Delayed postpartum preeclampsia: an experience of 151 cases. Am J Obstet Gynecol 190:1464–1466
Modi M, Modi G (2000) Case reports: postpartum cerebral angiopathy in a patient with chronic migraine with aura. Headache 40:677–681
Morgans D (1995) Bromocriptine and postpartum lactation suppression. Br J Obstet Gynaecol 102:851–853
Newbould S (2002) Postpartum eclampsia. Am Fam Physician 66:1
No authors listed (1984) Postpartum hypertension, seizures, strokes reported with bromocriptine. FDA Drug Bul 14:3–4
Qureshi AI, Frankel MR, Ottenlips JR, Stern BJ (1996) Cerebral hemodynamics in preeclampsia and eclampsia. Arch Neurol 53:1226–1231
Raps EC, Galetta SL, Broderick M, Atlas SW (1993) Delayed peripartum vasculopathy: cerebral eclampsia revisited. Ann Neurol 33:222–225
Raroque HG Jr, Tesfa G, Purdy P (1993) Postpartum cerebral angiopathy. Is there a role for sympathomimetic drugs? Stroke 24:2108–2110
Ringer AJ, Qureshi AI, Kim SH, Fessler RD, Guterman LR, Hopkins LN (2001) Angioplasty for cerebral vasospasm from eclampsia. Surg Neurol 56:, 73–8; discussion 378–379
Roh JK, Park KS (1998) Postpartum cerebral angiopathy with intracerebral hemorrhage in a patient receiving lisuride. Neurology 50:1152–1154
Sekimoto E, Kunishige M, Kuriwaka R, Shinohara M, Ebisutani D, Kitamura K, Doi T, Kushiki N, Mitsui T (2002) Delayed vasospasm in late postpartum cerebral angiopathy after withdrawal of methylergometrine. Cerebrovas Dis 13:288–289
Sengar AR, Gupta RK, Dhanuka AK, Roy R, Das K (1997) MR imaging, MR angiography, and MR spectroscopy of the brain in eclampsia. AJNR Am J Neuroradiol 18:1485–1490
Singhal AB (2004a) Cerebral vasoconstriction syndromes. Top Stroke Rehabil 11:1–6
Singhal AB (2004b) Postpartum angiopathy with reversible posterior leukoencephalopathy. Arch Neurol 61:411–416
Song JK, Fisher S, Seifert TD, Cacayorin ED, Alexandrov AV, Malkoff MD, Grotta JC, Campbell MS.(2004) Postpartum cerebral angiopathy: atypical features and treatment with intracranial balloon angioplasty. Neuroradiology
Sugiyama Y, Muroi A, Ishikawa M, Tsukamoto T, Yamamoto T (1997) A benign form of isolated angiitis of the central nervous system in puerperium: an identical disorder to postpartum cerebral angiopathy? Intern Med 36:931–934
Tank PD, Chauhan AR, Bhattacharya MS, Warke HS, Raut VS (2004) Neurological complications in eclampsia: a case series. Int J Fertil Womens Med 49:61–69
Thomas SV (1998) Neurological aspects of eclampsia. J Neurol Sci 155:37–43
Trommer BL, Homer D, Mikhael MA (1988) Cerebral vasospasm and eclampsia. Stroke 19:326–329
Ursell MR, Marras CL, Farb R, Rowed DW, Black SE, Perry JR (1998) Recurrent intracranial hemorrhage due to postpartum cerebral angiopathy: implications for management. Stroke 29:1995–1998
Ursell MR, Marras CL, Farb R, Rowed DW, Black SE, Perry JR (1995) Recurrent intracranial hemorrhage due to postpartum cerebral angiopathy: implications for management. Stroke 29:1995–1998
Veltkamp R, Kupsch A, Polasek J, Yousry TA, Pfister HW (2000) Late onset postpartum eclampsia without pre-eclamptic prodromi: clinical and neuroradiological presentation in two patients. J Neurol Neurosurg Psychiatry 69:824–827
Yasuda Y, Matsuda I, Kang Y, Saiga T, Kameyama M (1993) Isolated angiitis of the central nervous system first presenting as intracranial hemorrhage during cesarean section. Intern Med 32:745–748
Zunker P, Golombeck K, Brossmann J, Georgiadis D, Deuschl G (2002) Post-partum cerebral angiopathy: repetitive TCD, MRI, MRA, and EEG examinations. Neurol Res 24:570–572
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Williams, T.L., Lukovits, T.G., Harris, B.T. et al. A fatal case of postpartum cerebral angiopathy with literature review. Arch Gynecol Obstet 275, 67–77 (2007). https://doi.org/10.1007/s00404-006-0194-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00404-006-0194-3